Simple acyclic molecules containing a single charge-assisted O–H group can recognize anions in acetonitrile : water mixtures†
Abstract
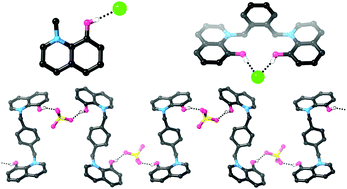
Hydroxypyridinium and hydroxyquinolinium compounds containing acidic O–H groups attached to a cationic aromatic scaffold were synthesized, i.e. N-methyl-3-hydroxypyridinium (1+) and N-methyl-8-hydroxyquinolinium (2+). These very simple compounds are capable of binding to chloride very strongly in CD3CN and with moderate strength in 9 : 1 CD3CN : D2O. Comparison with known association constants reveals that 1+ and 2+ bind chloride in CD3CN or CD3CN : D2O with comparable affinities to receptors containing significantly more hydrogen bond donors and/or higher positive charges. Crystal structures of both compounds with coordinating anions were obtained, and feature short O–H⋯anion hydrogen bonds. A receptor containing two hydroxyquinolinium groups was also prepared. While the low solubility of this compound caused difficulties, we were able to demonstrate chloride binding in a competitive 1 : 1 CD3CN : CD3OD solvent mixture. Addition of sulfate to this compound results in the formation of a crystallographically-characterised solid state anion coordination polymer.
- This article is part of the themed collections: The Supramolecular Chemistry of Anions and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...