Self-assembly of neutral platinum complexes possessing chiral hydrophilic TEG chains†
Abstract
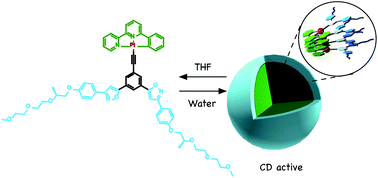
Neutral platinum complexes that possess chiral triethylene glycol (TEG) moieties were synthesized. The platinum complexes formed helically twisted stacked assemblies in chloroform and toluene, which were studied by 1H NMR, UV/vis spectroscopy, and emission spectroscopy. On the other hand, emissive micellar aggregates were observed in a THF/water mixed solvent. Dynamic light scattering (DLS) experiments revealed that micellar aggregates with a diameter (d) of ≈100 nm emitted strong light, whereas the monomeric form and large aggregates (d > 500 nm) did not show luminescence efficiently. Furthermore, the micellar aggregates were twisted chirally, where the twisted direction was determined by the chirality of the TEG moieties. The assemblies were observed to be solvent responsive, which allows for the modulation of the nanostructure by changing the solvent polarity.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...