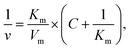A dual enzyme-mimicking radical generator for enhanced photodynamic therapy via series–parallel catalysis†
Zhenbang
Cao‡
ab,
Liang
Zhang‡
c,
Jianxin
Liu
d,
Dong
Wang
*c,
Kang
Liang
 abe,
Yu
Chen
abe,
Yu
Chen
 f and
Zi
Gu
f and
Zi
Gu
 *ab
*ab
aSchool of Chemical Engineering, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: zi.gu1@unsw.edu.au
bAustralian Centre for NanoMedicine (ACN), University of New South Wales, Sydney, NSW 2052, Australia
cDepartment of Ultrasound, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400010, P.R.China. E-mail: wang57554@163.com
dUltrasound Department, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430014, P.R.China
eGraduate School of Biomedical Engineering, University of New South Wales, Sydney, NSW 2052, Australia
fSchool of Life Sciences, Shanghai University, Shanghai 200444, P.R.China
First published on 14th September 2021
Abstract
Tumor hypoxia hampers the therapeutic efficacy of photodynamic therapy (PDT) by hardly supplying sufficient oxygen to produce cytotoxic compounds. Herein a dual enzyme-mimicking radical generator has been developed for the in situ generation of oxygen and abundant radical oxygen species to enhance PDT efficacy under photoacoustic imaging guidance. A manganese-incorporating and photosensitizer-loaded metal organic framework exhibited both catalase-like and peroxidase-like catalytic activities specifically at the tumor microenvironment, leading to simultaneous series catalysis and parallel catalysis pathways. As a result, the MOF-based radical generator nanoparticles can not only supply oxygen for PDT to produce singlet oxygen, but also generate hydroxyl radicals, thus further enhancing the anti-cancer effect of PDT. In vitro and in vivo evaluation of the radical generator nanoparticles demonstrated the relieved tumor hypoxia microenvironment, remarkably increased level of reactive oxygen species, and significantly improved anti-cancer effect with desirable PA imaging capacity. This work presents a “series–parallel catalysis” strategy enabled by a MOF nanozyme to enhance PDT efficiency and provides new insights into a highly efficient and low-toxic anti-cancer approach.
1. Introduction
Photodynamic therapy (PDT), being approved for clinical use in oncology, is an important non-invasive cancer treatment modality with high accuracy and safety.1 PDT involves the combination of light, photosensitizer and oxygen (O2). Upon light irradiation, the photosensitizer is excited from the ground state to the first excited single state and then converted to the triplet state. The excited triplet state can react with O2via electron transfer to generate singlet oxygen (1O2) that is able to kill cancer cells.2 However, PDT efficacy is largely restricted by hypoxia (a hallmark of solid tumor in which the tissues are deprived of adequate O2).3,4 To address the issue of insufficient oxygen supply, a few strategies have been developed, including O2 delivery (using hemoglobin,5,6 perfluorocarbon7) and O2 generation (using catalase-like nanoparticles such as MnO2,8,9 CaO2,10 Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11,12 to catalyze H2O2 into O2 to alleviate tumor hypoxia). The catalase-like nanoparticle-based O2 generation approach has been intensively explored in recent years, given that the involved nanomaterials not only act as the source to supply O2 but also provide a versatile platform for multi-channeled treatment.13 However, these nanoparticles suffer from low photosensitizer loading capacity, which implies a high dosage of nanoparticles required to achieve desirable therapeutic efficacy.
11,12 to catalyze H2O2 into O2 to alleviate tumor hypoxia). The catalase-like nanoparticle-based O2 generation approach has been intensively explored in recent years, given that the involved nanomaterials not only act as the source to supply O2 but also provide a versatile platform for multi-channeled treatment.13 However, these nanoparticles suffer from low photosensitizer loading capacity, which implies a high dosage of nanoparticles required to achieve desirable therapeutic efficacy.
Another critical challenge that limits the practical application of PDT is the relatively weak oxidation ability of 1O2, a therapeutic reactive oxygen species (ROS) generated through PDT. ROS, at a high level, can break the redox homeostasis and induce irreversible cancer cell damage, while a low level of ROS can stimulate cancer cell growth or be easily neutralized by endogenous antioxidants.14,15 Therefore, the production of sufficient, highly active ROS is vital in PDT induced anticancer therapy. However, the main type of ROS in PDT is limited to 1O2 (E0(1O2/O2˙−) = 0.65 V), which has a weaker oxidation ability than many other types of ROS such as hydroxyl radicals (E0(˙OH, H+/H2O) = 2.33 V). Thus, the production of highly oxidative ˙OH is ideal to be incorporated into the rational design of the PDT nanoplatform to boost therapeutic efficiency.
