Recent advances in decarbonylative annulation reactions
Abstract
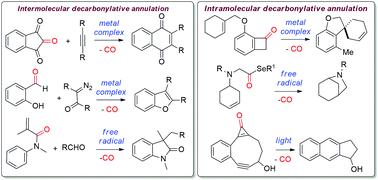
In recent years, decarbonylative annulation reactions have emerged as one of the most efficient routes to construct a variety of important carbocyclic and heterocyclic scaffolds. The main advantages of this type of reaction are, first, the carbonyl compounds are used as the starting materials which are abundant in nature and second, the major by-product of this reaction is carbon monoxide. In the last two decades, various intramolecular and intermolecular decarbonylative annulation reactions have been performed using carbonyl compounds and alkenes/alkynes/arenes/isocyanates etc. as the annulation partners. These reactions are typically performed either in the presence of or in the absence of a metal catalyst. However, these reactions are still in their infancy as a very little progress has been achieved in these reactions. Through this review article, it is attempted to highlight the recent developments on various decarbonylative annulation reactions which ultimately lead to the formation of important structural moieties.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...