Open Access Article
Open Access ArticleReview of emerging nanotechnology in bone regeneration: progress, challenges, and perspectives
Hadi
Hajiali
 *a,
Liliang
Ouyang
bc,
Virginia
Llopis-Hernandez
d,
Oana
Dobre
d and
Felicity R. A. J.
Rose
*a,
Liliang
Ouyang
bc,
Virginia
Llopis-Hernandez
d,
Oana
Dobre
d and
Felicity R. A. J.
Rose
 *a
*a
aDivision of Regenerative Medicine and Cellular Therapies, School of Pharmacy, University Park, University of Nottingham, NG7 2RD, UK. E-mail: hadi.hajiali@nottingham.ac.uk; felicity.rose@nottingham.ac.uk
bDepartment of Materials, Imperial College London, London, SW7 2AZ, UK
cDepartment of Mechanical Engineering, Tsinghua University, Beijing, 100084, China
dCentre for the Cellular Microenvironment, University of Glasgow, Glasgow, G12 8LT, UK
First published on 28th May 2021
Abstract
The application of nanotechnology to regenerative medicine has increased over recent decades. The development of materials that can influence biology at the nanoscale has gained interest as our understanding of the interactions between cells and biomaterials at the nanoscale has grown. Materials that are either nanostructured or influence the nanostructure of the cellular microenvironment have been developed and shown to have advantages over their microscale counterparts. There are several reviews which have been published that discuss how nanomaterials have been used in regenerative medicine, particularly in bone regeneration. Most of these studies have explored this concept in specific areas, such as the application of glass-based nanocomposites, nanotechnology for targeted drug delivery to stimulate bone repair, and the progress in nanotechnology for the treatment of osteoporosis. In this review paper, the impact of nanotechnology in biomaterials development for bone regeneration will be discussed highlighting specifically, nanostructured materials that influence mechanical properties, biocompatibility, and osteoinductivity.
Introduction
Nanotechnology as an applicable tool for regenerative medicine has gained interest as our understanding of the interactions between cells and biomaterials at the nanoscale has grown. Materials that are either nanostructured or influence the nanostructure of the cellular microenvironment have been studied and demonstrated to have advantages over their microscale counterparts.1 We focus here on the application of nanotechnology for bone tissue regeneration as this is an extensive field of study with an ongoing need for efficacious, novel treatments as bone regeneration therapies. This review will consider the many related research papers published in recent years with the common aim of realising the potential that nanotechnology provides to develop new strategies in bone regeneration.Loss of bone as a result of non-healing fractures following trauma, disease such as osteoporosis, and as a result of tumours affect millions of people globally, and that is one of the leading causes of disability worldwide.2 Current materials used in the clinic include metals, ceramics, polymers and their composites and despite their long history and widespread use, they have drawbacks including low biocompatibility, poor bone formation, and a mismatch of mechanical properties with the surrounding native bone. Regenerative medicine has become an attractive field of study particularly based on the increasing demand for new solutions to treat significant tissue loss. This field of study has developed new treatments which involve the use of cells, growth factors, and biomaterials either alone or in combination for any tissue type, including bone.3 Many research projects have focussed on the development of advanced biomaterials that accelerate and promote bone regrowth in defect sites including, more recently, the use of nanomaterials.4
The field of nanotechnology and the application of nanomaterials to regenerative medicine have significantly advanced in recent years. There are several reviews studies which have been published to demonstrate how nanomaterials have been used in medicine1,5–7 particularly in bone regeneration.8–17 Most of these papers have reviewed specific areas; for instance, Boccaccini et al. reviewed the application of glass-based nanocomposites in bone regeneration,9 Gu et al. studied the application of nanotechnology in the targeted drug delivery for bone regeneration,11 the progress in nanotechnology for the treatment of osteoporosis was evaluated in another review paper,15 and Wang et al., in their review study, investigated the interaction of nanomaterials with growth factors and cells for bone repair.16
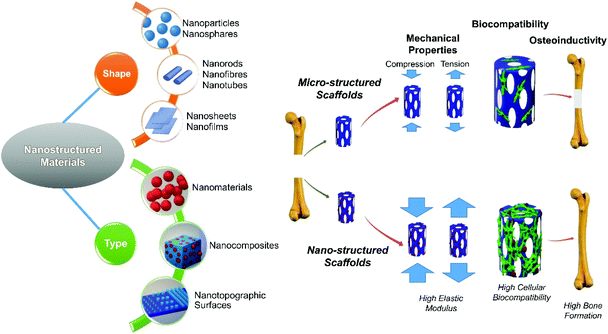
Properties of nanostructured biomaterials should be extensively studied for successful bone regeneration. It has been known that the mechanical properties, biocompatibility, and osteoinductivity of biomaterials are important criteria to influence bone regeneration.18,19 The bone formation response can also be changed by altering the mechanical environment.20 In addition, the biocompatibility of biomaterials play an important role in their performance for bone healing, and their osteoinductivity would be the most important property of biomaterials which induces new bone formation.21 Therefore, we consider how nanotechnology has been applied to modify tissue engineering scaffolds for bone in three key areas; mechanical properties, biocompatibility, and osteoinductivity, in this paper (Fig. 1). Furthermore, the current challenges and future directions are discussed.