Aside from therapeutic consideration, PDT is a local cancer treatment operation that requires bio-imaging guidance.16,17 Photoacoustic (PA) imaging is a newly emerging noninvasive biomedical imaging modality based on the generation of ultrasonic wave of ultrasound imaging, thus being able to provide high contrast imaging of the fine structure of deep tumors and anatomical, functional and metabolic information for tumor localization to guide local treatment such as PDT.18,19
Recently, metal–organic framework (MOF) nanomaterials have attracted wide attention and stood out among various nanomaterials for biomedical applications, by virtue of their unique structural, photonic and bio-nano interface characteristics.20 MOFs are hybrid crystals composed of metal ions or clusters connected by multidentate organic ligands to form metal–organic arrays possessing a structure with very high porosity and enormous surface area that enable high drug loading capacity. MOFs are highly tunable in terms of chemical composition, crystal structure, morphology and porosity, which renders them the capacity to incorporate various functional metal ions and therapeutic agents for drug delivery, bio-imaging, and multifunctional biomedical applications.21,22 In this work, we developed a MOF74-based dual-enzyme mimicking radical generator (ICG@PEG-MnMOF74) that demonstrated enhanced PDT treatment of tumors (Fig. 1). The radical generator ICG@PEG-MnMOF74 particle consists of three key components that are manganese ion-doped MOF74, photosensitizer indocyanine green (ICG), and stabilizer polyethylene glycol (PEG). The key aspects of ICG@PEG-MnMOF74 directly attributed to the enhanced PDT performance include: (1) well-dispersed and nanosized ICG@PEG-MnMOF74 particles acting as a catalyst and a photosensitizer to trigger oxygen generation and subsequent self-oxygen supplement PDT that generated a large amount of cytotoxic 1O2 (“series catalysis route”); (2) ICG@PEG-MnMOF74 particles exhibited peroxidase-like catalytic activities that induced a Fenton-like reaction to generate abundant ˙OH to kill cancer cells. The simultaneous production of 1O2 and ˙OH leads to a “parallel catalysis route”; (3) both series and parallel catalysis routes occur in response to the tumor microenvironment conditions, leading to high tumor selectivity and specificity; and (4) ICG@PEG-MnMOF74 exhibited PA imaging functionality that enabled the accurate diagnosis and precision localization of tumors, serving as an imaging tool to guide PDT. Overall, the radical generator-mediated PDT approach via series and parallel catalysis routes not only makes up for the deficiencies of additional PDT but also introduces new therapeutic and imaging features, opening up an avenue for local cancer treatment.
 | ||
| Fig. 1 Schematic illustration of the synthesis and enhanced photodynamic therapy (PDT) of ICG@PEG-MnMOF74 radical generator nanoparticles via series–parallel catalysis. | ||
2. Experimental methods
2.1. Materials
All chemicals, including manganese acetate tetrahydrate (>99.9%), 2,5-dihydroxyterephthalic acid (>98%), N,N-dimethylformamide, NaOH (≥98%), 3,3′,5,5′-tetramethylbenzidine (TMB), hydrogen peroxide (H2O2, 29.0–32.0%), hydrochloric acid (37%), acetic acid (≥99%), sodium acetate anhydrous (≥99%), phosphate buffered saline (PBS), paraformaldehyde, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, ≥98%) were purchased from Sigma-Aldrich. Dulbecco's modified Eagle's medium (DMEM), penicillin/streptomycin, and fetal calf serum (FCS) were purchased from Gibco. Phosphonic acid terminated poly(ethylene glycol) was synthesized using a controlled/living radical polymerization according to procedures described previously.23,24 Milli-Q water was used in the experiments.2.2. Synthesis of MnMOF74
Manganese acetate (1.3 mmol) and 2,5-dihydroxyterephthalic acid (dhtp) (0.5 mmol) were dissolved in 5 mL and 2.648 mL of N,N-dimethylformamide (DMF) respectively (the molar ratio of linker to metal = 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).25 dhtp solution was added into manganese acetate solution in a dropwise manner under vigorous stirring at room temperature. After stirring for 20 h, the resultant was centrifuged with DMF, and then washed and centrifuged twice using methanol.
1).25 dhtp solution was added into manganese acetate solution in a dropwise manner under vigorous stirring at room temperature. After stirring for 20 h, the resultant was centrifuged with DMF, and then washed and centrifuged twice using methanol.
2.3. PEGylation of MnMOF74
To synthesize PEGylated MOF nanoparticles, the MnMOF74 slurry was dispersed in water and mixed with phosphate group-terminated poly (ethylene glycol) (PEG) at a PEG to MOF mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was then sonicated in a water bath at room temperature for 30 min. The excessive PEG was removed via centrifugation. The resultant was dispersed in water and designated as PEG-MnMOF74.
1. The mixture was then sonicated in a water bath at room temperature for 30 min. The excessive PEG was removed via centrifugation. The resultant was dispersed in water and designated as PEG-MnMOF74.
2.4. ICG loading
Pre-dissolved ICG water solution was added into the PEG-MnMOF74 suspension at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After mixing for 24 h via continuous stirring, the resultant was washed and collected by centrifugation, and denoted as ICG@PEG-MnMOF74. The loading capacity of ICG was calculated as the proportion of ICG in the total amount of ICG@PEG-MnMOF74.
1. After mixing for 24 h via continuous stirring, the resultant was washed and collected by centrifugation, and denoted as ICG@PEG-MnMOF74. The loading capacity of ICG was calculated as the proportion of ICG in the total amount of ICG@PEG-MnMOF74.
