Open Access Article
Open Access ArticleCalixarene-decorated liposomes for intracellular cargo delivery†
Ilaria
Morbioli
a,
Alessandro
Casnati
 a,
Jeffrey D.
Esko
b,
Yitzhak
Tor
a,
Jeffrey D.
Esko
b,
Yitzhak
Tor
 *c and
Francesco
Sansone
*c and
Francesco
Sansone
 *a
*a
aDepartment of Chemistry, Life Sciences and Environmental Sustainability, Università degli Studi di Parma, Parco Area delle Scienze 17/A, 43124 Parma, Italy. E-mail: francesco.sansone@unipr.it
bDepartment of Cellular and Molecular Medicine, University of California, San Diego, La Jolla, California 92093, USA
cDepartment of Chemistry and Biochemistry, University of California, San Diego, La Jolla, California 92093, USA. E-mail: ytor@ucsd.edu
First published on 16th July 2021
Abstract
Amphiphilic calix[4]arenes, functionalized with guanidinium groups, are used to decorate the outer surface of liposomes and significantly improve the cellular uptake of a cargo compared to plain liposomes. The improved uptake is elicited and mediated by the interaction between the cationic polar heads of the macrocycle units embedded in the liposome bilayer and anionic heparan-sulfate proteoglycans surrounding the exterior of cells.
Drug development efforts have been driven by the continuous discovery of new pharmacological targets. In addition to bioactive small molecules (both natural and synthetic), high molecular weight effectors, such as proteins1,2 and nucleic acids,3,4 have been drawing attention as therapeutic tools. The efficacy of therapies and the associated side effects are related, among other factors, to the proper delivery of the drugs to their intended target. The selective and effective internalization of drugs into cells remain a major challenge. Additionally, issues of size, charge, solubility and stability of the therapeutic agents need optimization for their satisfactory administration.1,2,4,5 Moreover, widespread non-selective distribution in both healthy and injured tissues ultimately requires higher doses, which could be associated with side effects and immune response activation, besides being less economically viable and sustainable.
The active transport of therapeutic agents by suitable carriers can potentially overcome some or all of the mentioned limitations. Depending on its structure, the carrier can protect the bioactive component from degradation and improve its solubility, while masking it from the immune system and potentially elevating targeted delivery. Certain carriers can also facilitate cell membrane permeation and impact the cellular distribution among organelles. Among the strategies that have been exploited for drug delivery, liposomes are the most successful system known to date,6 satisfying many of the criteria listed above,7 with several clinically approved liposomal formulation.8 An important advantage liposomes possess is the simple functionalization of their surface. By embedding specific ligands, “smart” systems can be programmed, as in the case of folate-modified liposomes,9–12 which preferentially target cancer cells overexpressing folate receptors. Alternatively, the incorporation of probes facilitates tracking and diagnostic imaging.13–16 Long-circulating liposomes can be obtained by covering their surface with carbohydrates or polymers.17
Unfortunately, the size of liposomes is an obstacle to their uptake. Since easy permeation through the cell membrane is not possible, alternative internalization mechanisms need to be implemented. Decorating liposome bilayers with cell-penetrating peptides (e.g., Tat peptide) has been reported to enhance their cellular uptake.18–21 Analogously, liposomes decorated with guanidinium-rich molecules such as guanidinylated neomycin (then termed GNeosomes) have been shown to effectively enter cells and facilitate the lysosomal delivery of both small molecules and bioactive proteins.22 The effective cellular uptake and delivery have been attributed to the affinity of guanidinoglycosides to cell surface heparin-sulfate proteoglycans (HSPGs).22–28
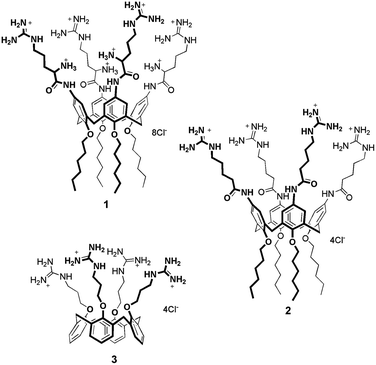
Amphiphilic calixarenes functionalized with guanidinium groups29 have been shown to be efficient carriers for gene delivery and transfection.29–32 One derivative, in particular, bearing four arginine units at the upper rim, demonstrated to be a potent non-viral vector, capable of delivering DNA plasmids,32 microRNAs33 and peptide nucleic acids (PNA)34 across cell membrane. (Bola)Amphiphilic calixarenes have also been successfully embedded in liposome lipid bilayers.35–39 We therefore envisioned that decorating the outer surface of liposomes with guanidinylated calixarenes would provide hybrid systems synergistically leading to molecular carriers with improved cell penetration properties. The specific use of properly functionalized calixarenes can be particularly advantageous since these multivalent macrocycles demonstrated added values with respect to monovalent and/or non-macrocyclic analogues.32,40 Moreover, in perspective, the presence of their cavity could be exploited to provide the liposomes with additional functions thanks to non-covalent host–guest phenomena. Here we describe how three different amphiphilic cone-shaped calixarenes variably functionalized with guanidinium groups (Fig. 1) were embedded in liposome bilayers and how they affect the cellular uptake properties for the modified liposomes obtained.
