Chemoselective electrochemical reduction of nitroarenes with gaseous ammonia†
Abstract
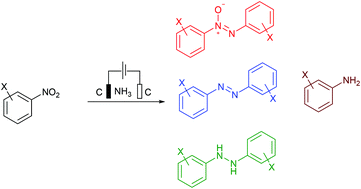
Valuable aromatic nitrogen compounds can be synthesized by reduction of nitroarenes. Herein, we report electrochemical reduction of nitroarenes by a protocol that uses inert graphite felt as electrodes and ammonia as a reductant. Depending on the cell voltage and the solvent, the protocol can be used to obtain aromatic azoxy, azo, and hydrazo compounds, as well as aniline derivatives with high chemoselectivities. The protocol can be readily scaled up to >10 g with no decrease in yield, demonstrating its potential synthetic utility. A stepwise cathodic reduction pathway was proposed to account for the generations of products in turn.
- This article is part of the themed collections: Synthetic electrochemistry and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...