Open Access Article
Open Access ArticleThe broad amine scope of pantothenate synthetase enables the synthesis of pharmaceutically relevant amides†
Mohammad Z.
Abidin
 ab,
Thangavelu
Saravanan
ab,
Thangavelu
Saravanan
 *ac,
Erick
Strauss
*ac,
Erick
Strauss
 d and
Gerrit J.
Poelarends
d and
Gerrit J.
Poelarends
 *a
*a
aDepartment of Chemical and Pharmaceutical Biology, Groningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands. E-mail: g.j.poelarends@rug.nl
bDepartment of Animal Products Technology, Gadjah Mada University, Bulaksumur, Yogyakarta 55281, Indonesia
cSchool of Chemistry, University of Hyderabad, P.O. Central University, Hyderabad 500046, India. E-mail: tsaravanan@uohyd.ac.in
dDepartment of Biochemistry, Stellenbosch University, Private Bag X1, Matieland, 7602, South Africa
First published on 24th April 2021
Abstract
Pantothenate synthetase from Escherichia coli (PSE. coli) catalyzes the ATP-dependent condensation of (R)-pantoic acid and β-alanine to yield (R)-pantothenic acid (vitamin B5), the biosynthetic precursor to coenzyme A. Herein we show that besides the natural amine substrate β-alanine, the enzyme accepts a wide range of structurally diverse amines including 3-amino-2-fluoropropionic acid, 4-amino-2-hydroxybutyric acid, 4-amino-3-hydroxybutyric acid, and tryptamine for coupling to the native carboxylic acid substrate (R)-pantoic acid to give amide products with up to >99% conversion. The broad amine scope of PSE. coli enabled the efficient synthesis of pharmaceutically-relevant vitamin B5 antimetabolites with excellent isolated yield (up to 89%). This biocatalytic amide synthesis strategy may prove to be useful in the quest for new antimicrobials that target coenzyme A biosynthesis and utilisation.
1. Introduction
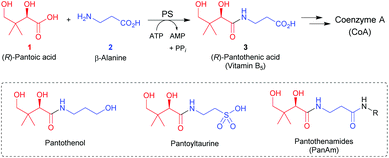
Amide bond-containing natural products are some of the most important biologically active molecules.1,2 In view of that, the development of versatile and sustainable catalytic strategies for amide bond formation is of high industrial and academic interest.3 Biocatalysis has emerged as a sustainable catalytic methodology for amide bond formation, and several enzyme classes have been explored for their usefulness in the preparation of pharmaceutically relevant amides.4–7 In this context, ligase enzymes that use adenosine triphosphate (ATP) to catalyze the formation of amides from carboxylic acids and amines in aqueous media may prove particularly useful.8 A well-known example is pantothenate synthetase (PS), an enzyme that catalyzes the ATP-dependent condensation of (R)-pantoic acid (1) and β-alanine (2) to produce (R)-pantothenic acid (vitamin B5, 3), the biosynthetic precursor of the central metabolic cofactor coenzyme A (CoA) (Scheme 1).9 The PS-catalyzed reaction proceeds via a pantoyl-adenylate intermediate that is attacked by β-alanine.9Enzymes from the CoA biosynthetic pathway have been identified as attractive targets for new antimicrobial compounds.10,11 One of the first strategies that was explored for the inhibition of CoA biosynthesis relied on the preparation of pantothenic acid antimetabolites,12 such as pantothenol and pantoyltaurine (Scheme 1). These compounds maintained the core structure of the vitamin (its pantoic acid moiety) but had its β-alanine portion replaced with related amines that lacked the –COOH group that is required for synthesis of CoA. As such, they would likely be accepted as alternate substrates for CoA biosynthesis, but would block the process at a critical step. This mode of action is the same as that employed by the sulphonamide antimicrobials, which mimic the p-aminobenzoic acid (PABA) substrate of folic acid biosynthesis.13 Importantly, pantothenol, as well as pantoyltaurine and its amides, have shown some promise as antiplasmodial agents, with the pantoyltauramides also exhibiting selective activity against selected streptococcal infections.10
The pantothenamides (PanAms, Scheme 1) are a more recently developed class of pantothenic acid analogues that show potent antimicrobial activity against a variety of pathogenic microorganisms, including Staphylococcus aureus and the malaria parasite Plasmodium falciparum.11,14 Unfortunately the PanAms, which are secondary or tertiary amides of pantothenic acid, are metabolically labile and are prone to hydrolysis by serum enzymes such as the pantetheinases.15 Several strategies have been explored to counter or reduce this degradation to increase the clinical potential of these molecules. One that has shown some promise was the introduction of an α-methyl substituent in the β-alanine portion; the resulting α-methyl-PanAms showed increased stability (and consequently also potency) in comparison to the parent compounds.16 Clearly, the ability to substitute the β-alanine moiety by structurally diverse amines enabling the enzyme-catalyzed production of a wide variety of structurally diverse pantothenic acids as antimetabolite antimicrobials (and precursors of such antimetabolites) is of high interest.
