Lan Zhao, Changfu Qiu, Lixin Zhao, Guangwei Yin, Fangyi Li, Chunhua Wang and Zheng Li
Org. Biomol. Chem., 2021,19, 5377-5382
DOI:
10.1039/D1OB00731A,
Paper
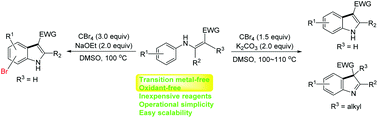
Described here is a general and highly efficient method for the synthesis of 1H- and 3H-indoles. In the presence of CBr4 and a suitable base, the cyclization of N-aryl enamines proceeds with high efficiency. Unlike previous intramolecular cross dehydrogenative coupling (CDC) of the same substrates, this process does not require the use of either a transition metal or a stoichiometric amount of oxidant. This method also features operational simplicity, easy scalability and good substrate tolerability. Control experiments indicate the reactions may proceed in a tandem sequence of bromination and intramolecular Friedel–Crafts alkylation in a simple one-pot procedure.