Asymmetric synthesis of (−)-dehydro-exo-brevicomin with a photoisomerisation–intramolecular acetalisation sequence†
Abstract
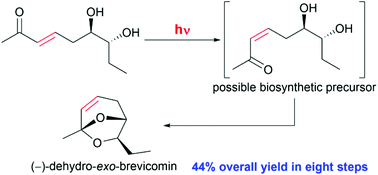
We herein report a novel, short asymmetric synthesis of (−)-dehydro-exo-brevicomin (DHB, 1), a sex pheromone isolated from house mice, in 44% overall yield, the highest yield reported so far, over eight steps from trans-3-hexen-1-ol (7). We successfully prepared the target molecule (−)-1 from spontaneous intramolecular acetalisation after the photoisomerisation of trans-enone 6, which generated the corresponding cis-enone 5in situ, the possible biosynthetic precursor of DHB.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...