Construction of fully substituted carbon centers containing a heteroatom and a CF3 group via in situ generated p-quinone methides†
Abstract
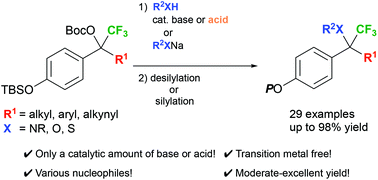
1,6-Conjugate additions of in situ generated δ-CF3-δ-substituted p-quinone methides have been achieved with a variety of heteronucleophiles under mild conditions, which led to facile and practical construction of fully substituted carbon centers including a heteroatom and a CF3 group. In particular, it was revealed that some amines themselves worked for efficient cleavage of the TBS protective group, and addition of a catalytic amount of an appropriate Brønsted acid was found to sometimes improve the progress of the desired process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...