Synthesis of enantiopure α-Tfm-proline and α-Tfm-pipecolic acid from oxazolo-pyrrolidines and -piperidines†
Abstract
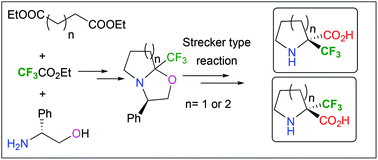
Enantiopure α-Tfm-proline and α-Tfm-pipecolic acid were synthesized starting from commercially available diesters and ethyl trifluoroacetate. A Strecker type reaction on intermediate chiral Tfm-oxazolo-pyrrolidine and -piperidine provided the corresponding nitrile precursor of enantiopure (R) and (S) α-Tfm-proline and α-Tfm-pipecolic acid. The C-terminal peptide coupling reaction of α-Tfm-pipecolic acid has been successfully achieved.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...