Synthesis of calixarene derivatives
Calixarenes 132 and 331 were synthesized accordingly to published procedures. Calixarene 2 was prepared (Scheme S1†) from 5,11,17,23-tetraamino-25,26,27,28-tetrahexyloxycalix[4]arene29 that was coupled with Boc protected 5-aminopentanoic acid using HBTU as coupling reagent. After removal of the Boc protecting groups, the derivative was treated with bis-Boc-triflyl guanidine. Final acidic deprotection provided calixarene 2 containing the 5-guanidiniumpentanoic acid units.Calixarene 1 presents four arginine units at the upper rim and hexyl chains at the lower rim, while 2 bears 5-guanidinylated pentanoyl units instead of Arg residues at the upper rim. This latter derivative was designed to evaluate the significance of the amino acid primary α-amino groups for the internalization process. Compound 3 displays the guanidinium groups at the lower rim and does not present aliphatic tails. The lipophilic portion, which confers the amphiphilic character to this derivative, is represented by the aromatic skeleton of the macrocycle.31
The tetraarginino derivative 1 was previously shown to self-assemble into spherical aggregates in water.32 Analogously, calixarene 2 tends to aggregate as verified by 1H NMR in D2O, showing spectra characterized by broadening of the signals even up to 80 °C (Fig. S1†). This behavior, common to both compounds, supported the hypothesis that they could be reasonably embedded into lipophilic liposomal bilayers. This was not necessarily obvious for derivative 3 that does not present linear aliphatic chains and indeed aggregates in aqueous environment only in presence of high salt concentrations.31
Liposomes preparation
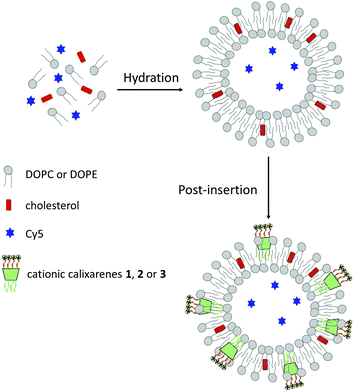
Liposomes, encapsulating a fluorescent dye for monitoring uptake, were prepared following an optimized procedure previously developed (Fig. 2).26 Briefly, lipid films containing 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and cholesterol in a 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 ratio, were hydrated with a 100 μM aqueous solution of a positively charged Cy5 cyanine dye (in ESI† the structure). The suspension formed was sonicated, freeze–thawed and extruded through a polycarbonate membrane to afford 100 nm liposomes. The non-encapsulated dye was removed by size exclusion chromatography. Efficiency of Cy5 dye encapsulation was determined by measuring the fluorescence intensity of solutions in MeOH of the liposomes at 0.1 mg mL−1 concentration, before and after size exclusion chromatography (excitation and emission wavelength at 640 and 672 nm, respectively). A dye encapsulation efficiency of 15% was estimated by the ratio between the two fluorescence intensities.