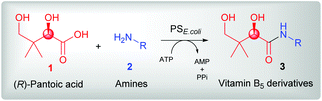
PS enzymes have been isolated from different organisms including bacteria, fungi, and plants.17–20 Although several elegant studies have focused on the functional and mechanistic characterization of PS enzymes,21–24 limited information on the substrate scope of these enzymes and their usefulness for biocatalytic synthesis of a wide variety of distinct pantothenic acid derivatives is available. Recently, we reported that pantothenate synthetase from Escherichia coli (PSE. coli) was able to accept both the (R)- and (S)-enantiomers of α-methyl-substituted β-alanine (3-amino-2-methylpropanoic acid) as nonnative amine substrates for coupling to 1.25 This substrate promiscuity was exploited for the enzymatic cascade synthesis of both diastereoisomers of α-methyl-substituted pantothenic acid starting from a simple non-chiral building block. Encouraged by these findings, we aimed to systematically explore the amine substrate scope of PSE. coli. Herein we show that besides the native amine substrate β-alanine, the enzyme accepts a wide range of structurally diverse amines with different functional groups for coupling to the natural carboxylic acid substrate (R)-pantoic acid to give various amide products with up to >99% conversion. This broad amine scope of PS thus enables the rapid synthesis of pharmaceutically relevant amides, including known vitamin B5 antimetabolites.
2. Results and discussion
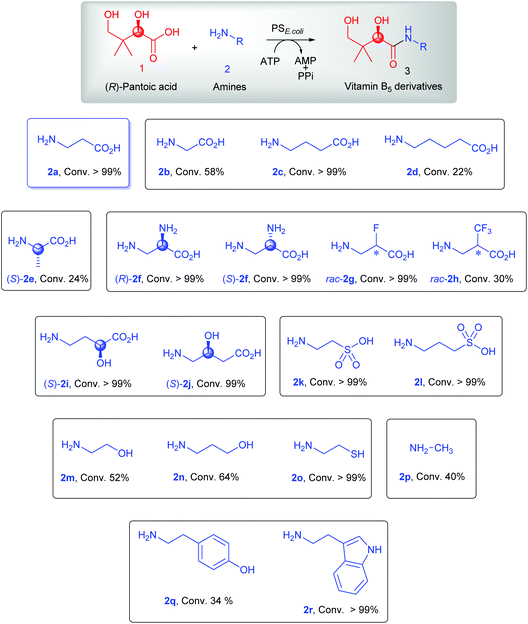
We started our investigations by testing the condensation of the native substrates 1 and 2a using previously reported conditions.25 With a 2-fold excess of 1 over 2a, full conversion of 2a into the corresponding amide product 3a was achieved (>99%; Scheme 2 & Fig. S1†). Using the same reaction conditions, the non-native amines 2b (glycine), 2c (γ-aminobutyric acid, GABA), and 2d (5-aminovaleric acid), which have different aliphatic linkers between the amino and carboxyl groups compared to 2a, were also accepted as substrates for amidation by PSE. coli. While full conversion was achieved with GABA 2c (>99%; Fig. S3†), moderate conversion was observed with the shorter glycine 2b (58%; Fig. S2†) and the extended 5-aminovaleric acid 2d (22%; Fig. S4†).Next, the chiral amines 2e–2j containing different substituents on the alkyl chain were tested as potential substrates for PSE. coli. Whereas the enzyme was able to use (S)-2e (L-alanine) for coupling to 1 (24% conversion, Fig. S5†), the opposite enantiomer (R)-2e (D-alanine) was not accepted as a substrate (Scheme S1†), illustrating the high enantioselectivity of PSE. coli for this substrate. In contrast, both the (R)- and (S)-enantiomers of 2f (3-amino-alanine) were