Mechanical properties
Progress
Mechanical forces can play important roles in bone reconstruction and formation.20,22 Bone cells are responsive to local mechanical stresses supplied during motion. These stresses can contribute to changes in cellular metabolism and influence bone remodelling by inducing signalling and biochemical cascades.20 Furthermore, it has been shown that biomaterials with an insufficient mechanical strength or with a higher mechanical strength than native bone can lead to bone repair failure and bone loss that might be as a result of weak interfacial bonding or stress shielding.21 Therefore, the mechanical properties of biomaterials for bone repair have to be considered extensively.There are many papers in the literature that have demonstrated how the mechanical properties of biomaterials can be modified by the application of nanotechnology. The results of recent studies are summarised in Table 1. Bone matrix, a natural nanocomposite structure, consists of two major phases at the nanoscale including organic (collagen) and inorganic (Hydroxyapatite (HAP)). There is a range of mechanical properties depending on the type of bone with the elastic modulus varying from 1 to 20 GPa, and the compressive strength ranging from 1 to 100 MPa dependent on its porosity, composition and structure.23,24 Various effects of nanomaterials on the mechanical properties of these nanocomposites have been reported and is dependent upon the nanoparticle dispersion state, surface area, polydispersity, and organo-modification to improve interface interaction between phases.25 Studies have shown how incorporation of nanosized materials, including HAP and bioglass(BG), can improve their mechanical properties compared to their microsized equivalents.26–29 We have also showed that the elastic modulus of porous scaffolds can be enhanced by the addition of nano BG to the polymeric matrix.30 The chemical and thermal analysis in our study demonstrated a favourable interaction between polymer and bioglass nanoparticles which enhanced the interaction of the two phases at the interface31 and consequently enhanced stiffness. It has also been shown that the homogenously dispersed nanoparticles are also effective in increasing the stiffness of synthetic polymer-based materials.25
| Materials | Fabrication method | Porosity | Mechanical properties results | Ref. |
|---|---|---|---|---|
| PLLA: poly-L-lactic acid; HAP: hydroxyapatite; PA: polyamide; PHB: poly (3-hydroxybutirate); n: nano; m: micro. | ||||
| Compact human bone | Natural development (nanocomposite of 20% collagen and 69% calcium phosphate) | 5–10% | Elastic modulus 17.0–18.9 GPa; compressive strength: 170–193 MPa; tensile strength: 124–174 MPa; bending strength: 160 MPa; shear strength: 54 MPa; fracture toughness: KIc: 2–12 MPa m1/2 | 23 and 24 |
| Cancellous human bone | 50–95% | Elastic modulus: 1–2 GPa; compressive strength: 1–100 MPa | ||
| PLLA/nHAP (50/50) | Solid–liquid | 85–87% | Elastic modulus: 0.014 GPa; compressive strength: 8.67 MPa | 29 |
| PLLA/mHAP (50/50) | Phase separation | Elastic modulus: 0.013 GPa; compressive strength: 4.61 MPa | ||
| PLLA | Freeze–dryer | Elastic modulus: 0.0017 GPa; compressive strength: 2.4 MPa | ||
| PA/nHAP (38%–64% nHAP) | Injection moulding | — | Elastic modulus: 3.6–5.6 GPa; compressive strength: 93–117 MPa; tensile strength: 65–87 MPa; bending strength: 77–95 MPa | 32 |
| Injection foaming | 71–80% | NA | ||
n-HAP/PA (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Thermally induced phase inversion | 52%–70% | Elastic modulus: 0.29–0.85 GPa; compressive strength: 13.2–33.9 MPa | 33 |
| nHAP-zirconia (1.5%) | Pressure-assisted sintering | — | Elastic modulus: 149 GPa; compressive strength: 766 MPa; Bending strength: 243 MPa | 26 and 27 |
| nHAP | Elastic modulus: 130 GPa; Compressive strength: 897 MPa; Bending strength: 193 MPa; fracture toughness: KIc: 1.3 MPa m1/2 | |||
| Conventional HAP | Elastic modulus: 120 GPa; compressive strength: 120–800 MPa; bending strength: 38–113 MPa; fracture toughness: KIc: 1 MPa m1/2 | |||
| PHB/nBG (10%–30%) | Solvent casting (dense composite) | — | Elastic modulus: 1.15–1.58 GPa | 28 |
| PHB/mBG (10%–30%) | Elastic modulus: 0.79–0.83 GPa | |||
| PHB | Elastic modulus: 1.01 GPa | |||
| PHB/nBG (0%–10%) | Salt leaching | 59%–85% | Elastic modulus: 0.007–0.048 GPa | 30 |
Challenge
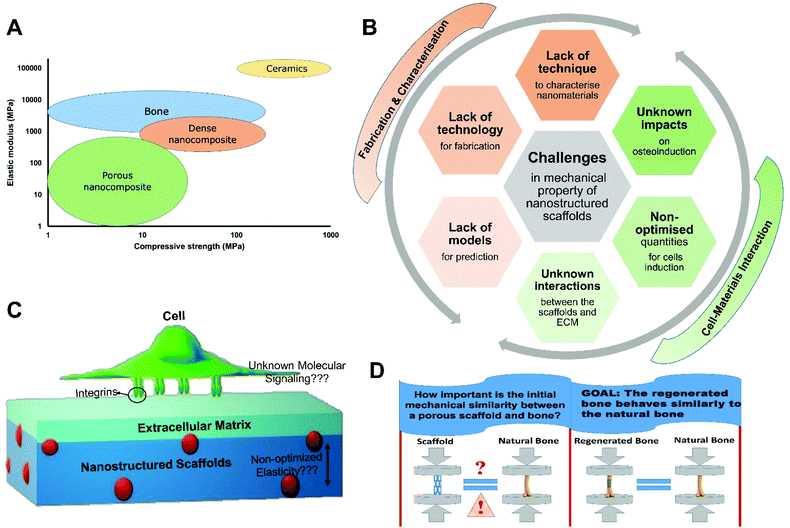
Despite the fact that there have been many attempts to fabricate scaffolds with appropriate mechanical properties, porous scaffolds that mechanically behave similarly to bone are yet to be reported (Fig. 2A). Although some dense nanocomposites have been developed that exhibit an elastic modulus and strength in the range of cancellous bone, regrowth of bone cannot be expected in such non-porous structures and these do not allow for tissue infiltration. It has been demonstrated that interconnected porous scaffold networks that enable the transport of nutrients, removal of wastes, and facilitate proliferation, migration of cells, and vascular ingrowth are essential for bone tissue engineering.34Although scaffolds with enhanced mechanical properties, through the incorporation of nanomaterials, have been reported by numerous researchers, our fundamental knowledge of the “nano effect” in terms of mechanical properties is not fully developed (Fig. 2B and C). Challenges in the visualisation at the nanoscale hinders our understanding of the extent and properties of the interphase region. The properties of the interphase layer in nanocomposites has therefore been mostly investigated by indirect methods such as thermal analysis.35 Furthermore, a variety of analytical and computational models have been developed to analyse and predict the mechanical behaviours of nanomaterials and nanocomposites,36 but the complexity of generating experimental data, the lack of knowledge about the interphase region, and the high computational costs have limited the development of models that can precisely predict the properties of the various types of nanocomposites.
