Photoelectrochemical H2 evolution on WO3/BiVO4 enabled by single-crystalline TiO2 overlayer modulations†
Eunoak
Park
a,
Santosh S.
Patil
a,
Hyeonkwon
Lee
b,
Vijay S.
Kumbhar
*c and
Kiyoung
Lee
 *a
*a
aDepartment of Chemistry and Chemical Engineering, Inha University, 22212 Incheon, Republic of Korea. E-mail: kiyoung@inha.ac.kr
bResearch Institute of Environmental Science & Technology, Kyungpook National University, 80 Daehak-ro, Buk-gu, Daegu, Republic of Korea
cApplied Chemistry, Graduate School of Sciences and Technology for Innovation, Yamaguchi University, Japan. E-mail: vijay1712phy@gmail.com
First published on 25th August 2021
Abstract
Tungsten oxide/bismuth vanadate (WO3/BiVO4) has emerged as a promising photoanode material for photoelectrochemical (PEC) water splitting owing to its facilitated charge separation state differing significantly from single phase materials. Practical implementation of WO3/BiVO4 is often limited by poor stability arising from the leaching of V5+ from BiVO4 during PEC operations. Herein, we demonstrate that the synthesis of a tungsten oxide/bismuth vanadate/titanium oxide (WO3/BiVO4/TiO2) heterostructure onto a fluorine-doped tin oxide-coated glass substrate through a combined simple hydrothermal-spin coating strategy will advance PEC performance while slowing water oxidation kinetics and improving photostability. We show that surface postmodification with a nanometer-thick layer of (1 0 1) monofacet-selective single-crystalline TiO2 provides stable photocurrent density, up to 1.04 mA cm−2 at 1.23 V (compared to a reversible hydrogen electrode in 0.5 M Na2SO4), with excellent quantum efficiency (45% at 460 nm) and long-term photostability (24 h). Interestingly, crystalline TiO2 activation layers behave differently from previous TiO2 amorphous layers, blocking surface defects while improving corrosion resistance, photostability, and the electron transfer process. These results indicate a ≈2.5 times enhancement in photoelectrocatalytic activity related to referenced WO3/BiVO4 photoanodes, encouraging the use of single-crystalline TiO2 modulations to develop a range of materials for PEC/photocatalytic applications.
Introduction
Converting solar energy into hydrogen (H2) energy via photoelectrochemical water splitting is a cost effective and sustainable approach for mitigating overreliance on fossil fuels and global warming issues.1,2 As H2 energy is renewable, it can be stored, transported, and even used in fuel cells to generate power and/or is an important feedstock in the chemical industry (e.g., in the catalytic conversion of CO2 into hydrocarbons, such as methanol, formic acid, methane, or ammonia synthesis).3–5 Photoelectrochemical (PEC) water splitting (H2O → H2 + 1/2 O2) is based on photogenerated electron–hole pairs in a semiconductor that subsequently catalyze the oxidation and reduction of a H2O molecule into H2 and O2 fuels.6 However, the practical implementation of PEC cells remains hindered by stringent material challenges that simultaneously require (i) excellent light harvesting capability, (ii) long-term stability, and (iii) charge-carrier separation and transfer. Since Fujishima Honda's7 pioneering work on titanium dioxide (TiO2) photocatalysts, various visible-light responsive metal oxides (such as tungsten oxide (WO3), bismuth vanadate (BiVO4), and iron(III) oxide (Fe2O3)) have been examined to absorb a major fraction of the solar spectrum and produce green H2. However, those single materials are insufficient to deliver high solar-hydrogen-conversion efficiency for practical application.A number of strategies have been developed by combining two or more semiconductors, co-catalysts for constructing heterostructures, integrated material designs, or composites that provide an opportunity to achieve multiple catalytic functionalities from different counterparts; these have enhanced PEC/photocatalytic performance and stability regarding water splitting and environmental remediation applications. For instance, a combination of WO3 and BiVO4 creates a type II heterojunction and enhances PEC performance,8,9 which mainly stems from the excellent transference of photogenerated electrons to produce a charge-separated state.10–12 Nonetheless, while WO3/BiVO4 is a relatively stable material, it often suffers from severe V5+ leaching out of the BiVO4 core during photoelectrocatalytic operations.13
Postmodification of a WO3/BiVO4 heterojunction with a surface protecting promoter is indispensable to realize potentially high PEC performance with long-term durability. Previous efforts have mainly relied on building an additional passivation layer onto a WO3/BiVO4 semiconductor pair.14–17 For example, Khoomortezaei et al.14 reported tungsten oxide/bismuth vanadate/bismuth ferrite (WO3/BiVO4/BiFeO3) heterojunctions with a layered coating of BiFeO3 that lowered the overpotential and charge transfer resistance of the photoelectrode. Baek et al.11 made a tin oxide/tungsten oxide/bismuth vanadate (SnO2/WO3/BiVO4) triple-layer planar photoelectrode that exhibited superior photoconversion efficiency during water decomposition. Recently, Seo et al.18 added a photocatalytically inactive amorphous TiO2 layer onto a WO3/BiVO4 electrode that reduced charge-carrier recombination by passivating the surfaces. Although previous studies have made remarkable developments, they used amorphous TiO2 overlayers and met with little success in attaining sufficient stability. Simple solution-processed synthesis of crystalline TiO2 can offer a means of creating intricately designed WO3/BiVO4 heterostructures that otherwise defy creation. We anticipate that depositing a nanometer-thick layer of single-crystalline TiO2 onto WO3/BiVO4 may grant proficient catalytic operations with long-term durability.