2.5. Michaelis–Menten kinetics by TMB assay
TMB assay was conducted to monitor the chromogenic reaction (λ = 650 nm) of the MOF/H2O2 system. A solution (pH = 4) was prepared using HAC/NaAC in the presence of NaHCO3 (25 mM). In an 800 μL buffer solution, 50 μg mL−1 of MOF suspension was mixed with TMB and H2O2 at the final concentrations of 800 μM and 1 mM respectively. The absorbance of the mixture was measured on a UV-vis spectrometer (CARY 300, Varian) equipped with a temperature controller. For the kinetic study, the concentrations of H2O2 (0.01, 0.05, 0.1, 0.2, 0.5, 1, 1.5, 2, 4, and 8 mM) were varied while the concentrations of TMB (800 μM) remained fixed. The Michaelis–Menten constant was calculated by using Lineweaver–Burk plots of the Michaelis–Menten equation:where v represents the initial velocity, Vmax the maximum reaction velocity, C the concentration of the substrate, and Km the Michaelis–Menten constant.
2.6. Evaluation of H2O2 consumption
H2O2 consumption was evaluated by using the titanium sulfate (Ti(SO4)2) colorimetric method. PEG-MnMOF74 (0.25 mg) was incubated at 37 °C with 0.5 mL of H2O2 (1 mM), followed by mixing with a 25 mL solution containing 1.33 mL of Ti(SO4)2 (24%) and 8.33 mL of H2SO4 for 10 min. The mixture was then centrifuged to collect the supernatant, and the concentration of H2O2 in the supernatant was obtained by measuring the absorbance at λ = 405 nm. The continuous catalytic effect was measured by the repetitive addition of H2O2 (1 mM) to the solution.2.7. Cell culture
Breast cancer cells (4T1 cells) were cultured in a growth medium (DMEM) supplemented with 10% fetal calf serum (FCS), streptomycin (100 mg mL−1) and penicillin (100 units mL−1). The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2 in air.2.8. In vitro and in vivo photoacoustic imaging
The PA imaging of ICG@PEG-MnMOF74 was conducted on a Vevo LAZR Photoacoustic Imaging System (VisualSonics Inc., Toronto, Canada) equipped with a LZ250 (fiber-optic bundles: 25.4 × 1.25 mm; focal depth: 10 mm; center frequency: 21 MHz; axial resolution: 75 μm). Prior to imaging, 100 μg mL−1 ICG@PEG-MnMOF74 was scanned under PA at different wavelengths from 690 nm–960 nm to determine the λmax. ICG of certain concentrations was subjected to PA imaging with regard to the region of interest (ROI). For the in vivo PA imaging, 4T1 tumor-bearing Balb/c mice (n = 3) were intravenously injected with 200 μL of saline containing ICG@PEG/MOF (4 mg kg−1 ICG, 10 mg kg−1 PEG-MnMOF74). After injection, PA images of tumor sites were recorded at λmax = 780 nm at 1, 2, 4, 6, 8 and 24 h. The intensities in the ROI were quantified using Vevo LAZR software.2.9. In vitro cell viability assessment
The in vitro cytotoxicity was assessed by CCK-8 assay. The 4T1 cells were treated with ICG and ICG@PEG-MnMOF74 at a concentration equivalent to 5, 10 or 15 μg mL−1 of ICG containing 25 mM NaHCO3. After 6 h of incubation, the cells were irradiated at a power of 1.5 W cm−2 for 3 min on ice (excluding the photothermal effect). The cell viability was measured by using a CCK-8 cell viability kit and calculated by comparing with the control group without treatment. The cell living status was also visualized by live and dead staining with calcein acetoxymethyl ester (CAM) and propidium iodide (PI) to differentiate live (green fluorescence) and dead (red fluorescence) cells. Images were recorded with a confocal laser scanning microscope (FV 1000, Olympus, Japan). Cell quantification was conducted using ImageJ. Live and dead cells in 3 randomly selected views with equivalent areas were counted. The percentages were calculated as live cell percentage = live cells/(live cells + dead cells) × 100%.2.10. In vitro oxidative stress assessment
The hydroxyl radical generation ability of PEG-MnMOF74 under the tumor microenvironment conditions was evaluated by using a hydroxyphenyl fluorescein (HPF) probe. The 4T1 cells were seeded at a density of 1 × 105 in DMEM in the φ 15 CLSM-exclusive culture disk and allowed to adhere overnight. The cells were treated with different agents (i.e., PBS, H2O2 (100 μM), PEG-MnMOF74 (25 μg mL−1), PEG-MnMOF74 (25 μg mL−1) + H2O2 (100 μM)). After incubation for 6 h, the cells were cultured with HPF solutions (10 μL of 1 mM per mL culture) for 30 min. The level of the intracellular hydroxyl radical was evaluated with confocal microscopy by detecting the fluorescence at λex = 488 nm and λem = 525 nm. The experiments were carried out in 25 mM NaHCO3 cultures with various pH values.The in vitro oxidative stress level was assessed using 2′,7′-dichlorofluorescein diacetate (DCFH-DA). The 4T1 cells were seeded at a density of 1 × 105 in DMEM in the φ 15 CLSM-exclusive culture disk and allowed to adhere overnight. The culture medium was then replaced with media containing ICG@PEG-MnMOF74 or ICG at a concentration equivalent to 10 μg mL−1 of ICG. Five treatments (i.e., PBS, H2O2 (100 μM), H2O2 (100 μM) + laser irradiation, ICG + H2O2 (100 μM) + laser irradiation, PEG-MnMOF74 + H2O2 (100 μM), and ICG@PEG-MnMOF74 + H2O2 (100 μM) + laser irradiation) were performed to evaluate the in vitro oxidative stress. After incubation for 6 h, the cells were incubated with DCFH-DA (30 μM) for 30 min. Laser irradiation was then conducted on selected groups at a power of 1.5 W cm−2 for 3 min on ice. The level of intracellular ROS was evaluated with confocal microscopy by detecting the fluorescence of DCF (λex = 488 nm, λem = 525 nm for DCFH-DA).