16 ratio, were hydrated with a 100 μM aqueous solution of a positively charged Cy5 cyanine dye (in ESI† the structure). The suspension formed was sonicated, freeze–thawed and extruded through a polycarbonate membrane to afford 100 nm liposomes. The non-encapsulated dye was removed by size exclusion chromatography. Efficiency of Cy5 dye encapsulation was determined by measuring the fluorescence intensity of solutions in MeOH of the liposomes at 0.1 mg mL−1 concentration, before and after size exclusion chromatography (excitation and emission wavelength at 640 and 672 nm, respectively). A dye encapsulation efficiency of 15% was estimated by the ratio between the two fluorescence intensities.
Part of the plain liposomes obtained in this way were then decorated with different calixarenes using a post-insertion method.26 This procedure presents two main advantages: (1) it can be applied to any sufficiently amphiphilic ligand, and (2) the inserted ligands are exclusively found in the outer layer with their polar heads facing the exterior of the liposomes. Experimentally, an aqueous solution (20 μL, 2.7 mol%) of the calixarene (1–3) was equilibrated for 1 h at room temperature with a suspension of the liposomes and the unincorporated calixarene molecules were removed by centrifuge gel filtration giving liposomes Lipo-1, Lipo-2 and Lipo-3, respectively.
Unmodified and decorated liposomes were analyzed for their size and Z-potential (Table S1†). The average hydrodynamic diameter of the liposomes does not significantly change after the insertion of the calixarenes. The Z-potential values measured following the treatment with the macrocycles is, however, significantly different from that of the plain liposomes. While the latter is near 0, a positive increase is observed, as expected, for Lipo-1, -2 and -3, supporting the presence of the positively charged calixarenes on the liposomal surface. The Z-potential values measured for Lipo-1 and Lipo-2 are very similar although 1 could potentially possess up to eight positive charges per molecule. This could be explained by either modulation of the pKa values of argininocalixarene 1 or its lower incorporation level. The higher overall charge at the upper rim of 1 makes it more polar than 2 and therefore it should be less prone to insertion into the lipid bilayer; additionally, the highly solvated and charged ammonium groups, which are closer than the guanidinium groups to the aromatic/lipophilic region of the macrocycle, could in part hamper the incorporation into the lipid bilayer. The Z-potential of Lipo-3, functionalized with the lower rim guanidinocalixarene 3 is lower, likely due to the lack of aliphatic tails, that makes its insertion into the membrane less efficient.
Cellular uptake
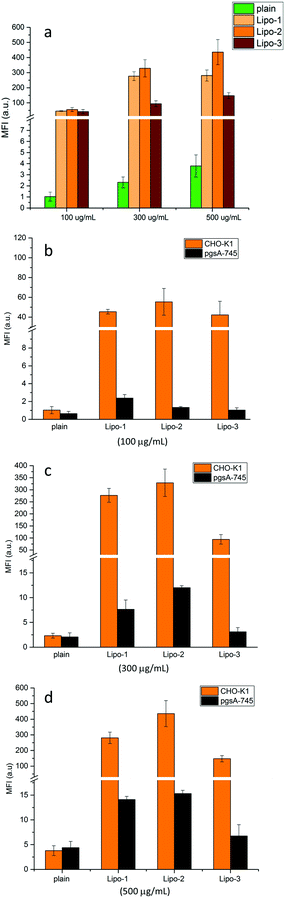
Cellular uptake was first evaluated in wild-type Chinese hamster ovary (CHO-K1) cells. These were incubated with solutions of plain liposomes and Lipo-1, -2 and -3 at different concentrations (100, 300 and 500 μg mL−1), for 20 minutes (37 °C, 5% CO2), then harvested and analyzed by flow cytometry to determine the mean fluorescence intensity (MFI) of the encapsulated Cy5.The MFI of cells treated with Lipo-1, -2 and -3 (Fig. 3 and Table S2†) is significantly higher than that of cells treated with the plain liposomes (between 100 to 450 fold), clearly demonstrating the role played by guanidinocalixarenes in enhancing cellular internalization. At the lowest concentration tested (100 μg mL−1), the cellular uptake of Lipo-1–3 is practically identical. At higher concentrations, higher uptake efficiency is observed for Lipo-1 and Lipo-2 when compared to Lipo-3. The poorer uptake of the latter could be related to its lower degree of functionalization as suggested by its lower Z-potential. This could also explain the slightly better uptake efficiency of Lipo-2 compared to Lipo-1. The observed differences further support the direct involvement of the calixarenes embedded at the outer surface of the liposomes. Comparing the results displayed by Lipo-1 and Lipo-2 also suggests a small detrimental effect of the primary α-ammonium groups on cellular uptake, likely due to its negative impact, mentioned above, on the upstream calixarene insertion into the liposome bilayer. This is consistent, indeed, with the zeta potentials, that, being essentially the same for Lipo-1 and Lipo-2 despite the high number of potentially charged groups in 1, support the hypothesis of a lower concentration of this latter on the liposome surface and then justify the corresponding lower effect on the uptake process.