The interactions between cells and their pericellular environment when growing within scaffolds of various stiffness remains relatively unexplored (Fig. 2C).37 However, it has been shown that cells can sense the rigidity of the extracellular matrix (ECM) in 2D environments through cell surface receptors such as integrins.38 Integrins can regulate cell migration, proliferation, and differentiation by connecting the intracellular cytoskeleton to the ECM. This activates focal adhesion kinase (FAK) and phosphoinositide 3-kinase (PI3K) signaling pathways to regulate the self-renewal and proliferation of cells, and has been demonstrated with mesenchymal stem cells.39 It is still not clear how the mechanical properties of scaffolds stimulate cells to secret the proteinaceous pericellular matrix associated with osteogenesis in a 3D environment. Therefore, there are no defined values for the desired elastic modulus and mechanical strength of scaffolds suitable for bone regeneration. The main challenges in the development of scaffolds with defined mechanical properties from nanostructured materials are summarised in Fig. 2B.
Perspective
Innovative fabrication methods, such as 3D printing,40 can be utilised to not only design and control scaffold porosity but also to improve mechanical properties through macroscale scaffold structure design. The application of different shapes of nanomaterials, including nanorods41,42 and nanowires,43 with homogeneous distribution and high interaction with the matrix interface can also enhance the strength and elastic modulus. Although, there are several ways to re-enforce porous scaffolds improve their mechanical properties, the main question, however, is how important is the initial mechanical similarity between a porous scaffold and bone in the defect site for bone regeneration? (Fig. 2D).It has been shown that the growth of bone in the implanted porous scaffolds can improve the mechanical properties of the regenerated bone in the defect site.44–47 Another study has suggested that stem cells take part in a bi-directional interaction between the features of their 3D surroundings and their own secreted pericellular matrix and these interplays induce cellular fate.37 Kolambkar et al. and Reichert et al. demonstrated that incorporation of growth factors in scaffolds with low mechanical strength (such as hydrogels) can induce bone regeneration with the desired resulting stiffness and torsional strength.45,47 It has also been illustrated that some gels can mechanically play the same role as autografts after implantation.46 These results reduce the importance of the initial similarity of the mechanical properties of the scaffold to native bone. They also suggest the feasibility of developing porous osteoinductive scaffolds, with lower mechanical properties than the surrounding bone, which can stimulate and accelerate new bone formation to such an extent that the new bone can quickly become load bearing.
Biocompatibility
Progress
It has been demonstrated that topographical features at different length scales, ranging from nano- to micrometre, can have significant impact on cell adhesion and morphology. In particular, it has been discussed in the literature that there could be an optimum size range in which cell adhesion would be enhanced most significantly but this is dependent on cell.48 We have reviewed previously how cellular focal adhesions interact with various nanotopographies and discussed how these interactions can be controlled to direct stem cell fate.49,50 In fact, cell migration, proliferation, and differentiation are all dependent upon adhesion.48–51 In normal physiological conditions, tissue re-organisation (e.g. during wound healing) is influenced by the bidirectional flow of information exchanged between cells and the ECM and this steers important cell functions such as adhesion, differentiation, and migration.51 There is opportunity for this phenomenon to be recreated on biomaterials. Because focal adhesions are assembled from the clustering of many integrins (on the nanoscale),49 the design of nanostructured materials (including nanomaterials, nanocomposites and nanotopographical features) can preferential guide the formation of focal adehsions and thereby regulate cell fate through changes in both cell biochemistry and cell morphology. Based on this, the application of nanomaterials as scaffolds should serve as an optimised reproducible environment for cell attachment, proliferation and function over other scaffold designs. The results of studies conducted to investigate the biocompatibility of nanomaterials and nanocomposites are summarised in Table 2.| Materials | Cell type | Tests | Results | Ref. |
|---|---|---|---|---|
| Nanomaterials | ||||
| Nanoapatite | MC3T3-E1 osteoblast-like cells | Cell viability with calcein-AM, ethidium-homodimer-1 | No significant difference in cell attachment and proliferation between nanoapatite scaffolds and controls. Cells infiltrate into the macro pores and anchor to the nano-apatite crystals | 55 |
| Nanobioglass(nBG) | MG63 osteoblast-like cells | LDH and mitochondrial activity, alkaline phosphatase (ALP) activity | High cytocompatibility of the nBG particles compared with micro BG | 59 |
| Nano fluorhydroxyapatite | Extracted osteoblast-like cells from adult rabbit, L929 mouse fibroblast cell line | Haemacytometer | Biocompatibility of fluorhydroxyapatite was higher than hydroxyapatite and fluorapatite | 60 |
| Gold nanoparticles-miRNA | Rat BMSCs | Alamar blue as say, ALP activity | High cellular biocompatibility of nanoparticles | 61 |
| Whitlockite inorganic nanoparticles (nWH: Ca18Mg2(HPO4)2(PO4)12) | Human tonsil-derived mesenchymal stem cells (hTMSCs) | LIVE/DEAD cell viability kit, click-iT EdU, flow cytometry assay, ALP activity | No significant difference in cell viability and proliferation between hydroxyapatite and WH nanoparticles. Significantly enhanced ALP activity in nWH compared to nHAP | 62 |
| Nanocomposites | ||||
| Nanohydroxyapatite/silk fibroin | Rat marrow mesenchymal cells | DNA contents, alkaline phosphatase (ALP) activity, the osteocalcin contents | Support cell proliferation, and osteogenic differentiation of the cells | 63 |
| Poly(3-hydroxybutyrate)/nHA nanofibers | Rat bone marrow stroma cells | Methylthiazol tetrazolium (MTT) assay, ALP activity | Positive effect on attachment, proliferation, and differentiation of cells | 64 |