2.11. In vivo biosafety study
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Chongqing Medical University and experiments were performed with the approval of the Animal Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. 7-Week female Balb/c mice (∼20 g) were intravenously injected with 200 μL of ICG@PEG-MnMOF74 saline solution at the dosages of 10, 40 and 80 mg kg−1 with the same volume of saline as the control. At 30-day post-treatment, blood was collected for hematological and biomedical index analysis. Mice were then sacrificed to collect their major organs (heart, liver, spleen, lungs, and kidneys) in 4% paraformaldehyde solution for histopathology analysis (hematoxylin and eosin (H&E) staining assay).2.12. In vivo anti-cancer effect evaluation
Xenografted tumors were generated in female Balb/c mice (∼20 g, 7 weeks old) by subcutaneously inoculating 1 × 106 4T1 cells suspended in PBS (100 μL). Once the tumor reached a volume of 100 mm3, mice per group were randomly allocated to 5 groups (n = 5): saline (group I), laser (group II), ICG + laser (group III), ICG@PEG-MnMOF74 (group IV), ICG@PEG-MnMOF74 + laser (group V). ICG and ICG@PEG-MnMOF74 in saline were injected via the tail vein (200 μL, 4 mg kg−1 ICG). After 6 h, the mice from group II, III and V were irradiated at the site of tumors with an 808 nm laser (1.5 W cm−2, on 20 s, off to room temperature, a total of 10 min on). Tumors were measured every two days with a digital caliper. The tumor volumes (V) were calculated as V = L × W2/2, and also normalized to the initial volume (V0) to obtain the relative tumor volume (V/V0). Mice were euthanized once the tumor volume reached 1000 mm3.2.13. In vivo detection of tumor hypoxia status
To visualize the hypoxia status of the 4T1 tumor, 6 mice were randomly divided into 2 groups (control and ICG@PEG-MnMOF74, n = 3 in each group). The two groups were intravenously injected with saline and ICG@PEG-MnMOF74 (4 mg kg−1 ICG, 10 mg kg−1 PEG-MnMOF74) respectively. The oxygenated hemoglobin levels of tumor tissues at 1, 2, 4, 6, 8, and 24 h post-injections were evaluated using a Vevo LAZR PA imaging system in oxy-hem mode and oxyhemoglobin saturation (sO2 Avr Total). It was recorded by measuring the ratios of oxygenated hemoglobin (λ = 850 nm) and deoxygenated hemoglobin (λ = 750 nm). At 6 h post-injection, the tumor tissues were collected for HIF-1α staining with the red fluorescence indicating hypoxia regions.2.14. Characterization
The size distribution and zeta potential were measured using a Malvern Zetasizer Nano Series. X-ray diffraction (XRD) measurements were performed on PEG/MOF powders using a PANalytical Empyrean II Co Source XRD instrument operated at 45 kV and 40 mA and a scan rate of 0.01° min−1 applied with a step size of 2θ = 0.0260°. The morphology of nanoparticles was observed using a transmission electron microscope (TEM, FEI Tecnai G2) at an acceleration voltage of 200 kV. Thermogravimetric analysis (TGA, TA Instruments, Q5000) was performed under nitrogen 40 mL min−1 from 30 to 800 °C with a rate of 20 °C min−1. The manganese content was measured on a PerkinElmer OPTIMA 7300 inductively coupled plasma optical emission spectrometer (ICP-OES) after the dissolution of the MOF in 1 M hydrochloride acid. The concentration of ICG was measured at a wavelength of 780 nm on a SpectraMax paradigm multimode microplate reader. O2 generation was monitored by using a dissolved oxygen meter (HANNA HI 98193). Electron spin resonance (ESR) spectra were obtained at room temperature in perpendicular mode on a Bruker EMX-8/2.7 spectrometer with the following settings: microwave frequency = 9.773 GHz, microwave power = 6.325 mW, modulation frequency = 100.00 kHz and modulation amplitude = 2.00 G. DMPO was used as a spin trap. The experiment was carried out in 25 mM NaHCO3/5% CO2 solution systems with various pH values.2.15. Statistical analysis
Quantitative data are presented as mean ± SEM and analyzed by two-way ANOVA with Tukey's multiple comparisons test using GraphPad Prism software; a P-value <0.05 was considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001).3. Results and discussion
3.1. Synthesis and physicochemical structure of ICG@PEG-MnMOF74
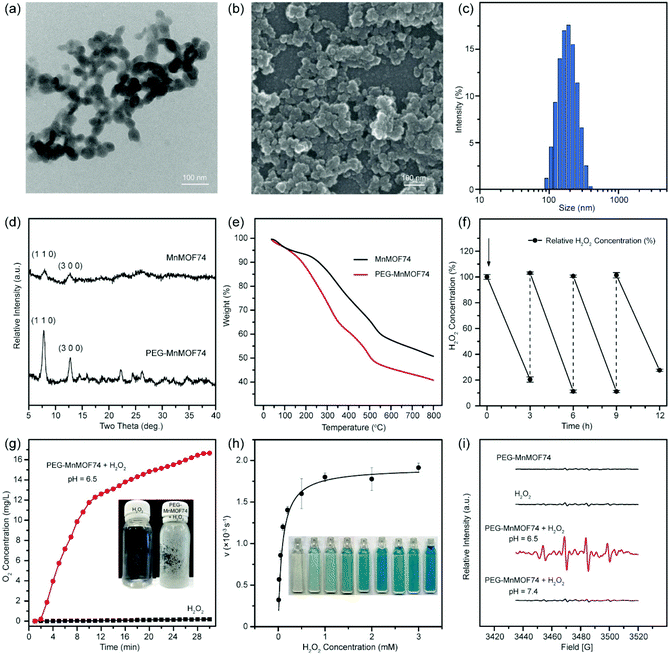
