Catalytic asymmetric umpolung reactions of imines via 2-azaallyl anion intermediates
Abstract
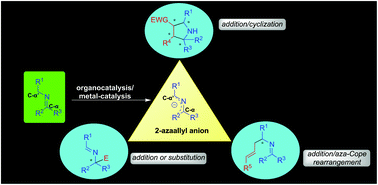
The imine umpolung is a relatively new and interesting strategy, especially in catalytic asymmetric synthesis. A significant development in organo- and transition metal-catalyzed umpolung of imines took place only in the recently concluded decade. A majority of the reports on the asymmetric umpolung of imines involve the initial generation of 2-azaallyl anion intermediates with the chiral catalysts, which serve as a significant driving force for the umpolung addition/substitution reactions. A variety of organocatalysts such as bifunctional cinchona alkaloids including squaramides and thioureas, chiral BINOL derived phosphoric acids, phase transfer catalysts (PTCs), phosphines, and transition metal-complexes of iridium, copper and palladium have been employed to achieve the excellent level of asymmetric induction in such types of umpolung reactions. The asymmetric imine umpolung strategy has been applied successfully to synthesize synthetic amino-acid derivatives and other useful chiral amines, including drugs and potentially bioactive molecules. This review summarizes all the significant recent development in catalytic umpolung reactions of imines involving a 2-azaallyl anion intermediate.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...