A fundamental role for cell surface HSPGs on the uptake of guanidinium-rich molecules has been proposed.41–46 To establish their role in the internalization of guanidinocalixarene-decorated liposomes, the same uptake experiments were repeated with pgsA-745 cells, a mutant CHO-K1 cell-line that does not express HSPGs.
For all the decorated liposomes, even at longer incubation times (1 h vs. 20 min), a substantially reduced uptake of Cy5 was observed in pgsA cells (Fig. 3b–d and Table S3†), roughly corresponding to around the 5% of the uptake observed with wild-type CHO-K1 cells and comparable to that of the plain liposomes in both cell-lines. HSPGs therefore play key roles in the internalization process of these calixarene-functionalized liposomes.
Cell viability in presence of the plain and modified liposomes was monitored using CellTiter BlueTM at the same concentrations employed for the uptake experiments. No significant toxicity was observed for the modified liposomes at these concentrations. Quite surprisingly, the percentage of viable cells was lower for the plain liposome with respect to the modified liposomes (Fig. S15†).
Conclusions
Amphiphilic calixarenes 1–3 have been demonstrated to be suitable for the functionalization of the outer surface of liposomes. Their presence does not alter the liposomes size but changes, as expected, the Z-potential from neutrality to +30–50 mV depending on the calixarene used. The functionalized liposomes Lipo-1–3 effectively internalize the Cy5 dye into cells. This effective cellular uptake, when compared to the limited uptake seen in cells treated with plain liposomes, illustrates the crucial role played by guanidinium-containing calixarenes in facilitating this process. Experiments with pgsA cells, a mutant CHO-K1 cell line which does not express heparan sulfate proteoglycans, showed substantially reduced dye uptakes, comparable to that of plain liposomes, not only confirming the involvement of these cell surface macromolecules in the internalization phenomenon, but also highlighting the role played by the cationic guanidinium groups of the three calixarenes. In perspective, these systems and in particular those decorated with 1 and 2, can be investigated for the delivery of pharmaceutically relevant molecules, as well as for exploring possible synergism between targeting and signalling functions due to host–guest complexation processes associated with the calixarene cavity.Author contributions
IM: investigation, visualization; AC: conceptualization, funding acquisition, resources, writing – review & editing; JDE: resources, writing – review & editing; YT: conceptualization, resources, supervision, writing – original draft, writing – review & editing; FS: conceptualization, funding acquisition, supervision, writing – original draft, writing – review & editing.Conflicts of interest
The authors declare the absence of any conflict of interest.Acknowledgements
FS and AC acknowledge the Centro Interdipartimentale Misure “G. Casnati” of Parma University for the use of NMR and Mass Spectrometry facilities. This work benefited from the equipment and framework of the COMP-HUB Initiative, funded by the ‘Departments of Excellence’ program of the Italian Ministry for Education, University and Research (MIUR, 2018–2022) and was partially supported by MIUR Project PRIN 2017E44A9P.Notes and references
- V. P. Torchilin, Drug Discovery Today: Technol., 2009, 5, 2 Search PubMed.