The radical generator for enhanced PDT was designed and synthesized by incorporating Mn ions and photosensitizer ICG into a MOF74 structure. Mn-containing MOF74 was firstly synthesized by the copolymerization of manganese ions with dimethylformamide links, and stabilized by the surface modification of PEG and loaded with photosensitizer ICG. Mn-MOF74 particles exhibited a suborbicular morphology with the particle size being about 50 nm (Fig. 2a and b), and PEG-MnMOF74 appeared less aggregated than MnMOF74 (ESI Fig. S1†). Such improved dispersity after PEG coating is also demonstrated by the relatively narrow hydrodynamic size distribution of PEG-MnMOF74 (176 nm in average size and 0.08 in PDI (Fig. 2c), compared with MnMOF74 being 342 nm in size and 0.27 in PDI). PEG modification not only increased MnMOF74 particle dispersity in water but also alleviated severe aggregation in electrolyte solutions (i.e. saline, PBS, culture medium containing serum) (ESI Table S1†). The XRD patterns of both PEG-MnMOF74 and MnMOF74 particles showed characteristic peaks at 2θ = 7.8° and 2θ = 12.7° that are associated with reflection from the (110) and (300) planes (Fig. 2d).26 The sharp peaks in the XRD pattern of PEG-MnMOF74 indicated that PEG-MnMOF74 particles were well crystallized. The XPS spectrum revealed the co-existence of Mn, O and C elements in both PEG-MnMOF74 and MnMOF74 (ESI Fig. S2†). ICP and TGA analysis showed that the Mn ions and PEG molecules account for 19.29 wt% and 11 wt% respectively in PEG-MnMOF74 (Fig. 2e). The successful loading of ICG in PEG-MnMOF74 was monitored by UV-vis spectroscopy, and the loading capacity of ICG was calculated to be approximately 28.6 wt%.3.2. Dual enzyme-like property of PEG-MnMOF74
Manganese is a versatile transition metal with bioactive catalytic activities. In nature, Mn functions in the oxygen-evolving complex of photosynthesis.27 Mn serves as a cofactor in many enzymes, and a trace metal required for the normal growth and development of human beings.27 The ability of Mn-containing nanomaterials to catalyze H2O2 to O2 has been recently explored for biomedical applications.28,29 Given that PEG-MnMOF74 particles have a high content of Mn (∼20 wt%), PEG-MnMOF74 can serve as a desirable catalase-mimicking nanozyme agent. The catalase-like activity of PEG-MnMOF74 was evaluated by measuring H2O2 consumption and recording the dissolved O2 level (Fig. 2f and g). The results from the H2O2 consumption study showed that ∼80% of H2O2 was consumed within 3 h after the first addition of H2O2, indicating the high degree of catalytic efficiency of PEG-MnMOF74 (Fig. 2f). After three cycles of H2O2 addition and consumption, PEG-MnMOF74 still remained efficient catalytic activity that was able to decompose ∼73% of H2O2. The ability of PEG-MnMOF74 to generate O2 from H2O2 was assessed by recording the amount of O2 production using an oxygen probe (Fig. 2g). The addition of PEG-MnMOF74 in H2O2 solution induced the rapid generation of O2 and reached 17 mg L−1 after 30 min reaction, while the dissolved O2 in water in the absence of PEG-MnMOF74 remained at a basal level.Based on the fact that Mn(II) is a Fenton-like catalyst,30,31 we investigated the peroxidase-like activity of PEG-MnMOF74 to disproportionate H2O2 to ˙OH. A typical chromogenic reaction of TMB was used to evaluate the peroxidase-like activity of PEG-MnMOF74 particles qualitatively and quantitatively (Fig. 2h). The TMB solution turned blue with the addition of PEG-MnMOF74 and H2O2, and the blue color became darker with the increase of either reaction time or H2O2 concentration. This phenomenon was caused by ˙OH generation during H2O2 decomposition that oxidizes TMB to a blue product (with light absorption λmax 650 nm). The absorbance at λ = 650 nm was plotted against time with the addition of H2O2 at different concentrations into PEG-MnMOF74, and the initial velocities were calculated. The initial velocities against H2O2 concentration were fitted into the Michaelis–Menten equation, and the Michaelis–Menten constant (Km) and maximum velocity (Vmax) were calculated as 0.055 mM and 1.86 × 10−6 M s−1 respectively, indicating the high catalytic activity of converting H2O2 to ˙OH. The Km value of 0.055 mM indicates that the PEG/Fe-LDH nanocatalyst could achieve 50% of maximum catalytic activity at the H2O2 concentration as low as 0.055 