| Poly(3-hydroxybutyrate)/nBG scaffolds | MG63 osteoblast-like cells | MTT assay, ALP activity | Improve cell proliferation, and induce a high level of ALP activity | 57 |
| Gelatin methacrylate/nanosilicates (LAPONITE®) hydrogels | MC3T3 E1–4 | Alamar blue assay, ALP activity | Nanocomposites are cytocompatible, enhanced ALP activity | 65 |
| Whitlockite/chitosan scaffolds | Human bone derived mesenchymal stem cells (hBMSCs) | Cell counting Kit-8 [CCK-8], ALP activity | Greatest proliferation and significantly higher ALP activity when cells cultured on WH/chitosan membranes compared to HAP/chitosan membranes and control | 66 |
An example is given by the study published by Webster and colleagues which demonstrated that osteoblast adhesion was significantly (p < 0.01) greater after 4 h when these cells were cultured on nanophase alumina, titania, and HAP than on microphase formulations of the same ceramics.52 Furthermore, another study demonstrated that osteoblast function and cellular activity were promoted on a nanostructured metallic surface in relation to a coarse-grained counterpart.53 Results of Palin and colleague's research also showed that nanostructured surfaces (nanophase titania and PLGA moulds of nanophase titania), without the influence of any other surface properties, improved adhesion and proliferation of osteoblasts.54 Xu and co-workers used injectable nano-apatite scaffolds for cell/growth factor delivery for bone regeneration. Created pores within the nano-apatite scaffolds were suitable for cell infiltration. The new scaffolds were biocompatible and promoted the adhesion and growth of osteoblast-like cells. The cells could infiltrate into the matrix pores, create cell–cell junctions, and adhere to the nano-apatite pores’ walls.55 Moreover, it has been shown that nano-bioglass can induce higher rates of cell proliferation and higher mRNA expression of osteogenic markers compared to micro-bioglass.56 Studies from our laboratories also showed that cells attach and proliferate highly on polymer/nBG scaffolds (PHB/nBG), and proved that there may be an optimal nanobioglass concentration for proliferation and osteoconduction.57
In another study, the effect of the various sizes of hydroxyapatite (HAP) nanoparticles (20, 40 and 80 nm), prepared as a film, on the proliferation of two bone-related cells, bone marrow derived mesenchymal stem cells (MSCs) and osteosarcoma cells (U2OS and MG63), were investigated. The results showed enhanced biocompatibility of films containing nanoparticles as compared with rod-like HAP with a length of hundreds of nanometres. The 20 nm nanoparticle films supported higher cell viability and proliferation of MSCs. But when the bone tumour cells were cultured on the HAP nanoparticles, the opposite phenomenon occurred. In fact, the proliferation of U2OS and MG63 cells was inhibited by the presence of the nanoparticles which was inversely proportional to the particle diameter.58
Furthermore, it has been demonstrated in a separate study that the orientation of cytoskeletal networks and expression of focal adhesion proteins and subsequently the adhesion, spreading and migration of SaOS-2 human osteoblast-like cells were affected by nano-TiO2 particles in a size dependent manner.67 They investigated the cells exposed to clinically relevant concentrations (0.05, 0.5, 5 mg L−1) of 5 and 40 nm spherical nano-TiO2. The actin and microtubule cytoskeletal networks were disrupted by treatment with nano-TiO2 leading to morphological modifications of SaOS-2 cells and the cell migration was significantly impaired in cells exposed to 5 nm nanoparticles compared to unexposed cells. Therefore, the biocompatibility and cytotoxicity of materials that incorporate nano-particles influences the subsequent cellular response and further studies are required prior to development for clinical application.
Challenge
Despite the progress made so far, there are still some challenges associated with the biocompatibility or cytotoxicity of the various nanotechnologies applied to bone regeneration (Fig. 3A). It is believed that the main mechanism of nanotoxicity is the high level of reactive oxygen species (ROS) produced in the cells,68 which might damage cells and cause sequent dysfunctions to the cellular microenvironment through downstream effects, including peroxidising lipids, disrupting DNA and altering gene transcription. Unlike regular chemical toxicants, nanomaterials cannot be fully defined for toxicity by determining their chemical composition and purity. Moreover, it has not been fully understood how and why nanomaterials differ from their bulk counterparts in toxicity, which also adds to the complexity of understanding nanotoxicity.The cytotoxicity of nanomaterials is synthetically affected by multiple particular characteristics, including chemical composition, size, shape, surface charge, solubility, and dose, which have been well reviewed by some papers.68,69 For instance, the size of the nanoparticle (NP) is critical for toxicity and it seems smaller NPs would compromise cell activity more, which is probably because of the increasing reactivity of NPs with decreasing size.70 The shape of nanomaterials would also make a difference and spherical NPs are usually easier and faster for endocytosis than tube- or fibre-like NPs.71 Despite the growing work in nanomaterials toxicity determination, there is still a demand for comprehensive and reliable characterisation of nanomaterials regarding their characteristics indicated above. For example, there are various methods to determine the size of NPs (e.g. dynamic light scattering, scanning electron microscopy, transmission electron microscopy, and atomic force microscopy), but it remains a challenge to unify the measurement because of the disagreement of values given by the different methods due to different principles behind these techniques.72 In addition, the measured size during preparation might not represent the actual size on site in application because of possible aggregation/agglomeration and other reactions caused by dispersion within the physicochemical environment. Such reactions also add to the difficulty of determining the actual effects of shape and surface charge on toxicity.