- R. Kintzing, M. V. Filsinger Interrante and J. R. Cochran, Trends Pharmacol. Sci., 2016, 37, 993 CrossRef PubMed.
- J. B. Opalinska and A. M. Gewirtz, Nat. Rev. Drug Discovery, 2002, 1, 503 CrossRef CAS PubMed.
- E. Mastrobattista, W. E. Hennink and R. M. Schiffelers, Pharm. Res., 2007, 24, 1561 CrossRef CAS PubMed.
- M. Cicerone, J. Giri, Z. Shaked and C. Roberts, Adv. Drug Delivery Rev., 2015, 93, 1 CrossRef CAS PubMed.
- B. Felice, M. P. Prabhakaran, A. P. Rodríguez and S. Ramakrishna, Mater. Sci. Eng., C, 2014, 41, 178 CrossRef CAS PubMed.
- G. Bozzuto and A. Molinari, Int. J. Nanomed., 2015, 10, 975 CrossRef CAS PubMed.
- D. K. Mishra, R. Shandilya and P. K. Mishra, Nanomedicine, 2018, 14, 2023 CrossRef CAS PubMed.
- X. Q. Pan, H. Wang and R. J. Lee, Pharm. Res., 2003, 20, 417 CrossRef CAS PubMed.
- R. J. Lee and P. S. Low, J. Biol. Chem., 1994, 269, 3198 CrossRef CAS.
- A. Gabizon, H. Shmeeda, A. T. Horowitz and S. Zalipsky, Adv. Drug Delivery Rev., 2004, 56, 1177 CrossRef CAS PubMed.
- Y. Lu and P. S. Low, Adv. Drug Delivery Rev., 2012, 64, 342 CrossRef.
- B. L. Viglianti, S. A. Abraham, C. R. Michelich, P. S. Yarmolenko, J. R. MacFall, M. B. Bally and M. W. Dewhirst, Magn. Reson. Med., 2004, 51, 1153 CrossRef CAS PubMed.
- A. Bao, B. Goins, R. Klipper, G. Negrete and W. T. Phillips, J. Nucl. Med., 2003, 44, 1992 CAS.
- V. Weissig, J. Babich and V. Torchilin, Colloids Surf., B, 2000, 18, 293 CrossRef CAS PubMed.
- V. P. Torchilin, Mol. Med. Today, 1996, 2, 242 CrossRef CAS PubMed.
- M. L. Immordino, F. Dosio and L. Cattel, Int. J. Nanomed., 2006, 1, 297 CrossRef CAS PubMed.
- V. P. Torchilin, R. Rammohan, V. Weissig and T. S. Levchenko, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8786 CrossRef CAS PubMed.
- V. P. Torchilin, T. S. Levchenko, R. Rammohan, N. Volodina, B. Papahadjopoulos-Sternberg and G. G. M. D'Souza, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1972 CrossRef CAS PubMed.
- Y.-L. Tseng, J.-J. Liu and R.-L. Hong, Mol. Pharmacol., 2002, 62, 864 CrossRef CAS PubMed.
- R. Khandia, A. Munjal, A. Kumar, G. Singh, K. Karthik and K. Dhama, Int. J. Pharmacol., 2017, 13, 677 CAS.
- L. Elson-Schwab, O. B. Garner, M. Schuksz, B. E. Crawford, J. D. Esko and Y. Tor, J. Biol. Chem., 2007, 282, 13585 CrossRef CAS PubMed.
- S. Sarrazin, B. Wilson, W. S. Sly, Y. Tor and J. D. Esko, Mol. Ther., 2010, 18, 1268 CrossRef CAS PubMed.
- A. V. Dix, L. Fischer, S. Sarrazin, C. P. H. Redgate, J. D. Esko and Y. Tor, ChemBioChem, 2010, 11, 2302 CrossRef CAS PubMed.
- E. Wexselblatt, J. D. Esko and Y. Tor, ACS Nano, 2015, 9, 3961 CrossRef CAS PubMed.
- K. M. Hamill, E. Wexselblatt, W. Tong, J. D. Esko and Y. Tor, J. Mater. Chem. B, 2016, 4, 5794 RSC.