mM, which is lower than the typical H2O2 concentration in the tumor microenvironment (0.1 mM). The Vmax of PEG-MnMOF74 indicates that the PEG-MnMOF74 could catalyze H2O2 at the maximum velocity of 1.86 × 10−6 M s−1. Next, the radical species from the catalytic reaction was identified by electron spin resonance (ESR) spectroscopy with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trap (Fig. 2i). The ESR spectrum of PEG-MnMOF74 solution with the addition of H2O2 under pH 6.5 showed a characteristic 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydroxyl radical signal, while this signal was not observed in the ESR spectra under pH 7.4. This acidity-triggered ˙OH generation indicates the potential use of PEG-MnMOF74 as a tumor-selective ROS generator to specifically kill cancer cells. The intracellular catalytic generation of ˙OH via PEG-MnMOF74 was verified by using a highly reactive oxygen radical probe hydroxyphenyl fluorescein (HPF). The cells displayed strong green fluorescence with PEG-MnMOF74 in the presence of H2O2 at pH 6.5 (Fig. 3c). In contrast, a low level of fluorescence signal was observed in the cells with the control treatments (Fig. 3c). The results indicate the stimuli-responsive intracellular hydroxyl radical generation ability of PEG-MnMOF74 in the tumor microenvironment.
1 hydroxyl radical signal, while this signal was not observed in the ESR spectra under pH 7.4. This acidity-triggered ˙OH generation indicates the potential use of PEG-MnMOF74 as a tumor-selective ROS generator to specifically kill cancer cells. The intracellular catalytic generation of ˙OH via PEG-MnMOF74 was verified by using a highly reactive oxygen radical probe hydroxyphenyl fluorescein (HPF). The cells displayed strong green fluorescence with PEG-MnMOF74 in the presence of H2O2 at pH 6.5 (Fig. 3c). In contrast, a low level of fluorescence signal was observed in the cells with the control treatments (Fig. 3c). The results indicate the stimuli-responsive intracellular hydroxyl radical generation ability of PEG-MnMOF74 in the tumor microenvironment.
3.3. In vitro assessment of enhanced PDT
The PDT assessment of ICG@PEG-MnMOF74 was performed on breast cancer 4T1 cells, and the cell viability was assessed by both CCK-8 assay and CAM/PI (live/dead) cell staining. From CCK-8 results (Fig. 3a), ICG@PEG-MnMOF74 demonstrated significantly stronger inhibition on 4T1 cell growth than pure ICG treatment at ICG concentrations of 5, 10 and 15 μg mL−1 under laser irradiation. For example, at an ICG concentration of 10 μg mL−1, cell viability was 11.9% in the ICG@PEG-MnMOF74 + laser group, which was remarkably lower than 59.8% in the ICG + laser group. In comparison, PEG-MnMOF74 demonstrated cell growth inhibition only at the concentrations being higher than 25 μg mL−1 (ESI Fig. S3†), indicating that the combination of ICG and PEG-MnMOF74 enhances the anticancer effect. By staining cells with living/dead cell dyes, negligible dead cells were found in the treatment groups of laser only and ICG@PEG-MnMOF74, as indicated by the strong green fluorescence of calcein-AM staining and few red fluorescence of PI staining (Fig. 3b). The treatment of ICG + laser led to an increased number of dead cells, suggesting that the combination of ICG and laser irradiation has shown a certain photodynamic effect. Notably, the ICG@PEG-MnMOF74 + laser exhibited a remarkably enhanced photodynamic effect that led to significantly increased cell death, in comparison to other treatments. In addition, results from the live cell quantification of the fluorescence images (ESI Fig. S4†) correspond to the cell viability results from the CCK-8 assay.To further explore the active compound that induces cell death, intracellular ROS generation was characterized by the DCFH-DA probe that could be rapidly oxidized by 1O2 and ˙OH and emit green fluorescence acting as a 1O2 and ˙OH sensor (Fig. 3d). The confocal images showed that only the ICG@PEG-MnMOF74 + laser group exhibited strong green fluorescence that was obviously much weaker in other control groups, indicating the significant production of ROS in 4T1 cells treated by ICG@PEG-MnMOF74 and laser. This phenomenon is in accordance with the aforementioned cell viability results, and highlights the indispensable role of MnMOF74 in the enhanced PDT approach.
