Recent advances in reactions using diacyl peroxides as sources of O- and C-functional groups
Abstract
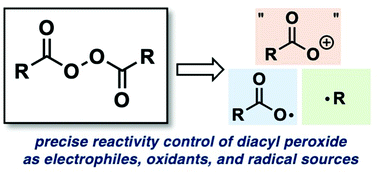
Diacyl peroxides, (RCO2)2, are readily available and widely used reagents for organic synthesis, because they can serve as electrophiles, oxidants, and radical sources. Recently, they have been used extensively as sources of O- and C-functional groups, in contrast to their classical applications as radical initiators. These novel reaction modes have greatly expanded the synthetic utility of diacyl peroxides by making it possible to simultaneously utilize plural functionalities of diacyl peroxides in unprecedented ways, with or without the aid of transition-metal catalysts. Here, we review recent advances in reactions utilizing diacyl peroxides as O- and C-sources, with examples illustrating how the reactivity of diacyl peroxides in organic reactions can be controlled.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...