- W. Tong, C. A. Dwyer, B. E. Thacker, C. A. Glass, J. R. Brown, K. Hamill, K. W. Moremen, S. Sarrazin, P. L. S. M. Gordts, L. E. Dozier, G. N. Patrick, Y. Tor and J. D. Esko, Mol. Ther., 2017, 25, 2743 CrossRef CAS PubMed.
- E. Wexselblatt, J. D. Esko and Y. Tor, J. Org. Chem., 2014, 79(15), 6766 CrossRef CAS PubMed.
- F. Sansone, M. Dudič, G. Donofrio, C. Rivetti, L. Baldini, A. Casnati, S. Cellai and R. Ungaro, J. Am. Chem. Soc., 2006, 128, 14528 CrossRef CAS PubMed.
- M. Dudič, A. Colombo, F. Sansone, A. Casnati, G. Donofrio and R. Ungaro, Tetrahedron, 2004, 60, 11613 CrossRef.
- V. Bagnacani, V. Franceschi, L. Fantuzzi, A. Casnati, G. Donofrio, F. Sansone and R. Ungaro, Bioconjugate Chem., 2012, 23, 993 CrossRef CAS PubMed.
- V. Bagnacani, V. Franceschi, M. Bassi, M. Lomazzi, G. Donofrio, F. Sansone, A. Casnati and R. Ungaro, Nat. Commun., 2013, 4, 1721 CrossRef PubMed.
- J. Gasparello, M. Lomazzi, C. Papi, E. D'Aversa, F. Sansone, A. Casnati, G. Donofrio, R. Gambari and A. Finotti, Mol. Ther.–Nucleic Acids, 2019, 18, 748 CrossRef CAS PubMed.
- J. Gasparello, A. Manicardi, A. Casnati, R. Corradini, R. Gambari, A. Finotti and F. Sansone, Sci. Rep., 2019, 9, 3036 CrossRef PubMed.
- S. Aleandri, A. Casnati, L. Fantuzzi, G. Rispoli, G. Mancini and F. Sansone, Org. Biomol. Chem., 2013, 11, 4811 RSC.
- Y.-X. Wang, Y.-M. Zhang, Y.-L. Wang and Y. Liu, Chem. Mater., 2015, 27, 2848 CrossRef CAS.
- Y.-C. Pan, H.-W. Tian, S. Peng, X.-Y. Hu and D.-S. Guo, Chin. Chem. Lett., 2017, 28, 787 CrossRef CAS.
- H.-W. Tian, Y.-C. Pan and D.-S. Guo, Supramol. Chem., 2017, 30, 562 CrossRef.
- H.-W. Tian, Y.-C. Liu and D.-S. Guo, Mater. Chem. Front., 2020, 4, 46 RSC.
- L. Baldini, A. Casnati and F. Sansone, Eur. J. Org. Chem., 2020, 5056 CrossRef CAS.
- T. Suzuki, S. Futaki, M. Niwa, S. Tanaka, K. Ueda and Y. Sugiura, J. Biol. Chem., 2002, 277, 2437 CrossRef CAS PubMed.
- S. Console, C. Marty, C. Garcia-Echeverria, R. Schwendener and K. Ballmer-Hofer, J. Biol. Chem., 2003, 278, 35109 CrossRef CAS PubMed.
- A. Ziegler and J. Seelig, Biophys. J., 2004, 86, 254 CrossRef CAS PubMed.
- J. P. Richard, K. Melikov, H. Brooks, P. Prevot, B. Lebleu and L. V. Chernomordik, J. Biol. Chem., 2005, 280, 15300 CrossRef CAS PubMed.
- I. Nakase, T. Takeuchi, G. Tanaka and S. Futaki, Adv. Drug Delivery Rev., 2008, 60, 598 CrossRef CAS PubMed.
- H. C. Christianson and M. Belting, Matrix Biol., 2014, 35, 51 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compounds characterization and NMR spectra; liposome characterization; tables with mean fluorescence intensity (MFI) values from uptake experiments. See DOI: 10.1039/d1ob01055g |
| This journal is © The Royal Society of Chemistry 2021 |