accepted as substrates by the enzyme with excellent conversion (>99%; Fig. S6 & S7†). Importantly, the β-amine group of (R)- and (S)-2f was exclusively used for amidation, demonstrating the high regioselectivity of the enzyme. Interestingly, F- and CF3-substituted β-alanines (rac-2g and rac-2h) were also accepted as substrates by PSE. coli with >99% and 30% conversion, respectively (Fig. S8 & S9†). In addition, the enzyme accepted both α- and β-hydroxyl-substituted GABAs [(S)-2i and (S)-2j] as substrates with excellent conversion (≥99%; Fig. S10 & S11†). The α-amino acids L-serine (2s), L-leucine (2t) and rac-3,3,3-trifluoroalanine (rac-2u) were not processed by the enzyme (Scheme S1†). Hence, it appears that α-amino acids are poor substrates for the PSE. coli enzyme, signifying that the distance between the amino and carboxyl groups is important as noted with amines 2a–2d and 2f.
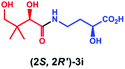
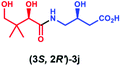
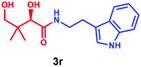
Having established that PSE. coli accepts various amino acids as non-native substrates, we investigated whether amine substrates 2k–2r (Scheme 2), in which the carboxylic acid group has been replaced by other functional groups, can be used as unnatural substrates for coupling to 1. Amines 2k (taurine) and 2l (homotaurine) are direct analogues of substrates 2a and 2c, respectively, and resulted in a similar enzymatic conversion (>99%; Fig. S12 & S13†). Analogues 2m and 2n were also accepted as non-native substrates by PSE. coli but resulted in lower conversion (52–64%; Fig. S14 & S15†). On the other hand, cysteamine (2o), in which the carboxyl group of 2a was replaced by a sulfur group, gave full conversion (Fig. S16†). The simple amine 2p (methylamine, 40% conversion, Fig. S17†) and the bulky amines 2q (tyramine, 34% conversion, Fig. S18†) and 2r (tryptamine, >99% conversion, Fig. S19†) were also accepted as substrates. Notably, benzylamine (2v) and histamine (2w) were not accepted as substrates by PSE. coli (Scheme S1†).
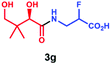
Overall, these results indicate that PSE. coli has a very broad substrate scope enabling the synthesis of various vitamin B5 antimetabolites. To further demonstrate the preparative usefulness of the PSE. coli enzyme, we performed semi-preparative scale synthesis of a few selected amides (3g, 84 mg; 3i, 22.1 mg; 3j, 21.4 mg; and 3r, 26 mg). Excellent conversions (>99%) and high product yields (up to 89%) were achieved (Table 1). Compared to previously reported chemical synthesis methods, this biocatalytic methodology offers an attractive alternative route towards difficult pantothenic acid derivatives.
