HFIP-mediated 2-aza-Cope rearrangement: metal-free synthesis of α-substituted homoallylamines at ambient temperature†
Abstract
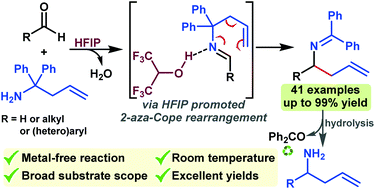
An efficient metal-free strategy for the synthesis of α-substituted homoallylamine derivatives has been developed via a 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)-promoted 2-aza-Cope rearrangement of aldimines, generated in situ by condensation of aldehydes with easily accessible 1,1-diphenylhomoallylamines. This reaction provides rapid access to α-substituted homoallylamines with excellent functional group tolerance and yields. The reaction takes place at room temperature and no chromatographic purification is required for product isolation. The synthetic utility of the current method is further demonstrated by the transformation of the obtained benzophenone ketimines into N-unprotected homoallylamines, an α-amino alcohol and an α-amino amide.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...