Recent advances in the synthesis and applications of β-keto sulfones: new prospects for the synthesis of β-keto thiosulfones
Abstract
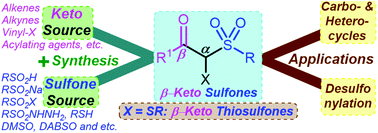
This review mainly focuses on recent developments in the preparation of β-keto sulfones and their extensive synthetic applications. New prospects for the synthesis of β-keto thiosulfones have also been highlighted. Over the last decade, there has been exponential growth in the direct construction of β-keto sulfones using a wide variety of keto and sulfonyl precursors. Of note, the most promising photoredox transformations and electrochemical synthesis methods of β-keto sulfones are also presented. Moreover, β-keto sulfones are versatile building blocks in organic synthesis due to their three essential functional groups: sulfonyl, carbonyl, and active methylene moieties. The convenient preparation of β-keto sulfones allows the synthesis of many valuable carbocyclic and heterocyclic compounds, and the effortless removal of the sulfonyl moiety via transformations is supported. The chemistry of β-keto sulfones (2013 to present) can be divided into several sections based on the sulfonyl surrogates, and ubiquitous synthetic strategies were systematically outlined.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...