3.4. In vivo assessment of enhanced PDT
In vivo anticancer behavior and photoacoustic imaging performance of ICG@PEG-MnMOF74 were evaluated using a 4T1 xenograft mouse model. Firstly, using ICG@PEG-MnMOF74 as a diagnostic agent has been explored by evaluating PA signals. The PA signal intensity increased in a linear relationship with the concentration of ICG@PEG-MnMOF74 (ESI Fig. S5†), demonstrating desirable PA responses. The in vivo PA images of tumor tissues showed that the PA signal increased gradually after the intravenous administration of ICG@PEG-MnMOF74, and the PA signal intensity reached the maximal at 6 h post-injection (Fig. 4a). The bio-imaging results imply the promising application of ICG@PEG-MnMOF74 to guide PDT treatment. Prior to performing PDT in vivo, we confirmed the ability of ICG@PEG-MnMOF74 to alleviate tumor hypoxia in vivo. The first line of evidence came from PA imaging in the oxyhemoglobin mode that was used to detect the oxygenated hemoglobin level of tumors before and after the administration of ICG@PEG-MnMOF74 (Fig. 4b). Before injecting ICG@PEG-MnMOF74, oxyhemoglobin showed weak signal intensity. The signal increased significantly after the administration of ICG@PEG-MnMOF74 and lasted for over 24 h. The PA imaging of tumor tissues in the oxyhemoglobin mode clearly showed that ICG@PEG-MnMOF74 could effectively improve oxygenation in hypoxic tumor regions. The hypoxia-inducible factor (HIF)-1α staining was conducted to form our second line of evidence to support the effect of ICG@PEG-MnMOF74 to alleviate tumor hypoxia. The tumors of mice treated with ICG@PEG-MnMOF74 exhibited very weak red fluorescence of HIF-1α and thus showed a low level of hypoxia, while the tumor tissues treated with the saline control exhibited a strong HIF-1α signal and a high level of hypoxia (Fig. 4c). All above results demonstrated that ICG@PEG-MnMOF74 could trigger in vivo localized oxygen generation efficiently and increase the level of endogenous oxygen level that can be beneficial to enhance PDT performance.In vivo PDT evaluation was conducted by treating 4T1-tumor bearing mice with different treatments including saline, laser only, ICG@PEG-MnMOF74 (14 mg kg−1 containing ICG 4 mg kg−1), ICG (4 mg kg−1) + laser, and ICG@PEG-MnMOF74 (14 mg kg−1) + laser. ICG@PEG-MnMOF74 exhibited the obvious inhibition of tumor growth after intravenous injection under laser irradiation, demonstrated by the significantly smaller tumor size than other groups (Fig. 5a–c). In particular, at the end of the treatment period, the average tumor volume of the ICG@PEG-MnMOF74 + laser group was about 2.7-fold smaller than that of the saline group (Fig. 5a). The enhanced anti-cancer effect induced by the ICG@PEG-MnMOF74 could be largely attributed to dual enzyme-like activities of PEG-MnMOF74 by simultaneously producing O2 and ROS (1O2 and ˙OH).
To evaluate in vivo biosafety, Balb/c mice were intravenously injected with ICG@PEG-MnMOF74 at a low dose of 10 mg kg−1, a medium dose of 40 mg kg−1, and a high dose of 80 mg kg−1. At 30-day post-injection, the major organs (heart, liver, spleen, lungs and kidneys) were collected for histological analysis. H&E staining images of tissues with the ICG@PEG-MnMOF74 treatment showed no observable pathological abnormalities (Fig. 5e), signifying the biocompatibility of ICG@PEG-MnMOF74. Also, the blood of mice was collected after a 30-day treatment period for complete blood count and biochemical index analysis including liver functional markers (ALT, AST, and TBIL), kidney functional markers (BUN and CR), and myocardial enzyme spectrum (CK and LDH-L). The results showed negligible variation among the different groups, indicating undetectable toxicity in a relatively long timeframe (ESI Fig. S6†). Moreover, the body weight of all mice increased regularly and demonstrated no significant change amongst groups (Fig. 5d). All these results indicated that the ICG@PEG-MnMOF74 has desirable in vivo biocompatibility.
4. Conclusions
This work presented a “series–parallel catalysis” strategy that simultaneously produced O2 and ˙OH to alleviate tumor hypoxia and generate abundant radicals for the enhanced PDT of cancers. It is realized by developing a dual-enzyme mimicking radical generator ICG@PEG-MnMOF74 that was constructed via a facile method and demonstrated both catalase-like and peroxidase-like activities. Both in vitro and in vivo experiments, using breast cancer 4T1 cell and animal models, exhibited the ability of ICG@PEG-MnMOF74 to overcome hypoxia and generate abundant ROS to specifically and efficiently kill cancer cells. The PA imaging functionality of ICG@PEG-MnMOF74 further guaranteed the precision localization of the tumor for improved PDT performance. We envisage that this multi-channeled radical generation strategy, along with the multifunctional dual-enzyme-like nanoplatform, will open the door to promote precise and effective PDT outcome.Author contributions
Z. Gu initiated and conceived the project. Z. Gu and D. Wang oversaw and were in charge of research activities at their own institutes. Z. Cao and L. Zhang performed the experiments and data analysis: Z. Cao was in charge of nanoparticle synthesis, characterisation and in vitro studies, and L. Zhang conducted animal experiments and part of cell studies. K. Liang, J. Liu and Y. Chen provided technical support on experimental design. All the authors contributed to manuscript writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Health and Medical Research Council of Australia (NHMRC) Early Career Fellowship (APP1073591), University of New South Wales (UNSW) Engineering Faculty Research Fund, UNSW-CAS (Chinese Academy of Sciences) Collaborative Research Grant, Chongqing Natural Science Foundation (cstc2019jcyj-zdxmX0019), and Health Commission of Hubei Province Scientific Research Project (Grant No. WJ2019M030). The authors acknowledge the UNSW Mark Wainwright Analytical Centre including facilities supported by the Australian Microscopy & Microanalysis Research Facility (AMMRF) at the Electron Microscope Unit.References
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535 CrossRef CAS.