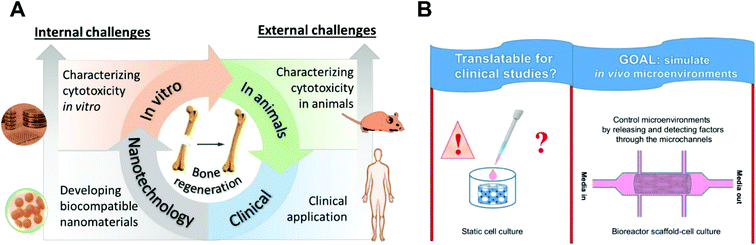
With respect to conducting biocompatibility assays, both in vitro (e.g. live/dead staining, MTT, DLH, and immunochemistry) and in vivo (e.g. blood compatibility and pharmacokinetics study) methods have been used. The in vitro assay, which is typically based on 2D cultured cells, is usually the first to be conducted because of its ease and quick outcome of the result. In vitro assays provide valuable quantitative and specific measurements for evaluating the toxicity of a new nanomaterial, but it cannot elucidate the mechanism of cell damage and death. In addition, in vitro results might not represent the in vivo outcomes because of their different microenvironments (2D versus 3D) and timescales of toxicity.73
With respect to bioactivity, the inclusion of nanoHAP (n-HAP) within scaffolds has gained much interest. HAP, as a ceramic component of natural bone, is osteoconductive (supporting bone formation) and not osteoinductive (stimulation of osteogenesis).74 HAP is able to support bone formation but cannot promote it, and the capability to stimulate osteogenesis is essential for materials to be used in bone regeneration. By implanting scaffolds fabricated from HAP, it cannot be expected that the newly formed bone demonstrates the same natural bone composition and structure, because the HAP is not highly degradable, and even if the cells migrate and grow inside the scaffolds, the arrangement of HAP around cells will be different from what we observe in natural bone. For example, Dasgupta and colleagues developed scaffolds by using gelatin-chitosan (GC) and GC composites containing 30 wt% n-HAP (GCH 30) which demonstrated that GCH 30 showed significant improvement in the compressive strength. MSC proliferation and differentiation on this scaffold observed in vitro, compared to the other GC scaffold formulations but when assesed in vivo, lacked bone formation following implantation, and no significant difference to the other GC scaffold formulations following 1, 2, 3 months after implantation.75 In another study, Kuttapan and colleagues fabricated a composite scaffold of silica coated nanohydroxyapatite (nHAP)-gelatin reinforced with electrospun poly(L-lactic acid) (PLLA) yarns, and also observed poor bone formation in the nanocomposte scaffolds when they were not loaded any growth factors following 4 and 12 weeks implantation in vivo.76 Nanostructured materials-tissue interactions in vivo therefore, are not necessarily predicted by in vitro assays due to the various components involved in regeneration that cannot be replicated easily in vitro.77
Perspective
There is an increasing tendency to introduce ions in n-HAP containing materials to improve the biocompatibility of HAP such as fluoride and magnesium ions. In particular, Whitlockite (WH) is a kind of calcium phosphate existing in natural bone tissue and contains Mg.78 Recent results from the incorporation of WH within scaffolds show higher cell proliferation and differentiation compared to scaffolds containing HAP alone. The incorporation of WH, HAP, bioglass or ceramic phase in the fabrication of scaffolds should be considered with respect to their degradability, bioresorbability, and their functionality during and after bone regeneration. We have previously reviewed how calcium and phosphate ion concentrations in the cells and in the ECM can promote and inhibit mineralisation. In addition, there are still several unanswered questions related to calcium phosphate particles that are associated with mineralisation.79 On the other hand, some studies have shown that scaffolds containing no ceramic phase, particularly hydrogels with incorporated growth factors, were also able to induce mineralisation.80,81 For instance, Yan and colleagues developed a hydrogel system by using hyaluronic acid (HA) and modulating BMP-2 release, and the in vivo experiments with these hydrogels clearly illustrated early mineralisation (in just two weeks).81 We have also demonstrated the application of an Alginate/ECM hydrogel system with incorporated stem cells and growth factors for in vivo bone formation in which the bone ECM component showed an increase in mineralisation compared to irradiated Alginate/ECM (U.V. irradiation was performed to inactivate endogenous growth factors within bone ECM).82 These studies suggest that if the cellular microenvironments is well designed, not only can the cells fabricate their own ECM-like collagen, but also the ceramic phase can be naturally formed in an appropriate arrangement around the cells, and potentially the bone can be functionally regenerated.In addition, as mentioned, several unsuccessful animal studies have been reported despite earlier promising in vitro data. There is therefore a need to develop better standards for cell characterisation and to design bioreactors which simulate tissue regeneration in vivo.83 In this regard, micro and nanofabrication technologies can be useful. The microenvironment can be simulated by the design of advanced microfluidic systems, and scaffolds can be tested and approved in these developed systems before in vivo implantation (Fig. 3B).
Though many nanomaterials seem to be nontoxic and even yield beneficial effects for the body, solid risk assessment is still necessary to address the possible concerns of the public and end-users. Regarding the internal challenges above, there is a great demand for establishing standards and guidelines to determine the biocompatibility of nanomaterials and nanotechnologies in biomedical applications, e.g. bone regeneration. The ultimate standards should cover all the aspects throughout the pathway from the comprehensive characterisation of nanotechnologies/nanomaterials to reliable toxicity assessment methods. The US National Institute of Standards and Technology (NIST) released the world's first reference material (RM) standards of gold nanoparticles (AuNPs) (10, 30 and 60 nm in diameter) in 2008 and has published some other RMs since then. Nelson et al.84 tested the genotoxicity of these NPs based on HepG2 cells and concluded that the NIST AuNPs could be potentially used as negative-control nanoparticle RMs for in vitro and in vivo genotoxicity studies. More RMs standards should be introduced together with other types of guidelines and protocols.
Osteoinductivity
Progress
Osteoinduction is defined as the ability to stimulate the differentiation of stem cells or osteoprogenitor cells to mature bone-forming cells,85,86 and it is the process by which osteogenesis is encouraged. Finally, it is defined as ‘action or process of stimulating osteogenesis’ agreed in the 2018 Chengdu consensus conference.86 It is necessary to conduct an in vivo test of any new biomaterial before classifying it as osteoinductive, even if the material had shown great potential in vitro. García-Gareta et al.87 have shown that osteoinductivity is an isolated phenomenon, which is influenced by the chemistry and structure of the biomaterials. Many studies investigating osteoinduction describe bone formation after 8 weeks implantation, but the time after implantation should be in direct correlation with the size of the animal model used due to the fact that bone formation in larger animals is a slower process. There are some studies that have investigated the application of nanotechnology to enhance the osteoinductive properties of new biomaterials in vivo; the results of these studies are summarised in Table 3.| Materials | Animal study | Defect size-test time | Controls | Results | Ref. |
|---|---|---|---|---|---|
| Nano-hydroxyapatite pullulan/dextran polysaccharide scaffolds | Mice-(subcutaneously) and | 4 mm–2, 15, 30, 60 days | Matrix without nHA empty | Nano-hydroxyapatite activates early calcification and osteoid tissue formation in non-osseous and osseous sites | 89 |
| Goats-(intramuscularly) | 10 mm–30 days | ||||
| Rats-femoral condyle | 6 mm–15, 30, 90 days | ||||
| Goats-mandible | 10 mm–30 days | ||||
| Goats-tibial osteotomy | 40 mm–30, 180 days | ||||
| n-TiO2/poly(ether-ether ketone) (PEEK) cylindrical implants | Dogs-tibia in the proximal diaphyseal region | 5 mm–28 days | PEEK | Enhanced bone regeneration around the implants by n-TiO2 | 88 |
| Nano-hydroxyapatite coated β-TCP scaffolds nano-hydroxyapatite coated poly caprolactone(PCL) scaffolds | Rabbits-iliac crest tuber sacrale of each leg | 20 mm–90 days | β-TCP PCL | New bone tissue occupying 33% for the nHAP-coated PCL scaffold and 68% for the nHAP-coated β-TCP after a 3-month test | 90 |
| Nanodiamond particles-modified poly(l-lactide-co-ε-caprolactone) (Poly(LLA-co-CL) scaffolds | Rats-central area of parietal bone | 5 mm–84 days | Poly(LLA-co-CL | Nanodiamond particles promote osteogenic metabolic activity and finally enhance osteoconductivity | 91 |
| Moxifloxacin hydrochloride + icariin/PCL nanofibers | Rabbits-middle radius of the right foreleg | 10 mm–30, 60, 90 days | PCL empty | Dual drug-loaded nanofibres possess a positive effect in enhancing the quality and quantity of bone formation when compared to the blank and PCL groups | 92 |
| DNA-loaded nano-calcium phosphate paste | Rabbits-right proximal medial rabbit tibia | 8 mm–14, 28, and 84 days | Non-loaded paste | Significantly accelerated bone healing and formation after 4 weeks in the DNA-loaded calcium phosphate group | 93 |
Wu et al. showed n-TiO2/polyether ether ketone (PEEK) could enhance bone regeneration around the implant in a canine model (implants were placed in each animal on the medial surface of each tibia in the proximal diaphyseal region).88 In another study, it has been shown that nHAP (as a composite of nanohydroxyapatite and pullulan/dextran polysaccharides) activates early calcification and osteoid tissue formation in non-osseous and osseous sites in different animal models such as the femoral condyle of rat, a transversal mandibular defect and a tibial osteotomy in goat.89
Novel nanohydroxyapatite sonocoated scaffolds were studied for bone regeneration by Rogowska-Tylman.90 A uniform layer of nanohydroxyapatite particles with a thickness within the range of 200 to 300 nm was sonocoated on two types of scaffolds: a porous β-TCP ceramic scaffold and a 3D-printed scaffold made of PCL fibers. In vivo tests in rabbits confirmed that the novel coating stimulated new tibial bone tissue formation, occupying 33% of the initial scaffold volume for the nHAP-coated PCL scaffold and 68% for the nHAP-coated β-TCP after 3 months.
M. Gong and colleagues92 have developed a core-sheath micro/nano fibre membrane consisting of a polycaprolactone core and gelatine shell loaded with the antibacterial drug moxifloxacin hydrochloride (MOX) and a traditional Chinese medicine called icariin (ICA) as biomimetic artificial periosteum to promote bone regeneration. They tested this material in vivo, in a rabbit radius defect model to test the formation of bone. They demonstrated that the dual drug-loaded nanofibers enhanced the quality and quantity of bone formation as well as maturation when compared to control groups.
An injectable DNA-loaded nano-calcium phosphate paste was prepared to be used as a bioactive bone substitution material by Schlickewei and colleagues.93 Calcium phosphate nanoparticles were loaded with BMP-7- and VEGF-A-encoding DNA prior to implantation in a rabbit critical-size tibial bone defect and observed over 2, 4 and 12 weeks following surgery. The calcium phosphate paste without DNA led to regular healing of the critical-size bone defect, but the healing was slower than the DNA-loaded paste. Thus, the in situ transfection with BMP-7 and VEGF-A significantly improved the potential of calcium phosphate as a pasty bone substitution material. Over the last decade, several nanotechnologies94,95 have been applied to biomaterials fabrication and these approved for use in clinical applications. For example, Vitoss (Orthovita), approved by the FDA in 2000, is a three-dimensional scaffold composed of ultraporous beta-tricalcium phosphate (TCP) NPs (mean size 100 nm). These nanoparticles are used in repairing bone defects by enhancing resorption and stimulating new bone growth. However, in a FDA document published in 2003 related to this product,96 Vitoss scaffolds were advised to not be used to treat large defects. In 2008,97 they reported the scaffolds functioned as intended in all cases, based on in vitro data, although the osteostimulatory nature of the scaffolds has not yet been correlated to human clinical experience. In addition, a clinical trial also demonstrated that the nanostructured biphasic calcium phosphate is biocompatible and osteoconductive, and that the implants remained in situ after 3 and 6 months without complications or loss of the implants and with preservation of the alveolar architecture.98 The number available studies regarding in vivo new bone formation in human is limited, which gives researchers a limited understanding of the detailed mechanism of bone development in these materials. This section of the review illustrates the lack of studies in this area as well as the need for further in vivo and clinical studies on this matter.
Challenge
Nonetheless, the application of nanotechnology to biomaterials development for bone has developed rapidly in recent years, and it has been shown that such materials can stimulate bone formation in vivo. However, there are still several challenges in the regeneration of critical sized bone defects (Fig. 4).99 The insufficient number of stem cells in the defect site for differentiation to osteocytes, lack of cell penetration and migration to the defect zone, lower levels of vascularisation to supply nutrients and exchange waste materials for the cells could be the factors that impact on inadequate bone regeneration in the critical bone defects.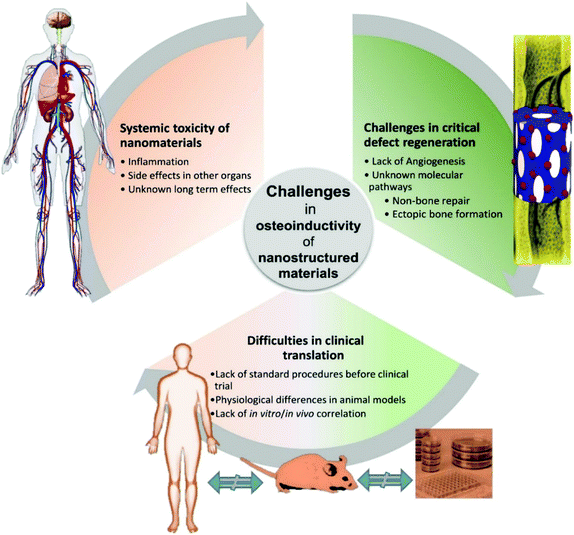 | ||
| Fig. 4 Associated challenges in terms of osteoinductivity for nanostructured materials in bone regeneration. | ||
Angiogenesis and osteogenesis are closely linked and they must be significantly considered for physiological bone function. It is demonstrated that changes in vascular growth can compromise physiological bone formation, for instance, leading to non-union fractures, and osteonecrosis.100,101 On the other hand, vascular endothelial growth factor (VEGF) is the main regulator of angiogenesis and has also been reported to inhibit osteoblast differentiation,102 and VEGF overexpression can also cause bone resorption.103 This competition between osteogenesis and angiogenesis inhibits formation of mature blood vessels and interrupts the coupling of angiogenesis and osteogenesis in bone regeneration. Furthermore, non-vascularisation in implanted scaffolds inhibits appropriate bone regeneration in defect sites.75 Therefore, the coupling of angiogenesis and osteogenesis is still one of the main challenges in bone regeneration, and both should be investigated when a nanomaterial is suggested for bone tissue engineering.
Animal models are used to study the osteoinductivity of nanostructured materials, however, the in vivo interaction of nanomaterials and biological systems are much more complex. In addition, bone regeneration in the commonly used animals for this work (rat, rabbit, sheep) is different compared to man due to species differences in bone regeneration and healing. Despite large investments in tissue engineering, the general success rate of biomaterials in clinical development remains low.99 The flawed preclinical research is one of the distinguished explanations, in which the use and outcome of animal models are critical for clinical translations. Hence, a validated and predictive animal model must be selected to address the clinical question. Small animal models are less expensive and are more straightforward to conduct. However, they do not always represent mechanistic responses compared to humans. For instance, mouse and rats show limited intracortical remodelling and their growth plates remain open. Therefore, the skeletal morphometric and mechanical data can be impacted by these differences.104
Most nanomaterials, such as gold nanoparticles, are currently being assessed in in vitro testing platforms without minimal development towards actual clinical applications.105 There are a few very important factors relevant to the use of nanoparticles (NPs) for in vivo applications, including their size and the stimulation of inflammatory response, together with their osteoinductive properties. If the NPs size is smaller than 10 nm they may be excreted through the kidneys, whereas NPs with sizes between 100 to 200 nm will be cleared by the liver and spleen. Moreover, NPs with sizes from 20 to 100 nm have been shown to diffuse through the endothelium of tumour tissues.95 Therefore, particle size plays an important role in materials development for the intended clinical application. Nano-TiO2 particles can enter the blood circulation and be deposited in the liver, changing biochemical indicators, leading to liver inflammation.106 It has been shown that long-term inflammation of the liver leads to cell activation, and multiple signalling pathways can promote the ECM deposition, causing liver fibrosis. Recently, the progress on murine liver injury induced by TiO2 has been investigated. The size of nano-TiO2, its dosage, exposure time, and surface properties can affect its toxicity. Therefore, the toxic effects of nano-TiO2 in humans should be investigated in greater depth, and in more in detail for nano-TiO2 based implants.
Regarding inflammation, some materials are tolerated by the body, such as polycaprolactone (PCL) or inert bioceramics that do not elicit a response from the host immune system.3 In the case of the polyesters, such as PCL, degradation results in acidic compounds that can affect the intracellular pH and alter cellular fate. The degradation of polymeric nanoparticles can induce an inflammatory reaction due to their reduced size and can be rapidly expelled by the body reducing their therapeutic efficacy.107 However, previous studies have demonstrated encouraging data in controlling inflammation in both in vitro and in vivo experiments. More extensive investigations should be carried out because the degraded products of the implants can exist for a long period of time in the body and more extensive screening of type, density, and duration of these signals should be conducted to evaluate their effects on macrophages.108 Finally, the bone engineering community must study the long-term toxicity of nanomaterials to add confidence to their inclusion in the biomedical field.
Perspective
Nanotechnology alone cannot provide a promising approach for bone regeneration. The importance of the application of growth factors such as BMP2, VEGF to enhance osteoinduction and vascularisation is more of concern in the case of critical-sized bone defects.76,109,110 VEGF levels must be controlled, because non-physiological doses can inhibit bone formation, directly influencing osteoblast differentiation and enhancing bone resorption.111 Current studies of growth factors’ functions should be aimed at further clarifying the molecular crosstalk between osteogenesis and angiogenesis, and the association of their dose in bone formation.112 The controlled release of growth factors and their concentrations are important parameters which should be optimised in bone regeneration.109,113,114 We have developed a new nanotechnology method by coating a nanolayer of poly (ethyl acrylate) on material surfaces, including biomaterials, that can increase the bioavailability of fibronectin domains for attachment of cells and growth factors and consequently, improve bone regeneration.115–117 In addition, the application of further nanosystem delivery methods for growth factors and other osteoinductive factors such as adenosine118 and ions119 can be explored to support complete bone regeneration. Therefore, it seems that if advanced fabrication methodologies (such as computer-aided design (CAD) and rapid prototyping) and growth factor and metabolite release systems are combined with nanotechnology, we can hopefully tackle the challenges of bone formation in critical-sized bone defects and provide efficacious treatment strategies.Conclusions
The field of nanotechnology and the application of nanomaterials to regenerative medicine has been a rapidly growing area of research in recent years. In this review paper, the impact of nanotechnology in biomaterials development for bone regeneration has been discussed. The review illustrates that mechanical properties, biocompatibility, and the osteoinductivity of biomaterials can be improved by the application of nanomaterials. However, there are still several challenges that need to be overcome. As this field moves forward, the combination of nanotechnology with factor delivery including growth factors, metabolites and ions will inform biomaterials design with the goal of achieving complete bone regeneration.Conflicts of interest
There are no conflicts to declare.References
- T. Vo-Dinh, Nanotechnology in biology and medicine: methods, devices, and applications, CRC Press, 2017 Search PubMed.
- R. A. Gosselin, D. J. Conway, J. J. Phillips and R. R. Coughlin, in Hunter's Tropical Medicine and Emerging Infectious Diseases, ed. E. T. Ryan, D. R. Hill, T. Solomon, N. E. Aronson and T. P. Endy, Content Repository Only!, London, 10th edn, 2020, pp. 114–119, DOI:10.1016/B978-0-323-55512-8.00013-2.
- A. Atala, R. Lanza, T. Mikos and R. Nerem, Principles of regenerative medicine, Academic Press, 2018 Search PubMed.
- Y. Yang, A. Chawla, J. Zhang, A. Esa, H. L. Jang and A. Khademhosseini, in Principles of Regenerative Medicine, ed. A. Atala, R. Lanza, A. G. Mikos and R. Nerem, Academic Press, Boston, 3rd edn, 2019, pp. 485–504, DOI:10.1016/B978-0-12-809880-6.00029-1.
- E.-S. Kim, E. H. Ahn, T. Dvir and D.-H. Kim, Int. J. Nanomed., 2014, 9, 1 CrossRef CAS PubMed.
- T. Rycroft, B. Trump, K. Poinsatte-Jones and I. Linkov, J. Nanopart. Res., 2018, 20, 52 CrossRef.
- T. Wu and M. Tang, J. Appl. Toxicol., 2018, 38, 25–40 CrossRef CAS PubMed.
- E. J. Harvey, J. E. Henderson and S. T. Vengallatore, J. Orthop. Trauma, 2010, 24, S25–S30 CrossRef PubMed.
- A. R. Boccaccini, M. Erol, W. J. Stark, D. Mohn, Z. Hong and J. F. Mano, Compos. Sci. Technol., 2010, 70, 1764–1776 CrossRef CAS.
- R. E. McMahon, L. Wang, R. Skoracki and A. B. Mathur, J. Biomed. Mater. Res., Part B, 2013, 101, 387–397 CrossRef PubMed.
- W. Gu, C. Wu, J. Chen and Y. Xiao, Int. J. Nanomed., 2013, 8, 2305 CrossRef PubMed.
- N. Gusić, A. Ivković, J. VaFaye, A. Vukasović, J. Ivković, D. Hudetz and S. Janković, Int. Orthop., 2014, 38, 1877–1884 CrossRef PubMed.
- G. G. Walmsley, A. McArdle, R. Tevlin, A. Momeni, D. Atashroo, M. S. Hu, A. H. Feroze, V. W. Wong, P. H. Lorenz and M. T. Longaker, Nanomedicine, 2015, 11, 1253–1263 CrossRef CAS PubMed.
- T. Gong, J. Xie, J. Liao, T. Zhang, S. