Diversification of α-ketoamides via transamidation reactions with alkyl and benzyl amines at room temperature†‡
Abstract
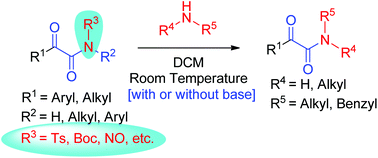
A wide range of N-tosyl α-ketoamides underwent transamidation with various alkyl amines in the absence of a catalyst, base, or additive. On the other hand, transamidation in N-Boc α-ketoamides was achieved in the presence of Cs2CO3. The reactions proceeded at room temperature and provided good to excellent yields of transamidation products under the optimized conditions. Broad substrate scope, functional group tolerance and quick conversions are the important features of the developed methodology.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...