- X. Qian, Z. Gu and Y. Chen, Mater. Horiz., 2017, 4, 800 RSC.
- T. Wang, H. Zhang, Y. Han, H. Liu, F. Ren, J. Zeng, Q. Sun, Z. Li and M. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 16367 CrossRef CAS.
- C. E. Weber and P. C. Kuo, Surg. Oncol., 2012, 21, 172 CrossRef PubMed.
- Z. Luo, M. Zheng, P. Zhao, Z. Chen, F. Siu, P. Gong, G. Gao, Z. Sheng, C. Zheng and Y. Ma, Sci. Rep., 2016, 6, 1 CrossRef CAS PubMed.
- S. Wang, F. Yuan, K. Chen, G. Chen, K. Tu, H. Wang and L. Wang, Biomacromolecules, 2015, 16, 2693 CrossRef CAS.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Hu, Nat. Commun., 2015, 6, 1 Search PubMed.
- W. Zhu, Z. Dong, T. Fu, J. Liu, Q. Chen, Y. Li, R. Zhu, L. Xu and Z. Liu, Adv. Funct. Mater., 2016, 26, 5490 CrossRef CAS.
- P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, R. S. DaCosta and X. Y. Wu, ACS Nano, 2014, 8, 3202 CrossRef CAS.
- L. H. Liu, Y. H. Zhang, W. X. Qiu, L. Zhang, F. Gao, B. Li, L. Xu, J. X. Fan, Z. H. Li and X. Z. Zhang, Small, 2017, 13, 1701621 CrossRef PubMed.
- J. Wei, J. Li, D. Sun, Q. Li, J. Ma, X. Chen, X. Zhu and N. Zheng, Adv. Funct. Mater., 2018, 28, 1706310 CrossRef.
- S. Xu, X. Zhu, C. Zhang, W. Huang, Y. Zhou and D. Yan, Nat. Commun., 2018, 9, 1 CrossRef PubMed.
- X. Li, N. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522 CrossRef CAS PubMed.
- H.-U. Simon, A. Haj-Yehia and F. Levi-Schaffer, Apoptosis, 2000, 5, 415 CrossRef CAS.
- K. Apel and H. Hirt, Annu. Rev. Plant Biol., 2004, 55, 373 CrossRef CAS PubMed.
- M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340 RSC.
- B. Yang, Y. Chen and J. Shi, Nanocatalytic Medicine, Adv. Mater., 2019, 31, 1901778 CrossRef.
- I. Steinberg, D. M. Huland, O. Vermesh, H. E. Frostig, W. S. Tummers and S. S. Gambhir, Photoacoustics, 2019, 14, 77 CrossRef.
- Q. R. Fu, R. Zhu, J. B. Song and H. H. Yang, Adv. Mater., 2019, 31, 1805875 Search PubMed.
- J. Yang and Y. W. Yang, Small, 2020, 16, 1906846 CrossRef CAS.
- W. Cai, H. Gao, C. Chu, X. Wang, J. Wang, P. Zhang, G. Lin, W. Li, G. Liu and X. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 2040 CrossRef CAS.
- Z. Guo, T. Wang, A. Rawal, J. Hou, Z. Cao, H. Zhang, J. Xu, Z. Gu, V. Chen and K. Liang, Mater. Today, 2019, 28, 10 CrossRef CAS.
- Z. Cao, L. Liang, K. Liang, S. Cheong, C. Boyer, J. J. Gooding, Y. Chen and Z. Gu, Adv. Sci., 2018, 5, 1801155 CrossRef.
- Z. Cao, N. N. K. Adnan, G. Wang, A. Rawal, B. Shi, R. Liu, K. Liang, L. Zhao, J. J. Gooding, C. Boyer and Z. Gu, J. Colloid Interface Sci., 2018, 521, 242 CrossRef CAS.
- M. Díaz-García, Á. Mayoral, I. Díaz and M. Sánchez-Sánchez, Cryst. Growth Des., 2014, 14, 2479 CrossRef.
- V. K. Yachandra, V. J. Derose, M. J. Latimer, I. Mukerji, K. Sauer and M. P. Klein, Science, 1993, 260, 675 CrossRef CAS PubMed.
- E. J. Martinze-Finley, S. Chakraborty and M. Aschner, in Encyclopedia of Metalloproteins, ed. R. H. Kretsinger, V. N. Uversky and E. A. Permyakov, Springer, New York, 2017, pp. 1227–1469 Search PubMed.
- S. Y. Yin, G. S. Song, Y. Yang, Y. Zhao, P. Wang, L. M. Zhu, X. Yin and X. B. Zhang, Adv. Funct. Mater., 2019, 29, 1901417 CrossRef.
- Y. Y. Chen, H. Zhong, J. B. Wang, X. Y. Wan, Y. H. Li, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 5773 RSC.
- X. Qian, J. Zhang, Z. Gu and Y. Chen, Biomaterials, 2019, 211, 1 CrossRef CAS.
- Y. Liu, M. Zhang and W. Bu, View, 2020, 1, e18 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr04104e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |