Concise catalytic asymmetric synthesis of (R)-4-amino Uhle's ketone†
Abstract
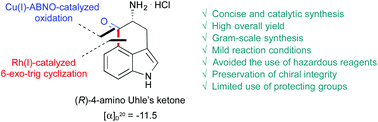
A practical and asymmetric synthesis of (R)-4-amino-5-oxo-1,3,4,5-tetrahydrobenz[cd]indole, an enantiopure framework shared by most ergot alkaloids, was accomplished. Our method involves a Rh(I)-catalyzed 6-exo-trig intramolecular cyclization of an appropriate 4-pinacolboronic ester D-tryptophan aldehyde followed by the oxidation of the resulting secondary benzylic alcohol with a Cu(I)-ABNO catalyst and final deprotection under acidic conditions. This new procedure offers significant advantages over previous synthetic approaches, including brevity, mild reaction conditions, preservation of chiral integrity, and high overall yield and avoids the use of stoichiometric amounts of strongly basic and pyrophoric organometallic reagents.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...