Herein, we present the synthesis of a WO3/BiVO4/TiO2 heterojunction using a simple hydrothermal-spin coating method. Postmodification with crystalline TiO2 was accomplished and played a key role in blocking surface defects in BiVO4 nanoparticles,19 leading to substantial improvements in electronic properties and photostability. The WO3/BiVO4/TiO2 heterojunction showed the best photocurrent density of 1.04 mA cm−2 at 1.23 V when compared to a reversible hydrogen electrode (RHE), an incident photon-to-current conversion efficiency (IPCE) of 25% in response to visible light at 460 nm, and exceptionally high durability (24 h) in 0.5 M Na2SO4 at 1.23 VRHE under standard 1 sun illumination (100 mW cm−2). According to gas chromatography analysis, the utmost H2 evolution rate of 78.8 μL cm−2 was achieved during PEC water splitting.
Results and discussion
The surface morphologies of WO3, WO3/BiVO4, and WO3/BiVO4/TiO2 electrodes synthesized through a hydrothermal-spin coating method were evaluated with scanning electron microscopy (SEM) images (Fig. 1). The prepared WO3 showed rough nanoplate shapes with relatively dense distributions ranging from nanometer to submicrometer scales (Fig. 1a). Interconnected nanoplates were grown nearly vertical to the fluorine-doped tin oxide (FTO) surface, creating several void spaces between them. The average length and thickness of the individual nanoplates were ∼400–600 nm and 100–160 nm, respectively, as confirmed by the cross-section and top view SEM images (Fig. 1a and d). Fig. 1c and f depict the top and cross-section views of SEM images after the deposition of BiVO4 nanoparticles (20 cycles) onto WO3via a spin coating method and subsequent air annealing at 500 °C. While BiVO4 nanoparticles with diameters ∼50–80 nm were uniformly coated onto WO3 nanoplate surfaces, overlayer thicknesses seemed irregular, indicating a possible overgrowth of BiVO4 nanoparticles (Fig. S1b and c in the ESI†). A thin top layer of TiO2 was formed onto the WO3/BiVO4 by spin coating a titanium butoxide (Ti(OCH2CH2CH2CH3)4) solution and subsequent annealing at 500 °C for 1 h (Fig. 1c and f). | ||
| Fig. 1 (a–c) Top view and (d–f) cross-sectional FE-SEM images of WO3, WO3/BiVO4, and WO3/BiVO4/TiO2, respectively. | ||
Crystal structures were analyzed further by acquiring X-ray diffraction (XRD) patterns. Fig. 2a shows the prominent diffraction peaks at 23.0°, 23.5°, 24.3°, 28.7°, 34.0°, 41.6°, 49.9°, 55.7°, and 76.4°; these can be assigned to the (0 0 2), (0 2 0), (2 0 0), (1 1 2), (2 2 0), (2 3 2), (−2 4 1), (4 0 2), and (0 6 1) planes, respectively, representing monoclinic WO3 (JCPDS #89-4476).20,21 The WO3/BiVO4 heterojunction showed preservation of the WO3 phase and Bragg diffractions at 18.8°, 28.9°, 30.4°, and 39.8°; these correspond to the (1 0 1), (1 1 2), (0 0 4), and (2 1 1) planes, respectively, suggesting the generation of a monoclinic BiVO4 form (JCPDS #75-1866).22,23 As illustrated in Fig. 2b (a magnified view of an XRD pattern), the one-cycle coated TiO2 shows a Bragg peak at 25.3°, confirming the generation of (1 0 1) selective single-crystalline anatase TiO2 (JCPDS #89-4921) nanoparticles.24,25 The XRD pattern of a five-cycle spin coated TiO2 (Fig. S3†) revealed a diffraction peak at 48.1°, implying the (2 0 0) plane of anatase TiO2 together with the heightened peak intensity of the (1 0 1) crystal plane.
 | ||
| Fig. 2 XRD patterns of (a) FTO, WO3, WO3/BiVO4, and WO3/BiVO4/TiO2 samples and (b) the corresponding magnified view. | ||
High-angle annular dark-field STEM (HAADF-STEM) was employed to investigate crystalline contacts between WO3/BiVO4/TiO2 components. The area marked A in Fig. 3a illustrates a high-resolution transmission electron microscopy (TEM) image with measured lattice spacings of 0.263 nm and 0.164 nm; this agrees well with the (2 2 0) and (4 0 2) planes of WO3. The selected area electron diffraction (SAED) pattern (Fig. 3d) confirms a polycrystalline nature, and the (2 2 0) and (4 0 2) planes correspond to monoclinic WO3. Fig. 3e (the area marked B in Fig. 3a) shows clear lattice fringes with lattice spacings of 0.308 nm and 0.349 nm that correspond with the (1 1 2) and (1 0 1) planes of monoclinic BiVO4 and anatase TiO2, respectively. The corresponding SAED pattern (Fig. 3f) reveals that single-crystalline TiO2 is oriented along the (1 0 1) facet. Energy dispersive X-ray spectroscopy (EDS) elemental maps were acquired (Fig. 3b1–b5) to investigate the composition of WO3/BiVO4/TiO2, demonstrating that five elements (W, O, Bi, V, and Ti) were uniformly distributed throughout the entire structure.
X-ray photoelectron spectroscopy (XPS) was performed to verify chemical states and compositions. Fig. 4a depicts the survey spectra, confirming the presence of Ti, W, Bi, V, and O elements in the WO3/BiVO4/TiO2 heterojunction. High-resolution XPS spectra of W 4f (Fig. 4b) show main peaks at binding energies of 35.9 and 38 eV, which can be attributed to the respective W 4f7/2 and W 4f5/2 states of the W6+ valence state.26Fig. 4c shows two peaks at binding energies of 159.6 and 164.8 eV, demonstrating that the respective Bi 4f7/2 and Bi 4f5/2 orbits exhibit a 3+ valence state in BiVO4.27 The convoluted signals of V 2p3/2 and V 2p1/2 peaks (shown in Fig. 4d) at 517.1 and 524.6 eV, respectively, clearly reveal an oxidation state of V5+ in BiVO4.28 The spin orbit coupling of Ti 2p3/2 and Ti 2p1/2 peaks (see Fig. 4e) showed binding energies of 459.4 and 465 eV, respectively, demonstrating the presence of TiO2 with a valence state of Ti4+.29 The asymmetric peak of O 1s at ∼530.7 eV, shown in Fig. 4f, can be assigned to lattice oxygen (O2−) and surface hydroxyl species.30
 | ||
| Fig. 4 XPS survey of (a) full spectra, (b) W 4f, (c) Bi 4f, (d) V 2p, (e) Ti 2p, and (f) O 1s region of WO3/BiVO4/TiO2. | ||
We employed ultraviolet–visible (UV-vis) absorption spectroscopy to understand the light absorption characteristics. As seen from Fig. 5a, the strong light absorption within a range of 325–460 nm was revealed for pristine WO3 electrodes, whereas WO3/BiVO4 and WO3/BiVO4/TiO2 exhibited longer wavelength light absorption in the range of 360–500 nm, demonstrating enhanced light absorption ability in the latter. IPCE spectra vs. wavelength was measured with various band-pass filters in a xenon (Xe) lamp, showing a red shift in the light absorption (Fig. 5b). The band gap values were ∼2.60 eV, 2.46 eV, and 2.44 eV, for WO3, WO3/BiVO4 and WO3/BiVO4/TiO2, respectively, as shown by the plot in Fig. 5c of (IPCE·hν)1/2vs. hν; this is in close agreement with band gap values estimated by the Tauc plot (ESI Fig. S4†) acquired from previous optical absorption spectroscopy (Fig. 5a).
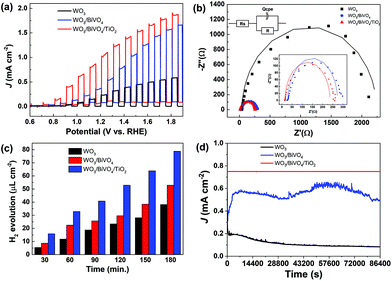
Considering the absorption in the visible region, we measured photoelectrochemical performances of WO3/BiVO4/TiO2 electrodes for water splitting and compared them with those of referenced WO3/BiVO4 and WO3 electrodes in 0.5 M Na2SO4 under standard simulated sunlight illumination (1 sun) at 1.23 VRHE. Fig. 6a shows the linear sweep voltammetry (LSV) curves of the prepared photoanodes, which were recorded at room temperature under chopped light steps (10 s). The photocurrent density at 1.23 VRHE followed the order WO3/BiVO4/TiO2 (1.04 mA cm−2) > WO3/BiVO4 (0.43 mA cm−2) > WO3 (0.23 mA cm−2), indicating a substantial improvement in the photoelectrocatalytic performance with addition of BiVO4 and TiO2 layers. This ∼2.5 times enhancement can be associated with interparticle electron transfer between integrated electrode materials and suppressed charge recombination due to the heterojunction effect.31,32 In addition, WO3/BiVO4/TiO2 showed an onset potential of 500 mV vs. RHE, which was lower than that of WO3 (800 mV vs. RHE) and WO3/BiVO4 (550 mV vs. RHE). The shift of the onset potential toward the negative further facilitates the electron drive from the conduction band (CB) of TiO2 to BiVO4 and then BiVO4 to WO3.33 This enhancement in anodic photocurrents was consistent with IPCE results (see Fig. 5b) of considerably higher values (45%) for the WO3/BiVO4/TiO2 electrode, indicating that the photocurrent is dependent on light absorption and consistent with improved performance. To determine the flat band potentials and donor density, a Mott–Schottky analysis was performed in 0.5 M Na2SO4. As seen in Fig. 5d, the electrode has a positive slope, suggesting n-type semiconductor behavior.34 The flat band potential values can be estimated using eqn (4); they were 0.35 V, 0.39 V, and 0.46 V vs. RHE for the WO3/BiVO4/TiO2, WO3/BiVO4, and WO3 electrodes, respectively.
As a control experiment, an amorphous TiO2 layer was coated onto our WO3/BiVO4 electrode by adopting a previously reported procedure.18,35 Results showed reduced photocurrent density, representing weaker interaction between host (WO3/BiVO4) and guest (amorphous-TiO2) counterparts (Fig. S5†). This reduction in the photocurrent density of WO3/BiVO4/TiO2 (amorphous) was presumably due to the low electric conductivity and defective amorphous TiO2 reported previously.36,37 In addition, a 5-cycle coated TiO2 electrode exhibited low photocurrent density (Fig. S6†), presumably due to the presence of high thickness and defective polycrystalline TiO2, as evident from previous XRD results.37
To gain more insights into charge transfer kinetics at the interface of the photoanode/electrolyte, electrochemical impedance spectroscopy (EIS) was performed. Fig. 6b shows the EIS Nyquist plots of WO3, WO3/BiVO4, and WO3/BiVO4/TiO2, which were fitted to the equivalent circuit (Fig. 6b) and consisted of solution resistance (Rs), charge transfer resistance (Rct), and constant phase element (QCPE).38 The equivalent circuit-fitted values are summarized in Table 1. It is apparent that WO3/BiVO4/TiO2 displays a smaller Rct value (228.3 Ω), when compared to WO3 (2063 Ω) and WO3/BiVO4 (244.1 Ω). This indicates that charge transfer resistance was reduced after a coating of TiO2 nanoparticles was applied.
| Parameter | WO3 | WB | WBT |
|---|---|---|---|
| R s (Ω) | 42.47 | 29.05 | 14.96 |
| R ct (Ω) | 2063 | 244.1 | 228.3 |
| Q CPE (F) | 42.32 × 10−6 | 0.18623 × 10−3 | 69.16 × 10−6 |
These features suggest that creating a heterojunction with BiVO4 and crystalline TiO2 is an effective way to optimize the separation efficiency of electron–hole pairs, which would promote overall photoelectrocatalytic performance.35 Photoelectrochemical H2 gas evolution was performed under standard sunlight irradiation (1 Sun) for a 3 h duration. The H2 evolution rates were 78.8 μL cm−2 and 52.9 μL cm−2 for WO3/BiVO4/TiO2 and WO3/BiVO4 photoelectrodes, respectively, at 1.23 V vs. RHE under simulated solar light (AM 1.5, 100 mW cm−2); the larger HER rate represents the positive effect of crystalline TiO2 deposition (Fig. 6c). The durability test (J–T curve) of WO3/BiVO4/TiO2 displayed almost unchanged initial photocurrent density after 24 h of operation (Fig. 6d), whereas unmodified WO3/BiVO4 exhibited an initial drastic loss in photocurrent that the latter managed to retain after 0.5 h of operation, demonstrating robustness of ternary WO3/BiVO4/TiO2 as a photoelectrocatalyst for water splitting. The stable photocurrent density value of ∼0.76 mA cm−2 (Fig. 6d) obtained in this study is comparable to that of state-of-the-art WO3/BiVO4 photoanodes and other BiVO4-based photoanodes, such as WO3/BiVO4 (0.6 mA cm−2 at 1.23 VRHE, 0.1 M Kpi),39 SnO2/WO3/BiVO4 (1.5 mA cm−2 at 1.23 VRHE, Kpi with 0.1 M Na2SO3),40 WO3/BiVO4/BiFeO3 (1.1 mA cm−2 at 1.5 VRHE, 0.5 M Na2SO4),14 and SnO2/BiVO4/Co-Pi (0.8 mA cm−2 at 1.23 VRHE, 0.1 M Kpi).41 Please see Table S1† for more details.
Guided by observations and experimental results, a plausible electron transfer mechanism of a WO3/BiVO4/TiO2 photoelectrode is illustrated in Fig. 7. The band gap alignment was assigned on the basis of estimated optical band gap values and flat band potentials. Pristine WO3 is a relatively weak n-type material that can possess an optical absorption onset ∼2.7 eV, while WO3/BiVO4 has strong n-type behavior due to the heterojunction effect, producing large built-in potential at the interfaces and extended light absorption over wide band gap ranges (2.4–2.7 eV). When BiVO4 is in strong contact with WO3, a type II band offset can be produced that transfers electrons from the BiVO4 CB (+0.02 V vs. RHE) to the WO3 CB (+0.41 V vs. RHE) and facilitates electron–hole separation.42 Interestingly, since the CB edge potential is more negative in a WO3/BiVO4/TiO2 heterostructure, TiO2 would begin by inducing an electron transfer from the TiO2 CB to the BiVO4 CB and then inject into WO3 electrode's CB. Thus, accumulated electrons would be injected into a current collector and migrate to the metallic cathode (Pt) electrode to participate in the hydrogen evolution reaction. We assume that introducing a thin crystalline TiO2 overlayer would not allow hole accumulation driving forces (higher valence band potential). However, it could block surface defects that are centric to electron–hole recombination and lower the overpotential of photoelectrochemical reactions, leading to improved reaction kinetics. As a result, hole transfer going from the BiVO4 valence band (VB) to the WO3 VB would occur at the photoelectrode/electrolyte interface, leading to an oxygen evolution reaction with water molecules. In an account, the as-formed WO3/BiVO4/TiO2 photoelectrode with porous surface morphology produced from nearly vertical nanoplate-like structures provides a more accessible surface area, a type II heterojunction, and a thin crystalline TiO2 layer, which should synergistically boost the charge-carrier separation efficiency, photostability, and overall PEC performance without the aid of any co-catalyst or sacrificial agents.
Conclusions
We demonstrated an integrated design of WO3/BiVO4/TiO2 photoanode material by using a simple hydrothermal-spin coating strategy, which exhibited enhanced photoelectrocatalytic activity and photostability under simulated sunlight illumination. The WO3/BiVO4/TiO2 heterostructure displayed an outstanding photocurrent density of 1.04 mA cm−2 at 1.23 V vs. RHE and photostability over 24 h of operation. Postmodification with crystalline (1 0 1) TiO2 resulted in a ≈2.5 times improvement in PEC performance, substantially better than referenced WO3/BiVO4. This improvement in PEC performance can be attributed to fast electron transfer at the interface due to a type II band alignment, a high light harvesting ability, and excellent corrosion robustness (characterized through EIS, HR-TEM, IPCE, and chronoamperometry analysis). A thin crystalline TiO2 overlayer was beneficial to the blocking of surface defects, increasing the surface charge-carrier separation efficacy to hypothetically behave differently from previous amorphous-TiO2 passivation overlayers during PEC water splitting operations. This work offers new avenues for employing crystalline TiO2 overlayer modulations when developing commercially plausible visible-light driven photocatalyst materials.Experimental
Methods and materials
All chemicals were of reagent grade and used without further purification. Sodium tungsten dihydrate (Na2WO4·2H2O, ≥99%), oxalic acid (C2H2O4, 98%), sodium sulfate (Na2SO4, ≥99%), ammonium metavanadate (NH4VO3, ≥99%), bismuth(III) nitrate pentahydrate (Bi(NO3)3·5H2O, 98%), citric acid (C6H8O7, ≥99.5%), polyvinyl alcohol ((C2H4O)x), and titanium(IV) butoxide (Ti(OCH2CH2CH2CH3)4, 97%) were bought from Sigma Aldrich. Hydrochloric acid (HCl, 35–37%), nitric acid (HNO3, 60%), and isopropyl alcohol (C3H8O. 99.5%) were purchased from Samchun Chemicals.Synthesis of nanoplate-like WO3
WO3 nanostructures were fabricated on FTO coated glass substrates using a hydrothermal method. Prior to deposition, the substrates (2 × 4 cm2) were cleaned with acetone, ethanol, and deionized (DI) water using an ultrasonicator before being dried with N2 gas. They were then placed in a polyparaphenol (PPL)-lined reactor with their conducting sides facing downward. In typical synthesis, 0.16 g of Na2WO4·2H2O was dissolved in 20 mL DI water before adding 4 mL of 3 M HCl. Next, 20 mL of 35 mM C2H2O4 and 0.05 g of Na2SO4 (morphological agent) was added. The entire solution (30 mL) was then transferred into a PPL-lined autoclave and maintained at 140 °C for 2 h. After the reaction, the reactor was allowed to cool naturally to room temperature. The WO3-coated FTO electrode was cleaned using DI water and air annealed at 500 °C for 2 h.Synthesis of WO3/BiVO4 heterojunction photoelectrodes
A BiVO4 layer was deposited onto premade WO3/FTO (2 × 2 cm2) by spin coating the precursor solution at 3000 rpm for 30 s (20 cycles). In a typical synthesis, 1.17 g of NH4VO3, 10 mL of HNO3, and 20 mL of DI water were mixed together and stirred for 30 min to dissolve the precursors. Then, 4.85 g of Bi(NO3)3·5H2O and 1.92 g of C6H8O7 were added to the mixture before stirring to allow dissolution. When the solution color turned blue, 6 mL of the solution was mixed with 2 mL of (C2H4O)x solution (0.4 g in 40 mL DI water) and kept under sonication for 5 min. The as-obtained films were dried at 100 °C for 5 min and air annealed at 500 °C for 30 min.Synthesis of TiO2 thin layers onto the WO3/BiVO4 heterojunction
TiO2 precursor solution was prepared by dissolving 0.1 M Ti(OCH2CH2CH2CH3)4 in a mixed solvent of 20 mL C3H8O, 0.18 g DI water, and 0.331 g HNO3. The resultant solution was ultrasonicated for 1 h to allow complete dissolution. TiO2 was then deposited onto the as-obtained WO3/BiVO4 by spin coating the above solution mixture at 3000 rpm for 40 s and annealing it at 500 °C for 1 h.Characterization
The surface morphologies were examined by FE-SEM (JSM-6701 F, JEOL). Crystalline structures were characterized using an X-ray diffractometer (X'Pert Pro MRD, PANalytical) with a Cu-Kα radiation source and a high-resolution transmission electron microscope (HR-TEM; Titan G2 ChemiSTEM, FEI Co.). XPS (NEXSA, ThermoFisher) was used to analyze the elemental composition and oxidation states of the elements present within a heterojunction. Optical absorption spectra were recorded with a UV-vis spectrophotometer (UV-1900i, Shimadzu).PEC measurements
All PEC measurements were performed with a potentiostat (SP150, Bio-logic) under a solar simulator (LCS-100, Oriel Instruments) with a 100 W xenon arc lamp (100 mW cm−2). The electrolyte used was a 0.5 M Na2SO4 solution and the light exposed area of the photoanode was 1.5 cm2. A standard three-electrode cell, consisting of silver/silver chloride (Ag/AgCl) and Pt mesh, was used as reference and counter electrodes. The as-prepared photoanodes were used as working electrodes. LSV was performed at a scan rate of 5 mV s−1. IPCE (%) was measured at 1.23 V vs. RHE, using a light source and monochromator (MonoRa200, DONGWOO OPTRON) and the following equation: | (1) |
The measured potentials were converted into the RHE using the following equations:
 | (2) |
| EAg/AgCl (3 M KCl) = 0.1976 V at 25 °C. | (3) |
The Mott–Schottky plots were obtained using
 | (4) |
EIS measurements were performed within the frequency range of 50 kHz–100 MHz at 1.21 V vs. RHE. To examine evolved H2 gas during the PEC process, 200 μL of gas was taken from the sealed quartz tube system every half hour and injected into a gas chromatograph (Agilent 6850, Agilent Technologies).
Author contributions
E. Park: conceptualization, data curation, formal analysis, methodology, visualization, writing – original draft, and writing – review and editing; S. S. Patil: formal analysis, methodology, and writing – review and editing; H. Lee: formal analysis, methodology, and writing – review and editing; V. S. Kumbhar: conceptualization, supervision, writing – original draft, and writing – review and editing; K. Lee: conceptualization, supervision, writing – original draft, writing – review and editing, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea [grant numbers NRF-2019R1l1A3A01041454], funded by the South Korean Government particularly the Ministry of Education.References
- J. H. Kim and J. S. Lee, BiVO4-Based heterostructured photocatalysts for solar water splitting: A review, Energy Environ. Focus, 2014, 3(4), 339–353 CrossRef.
- M. G. Lee, J. S. Park and H. W. Jang, Solution-processed metal oxide thin film nanostructures for water splitting photoelectrodes: A review, J. Korean Ceram. Soc., 2018, 55(3), 185–202 CrossRef CAS.
- B. S. Kalanoor, H. Seo and S. S. Kalanur, Recent developments in photoelectrochemical water-splitting using WO3/BiVO4 heterojunction photoanode: A review, Mater. Sci. Energy Technol., 2018, 1(1), 49–62 Search PubMed.
- T. Hisatomi and K. Domen, Introductory lecture: Sunlight-driven water splitting and carbon dioxide reduction by heterogeneous semiconductor systems as key processes in artificial photosynthesis, Faraday Discuss., 2017, 198(0), 11–35 RSC.
- F. E. Osterloh, Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting, Chem. Soc. Rev., 2013, 42(6), 2294–2320 RSC.
- V. S. Kumbhar, H. Lee, J. Lee and K. Lee, Interfacial growth of the optimal BiVO4 nanoparticles onto self-assembled WO3 nanoplates for efficient photoelectrochemical water splitting, J. Colloid Interface Sci., 2019, 557, 478–487 CrossRef CAS PubMed.
- F. A. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238, 37–38 CrossRef PubMed.
- V. O. Smilyk, S. S. Fomanyuk, G. Y. Kolbasov, I. A. Rusetskyi and V. S. Vorobets, Electrodeposition, optical and photoelectrochemical properties of BiVO4 and BiVO4/WO3 films, Res. Chem. Intermed., 2019, 45(8), 4149–4161 CrossRef CAS.
- C. Liu, Y. Yang, J. Li, S. Chen, W. Li and X. Tang, An in situ transformation approach for fabrication of BiVO4/WO3 heterojunction photoanode with high photoelectrochemical activity, Chem. Eng. J., 2017, 326, 603–611 CrossRef CAS.
- L. Xia, J. Bai, J. Li, Q. Zeng, X. Li and B. Zhou, A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell, Appl. Catal., B, 2016, 183, 224–230 CrossRef CAS.
- J. H. Baek, B. J. Kim, G. S. Han, S. W. Hwang, D. R. Kim, I. S. Cho and H. S. Jung, BiVO4/WO3/SnO2 Double-heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell, ACS Appl. Mater. Interfaces, 2017, 9(2), 1479–1487 CrossRef CAS PubMed.
- P. Chatchai, Y. Murakami, S.-y. Kishioka, A. Y. Nosaka and Y. Nosaka, Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation, Electrochim. Acta, 2009, 54(3), 1147–1152 CrossRef CAS.
- Y. Park, K. J. McDonald and K.-S. Choi, Progress in bismuth vanadate photoanodes for use in solar water oxidation, Chem. Soc. Rev., 2013, 42(6), 2321–2337 RSC.
- S. Khoomortezaei, H. Abdizadeh and M. R. Golobostanfard, Triple layer heterojunction WO3/BiVO4/BiFeO3 porous photoanode for efficient photoelectrochemical water splitting, ACS Appl. Energy Mater., 2019, 2(9), 6428–6439 CrossRef CAS.
- Z. Zhang, B. Chen, M. Baek and K. Yong, Multichannel charge transport of a BiVO4/(RGO/WO3)/W18O49 three-storey anode for greatly enhanced photoelectrochemical efficiency, ACS Appl. Mater. Interfaces, 2018, 10(7), 6218–6227 CrossRef CAS PubMed.
- I. Grigioni, A. Corti, M. V. Dozzi and E. Selli, Photoactivity and Stability of WO3/BiVO4 Photoanodes: Effects of the contact electrolyte and of Ni/Fe oxyhydroxide protection, J. Phys. Chem. C, 2018, 122(25), 13969–13978 CrossRef CAS.
- X. Zhang, X. Wang, D. Wang and J. Ye, Conformal BiVO4-Layer/WO3-Nanoplate-array heterojunction photoanode modified with cobalt phosphate cocatalyst for significantly enhanced photoelectrochemical performances, ACS Appl. Mater. Interfaces, 2019, 11(6), 5623–5631 CrossRef CAS PubMed.
- S. S. Kalanur, I.-H. Yoo, J. Park and H. Seo, Insights into the electronic bands of WO3/BiVO4/TiO2, revealing high solar water splitting efficiency, J. Mater. Chem. A, 2017, 5(4), 1455–1461 RSC.
- T. Zhang, J. Su and L. Guo, Morphology engineering of WO3/BiVO4 heterojunctions for efficient photocatalytic water oxidation, CrystEngComm, 2016, 18(46), 8961–8970 RSC.
- Y. Zhang, D. Zhang, X. Xu and B. Zhang, Morphology control and photocatalytic characterization of WO3 nanofiber bundles, Chin. Chem. Lett., 2018, 29(9), 1350–1354 CrossRef CAS.
- C. Wang, X. Li, C. Feng, Y. Sun and G. Lu, Nanosheets assembled hierarchical flower-like WO3 nanostructures: Synthesis, characterization, and their gas sensing properties, Sens. Actuators, B, 2015, 210, 75–81 CrossRef CAS.
- J. Yin, S. Huang, Z. Jian, Z. Wang and Y. Zhang, Fabrication of heterojunction SnO2/BiVO4 composites having enhanced visible light photocatalytic activity, Mater. Sci. Semicond. Process., 2015, 34, 198–204 CrossRef CAS.
- Z. Jiao, H. Yu, X. Wang and Y. Bi, Ultrathin BiVO4 nanobelts: controllable synthesis and improved photocatalytic oxidation of water, RSC Adv., 2016, 6(77), 73136–73139 RSC.
- F. Bella, A. B. Muñoz-García, G. Meligrana, A. Lamberti, M. Destro, M. Pavone and C. Gerbaldi, Unveiling the controversial mechanism of reversible Na storage in TiO2 nanotube arrays: Amorphous versus anatase TiO2, Nano Res., 2017, 10(8), 2891–2903 CrossRef CAS.
- M. Tahir, B. Tahir and N. A. S. Amin, Gold-nanoparticle-modified TiO2 nanowires for plasmon-enhanced photocatalytic CO2 reduction with H2 under visible light irradiation, Appl. Surf. Sci., 2015, 356, 1289–1299 CrossRef CAS.
- D. a. e. Nagy, Effect of the morphology and phases of WO3 nanocrystals on their photocatalytic efficiency, R. Soc. Chem., 2016, 6, 33743–33754 CAS.