| Entry | Products | Conv.b (%) | Isolated yieldc (%) | ded (%) |
|---|---|---|---|---|
| a The reaction mixture consisted of PSE. coli (7 μM), (R)-pantoate [20 mM], an amine substrate [10 mM], ATP (20 mM), and MgCl2 (10 mM) in 10 mL Tris-HCl buffer (100 mM, pH 9), except for rac-2g (50 mL). b Conversion was determined by 1H NMR. c Isolated yield after silica gel column chromatography. d The diastereomeric excess (de) was determined by 1H NMR. | ||||
| 1 |
|
>99 | 71 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 |
|
>99 | 88 | >99 |
| 3 |
|
>99 | 86 | >99 |
| 4 |
|
>99 | 89 | — |
In contrast to its very broad amine scope, the PSE. coli enzyme was found to be highly specific for pantoic acid, with various substituted 4-hydroxybutanoic acids not accepted as alternative electrophiles. Detailed insight into the structural determinants of the substrate specificity of this enzyme awaits the determination of crystal structures in complex with various non-native substrates and/or products. Such structures will also aid in the optimization of PS enzymes for synthesis of various pharmaceutically relevant amides.
3. Conclusions
We have demonstrated that PSE. coli has a very broad substrate scope, accepting a wide variety of amine nucleophiles in the amidation of (R)-pantoate. The amines the enzyme accepted included fluorinated β-alanine, α- and β-hydroxylated GABAs, and tryptamine as non-native substrates, with excellent conversions (>99%), and the corresponding amides were obtained in high isolated yields (71–89%). This attractive biocatalytic strategy for rapid synthesis of diverse pantothenic acid derivatives—including known vitamin B5 antimetabolites that have proven to be difficult or tedious to synthesise—is likely to advance our ability to search for new antimicrobial compounds that target CoA biosynthesis and utilisation in a variety of pathogens.4. Experimental
4.1 Materials and methods
The compounds β-alanine (2a), glycine (2b), γ-aminobutyric acid (2c), 5-aminovaleric acid (2d), D-alanine [(R)-2e], L-alanine [(S)-2e], 3-amino-2-fluoropropionic acid (2g), (S)-4-amino-2-hydroxybutyric acid (2i), (S)-4-amino-3-hydroxybutyric acid (2j), taurine (2k), homotaurine (2l), ethanolamine (2m), 3-amino-1-propanol (2n), cysteamine (2o), methylamine (2p), tyramine (2q), tryptamine (2r), L-serine (2s), L-leucine (2t), 3,3,3-trifluoro-DL-alanine (rac-2u), benzylamine (2v) and histamine (2w) were purchased from Sigma-Aldrich Chemical Co. The compounds (R)-2,3-diaminopropionic acid hydrochloride [(R)-2f] and (S)-2,3-diaminopropionic acid hydrochloride [(S)-2f] were purchased from TCI Europe N.V. The compound 3-amino-2-(trifluoromethyl)-propionic acid (rac-2h) was purchased from 1ClickChemistry Inc. (USA). Solvents were purchased from Biosolve (Valkenswaard, The Netherlands) or Sigma-Aldrich Chemical Co. Ingredients for buffers and media were obtained from Duchefa Biochemie (Haarlem, The Netherlands) or Merck (Darmstadt, Germany). Dowex 50W X8 resin (100–200 mesh) was purchased from Sigma-Aldrich Chemical Co. Ni sepharose 6 fast flow resin and a HiLoad 16/600 Superdex 200 pg column were purchased from GE Healthcare Bio-Sciences AB (Uppsala, Sweden).Pantothenate synthetase from Escherichia coli (PSE. coli) was expressed and purified as described previously.25 Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions on precast gels (NuPAGE™ 12% Bis–Tris protein gels). The gels were stained with Coomassie Brilliant Blue. NMR analysis was performed on a Brucker 500 MHz machine at the Drug Design Laboratory, University of Groningen. Chemical shifts (δ) are reported in parts per million (ppm). Electrospray ionization orbitrap high-resolution mass spectrometry (HRMS) was performed by the Mass Spectrometry core facility, University of Groningen.
4.2 General procedure
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Netherlands Organization of Scientific Research (VICI grant 724.016.002) and from the European Research Council (PoC grant 713483). M. Z. A. acknowledges funding from the Indonesia Endowment Fund for Education (LPDP).References
- X. Wang, Nat. Catal., 2019, 2, 98–102 CrossRef
.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC
.
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed
.
- M. Lubberink, C. Schnepel, J. Citoler, S. R. Derrington, W. Finnigan, M. A. Hayes, N. J. Turner and S. L. Flitsch, ACS Catal., 2020, 10, 10005–10009 CrossRef CAS
.
- M. R. Petchey, B. Rowlinson, R. C. Lloyd, I. J. S. Fairlamb and G. Grogan, ACS Catal., 2020, 10, 4659–4663 CrossRef CAS PubMed
.
- Á. Mourelle-Insua, D. Méndez-Sánchez, J. L. Galman, I. Slabu, N. J. Turner, V. Gotor-Fernández and I. Lavandera, Catal. Sci. Technol., 2019, 9, 4083–4090 RSC
.
- M. R. Petchey and G. Grogan, Adv. Synth. Catal., 2019, 361, 3895–3914 CrossRef CAS
.
- A. Goswami and S. G. Van Lanen, Mol. Biosyst., 2015, 11, 338–353 RSC
.
-
E. Strauss, Comprehensive Natural Products II, ed. H.-W. Liu and L. Mander, Elsevier, Oxford, 2010, ch. 7, pp. 351–410 Search PubMed
.
- C. Spry, K. Kirk and K. J. Saliba, FEMS Microbiol. Rev., 2008, 32, 56–106 CrossRef CAS PubMed
.
- W. J. A. Moolman, M. de Villiers and E. Strauss, Biochem. Soc. Trans., 2014, 42, 1080–1086 CrossRef CAS PubMed
.
- S. Shapiro, J. Antibiot., 2013, 66, 371–386 CrossRef CAS PubMed
.
- D. I. Hammoudeh, Y. Zhao, S. W. White and R. E. Lee, Future Med. Chem., 2013, 5, 1331–1340 CrossRef CAS PubMed
.
- K. J. Saliba and C. Spry, Biochem. Soc. Trans., 2014, 42, 1087–1093 CrossRef CAS PubMed
.
- C. Spry, C. Macuamule, Z. Lin, K. G. Virga, R. E. Lee, E. Strauss and K. J. Saliba, PLoS One, 2013, 8, e54974 CrossRef CAS PubMed
.
- C. J. Macuamule, E. T. Tjhin, C. E. Jana, L. Barnard, L. Koekemoer, M. De Villiers, K. J. Saliba and E. Strauss, Antimicrob. Agents Chemother., 2015, 59, 3666–3668 CrossRef CAS PubMed
.
- K. Miyatake, Y. Nakano and S. Kitaoka, J. Nutr. Sci. Vitaminol., 1978, 24, 243–253 CrossRef CAS PubMed
.
- A. Pérez-Espinosa, T. Roldán-Arjona and M. Ruiz-Rubio, Mol. Genet. Genomics, 2001, 265, 922–929 CrossRef PubMed
.
- M. E. Webb and A. G. Smith, Adv. Bot. Res., 2011, 58, 203–255 CAS
.
- F. Tigu, J. Zhang, G. Liu, Z. Cai and Y. Li, Appl. Microbiol. Biotechnol., 2018, 102, 6039–6046 CrossRef CAS PubMed
.
- R. Zheng and J. S. Blanchard, Biochemistry, 2001, 40, 12904–12912 CrossRef CAS PubMed
.
- S. Wang and D. Eisenberg, Biochemistry, 2006, 45, 1554–1561 CrossRef CAS PubMed
.
- K. S. Chakrabarti, K. G. Thakur, B. Gopal and S. P. Sarma, FEBS J., 2010, 277, 697–712 CrossRef CAS PubMed
.
- A. Satoh, S. Konishi, H. Tamura, H. G. Stickland, H. M. Whitney, A. G. Smith, H. Matsumura and T. Inoue, Biochemistry, 2010, 49, 6400–6410 CrossRef CAS PubMed
.
- M. Z. Abidin, T. Saravanan, J. Zhang, P. G. Tepper, E. Strauss and G. J. Poelarends, Chem. – Eur. J., 2018, 17434–17438 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and NMR and LC-HRMS spectra. See DOI: 10.1039/d1ob00238d |
| This journal is © The Royal Society of Chemistry 2021 |