- Z. Ma, K. Song, L. Wang, F. Gao, B. Tang, H. Hou and W. Yang, WO3/BiVO4 Type-II Heterojunction arrays decorated with oxygen-deficient ZnO passivation layer: A highly efficient and stable photoanode, ACS Appl. Mater. Interfaces, 2019, 11(1), 889–897 CrossRef CAS PubMed.
- P. Guan, In situ anchoring Ag through organic polymer for configuring efficient plasmonic BiVO4 photoanode, Chem. Eng. J., 2019, 358, 658–665 CrossRef CAS.
- L. Shi, C. Xu, X. Sun, H. Zhang, Z. Liu, X. Qu and F. Du, Facile fabrication of hierarchical BiVO4/TiO2 heterostructures for enhanced photocatalytic activities under visible-light irradiation, J. Mater. Sci., 2018, 53(16), 11329–11342 CrossRef CAS.
- J. Z. Yin, S. B. Huang, Z. C. Jian, M. L. Pan, Y. Q. Zhang, Z. B. Fei and X. R. Xu, Enhancement of the visible light photocatalytic activity of heterojunction In2O3/BiVO4 composites, Appl. Phys. A, 2015, 120(4), 1529–1535 CrossRef CAS.
- S. S. Gujral, A. N. Simonov, M. Higashi, R. Abe and L. Spiccia, Optimization of titania post-necking treatment of TaON photoanodes to enhance water-oxidation activity under visible-light irradiation, ChemElectroChem, 2015, 2(9), 1270–1278 CrossRef CAS.
- N. Hirayama, H. Nakata, H. Wakayama, S. Nishioka, T. Kanazawa, R. Kamata, Y. Ebato, K. Kato, H. Kumagai, A. Yamakata, K. Oka and K. Maeda, Solar-driven photoelectrochemical water oxidation over an n-type lead–titanium oxyfluoride anode, J. Am. Chem. Soc., 2019, 141(43), 17158–17165 CrossRef CAS PubMed.
- A. P. Singh, N. Kodan, B. R. Mehta, A. Held, L. Mayrhofer and M. Moseler, Band edge engineering in BiVO4/TiO2 heterostructure: Enhanced photoelectrochemical performance through improved charge transfer, ACS Catal., 2016, 6(8), 5311–5318 CrossRef CAS.
- C. Liu, J. Su and L. Guo, Comparison of sandwich and fingers-crossing type WO3/BiVO4 multilayer heterojunctions for photoelectrochemical water oxidation, RSC Adv., 2016, 6(33), 27557–27565 RSC.
- D. Eisenberg, H. S. Ahn and A. J. Bard, Enhanced photoelectrochemical water oxidation on bismuth vanadate by electrodeposition of amorphous titanium dioxide, J. Am. Chem. Soc., 2014, 136(40), 14011–14014 CrossRef CAS PubMed.
- N. Wu, J. Wang, D. N. Tafen, H. Wang, J.-G. Zheng, J. P. Lewis, X. Liu, S. S. Leonard and A. Manivannan, Shape-enhanced photocatalytic activity of single-crystalline anatase tio2 (101) nanobelts, J. Am. Chem. Soc., 2010, 132(19), 6679–6685 CrossRef CAS PubMed.
- U. Shaislamov and B. L. Yang, CdS-sensitized single-crystalline TiO2 nanorods and polycrystalline nanotubes for solar hydrogen generation, J. Mater. Res., 2013, 28(3), 418–423 CrossRef CAS.
- D. Hongxing, L. Qiuping and H. Yuehui, Preparation of nanoporous BiVO4/TiO2/Ti film through electrodeposition for photoelectrochemical water splitting, R. Soc. Open Sci., 2018, 5(9), 180728 CrossRef PubMed.
- D. Coelho, J. Gaudêncio, S. A. Carminati, F. W. P. Ribeiro, A. F. Nogueira and L. H. Mascaro, Bi electrodeposition on WO3 photoanode to improve the photoactivity of the WO3/BiVO4 heterostructure to water splitting, Chem. Eng. J., 2020, 399, 125836 CrossRef CAS.
- S. S. M. Bhat, S. A. Lee, J. M. Suh, S.-P. Hong and H. W. Jang, Triple planar heterojunction of SnO2/WO3/BiVO4 with enhanced photoelectrochemical performance under front illumination, Appl. Sci., 2018, 8(10), 1765 CrossRef.
- J. Liu, J. Li, M. Shao and M. Wei, Directed synthesis of SnO2@BiVO4/Co-Pi photoanode for highly efficient photoelectrochemical water splitting and urea oxidation, J. Mater. Chem. A, 2019, 7(11), 6327–6336 RSC.
- I. Grigioni, K. G. Stamplecoskie, E. Selli and P. V. Kamat, Dynamics of photogenerated charge carriers in WO3/BiVO4 heterojunction photoanodes, J. Phys. Chem. C, 2015, 119(36), 20792–20800 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr04763a |
| This journal is © The Royal Society of Chemistry 2021 |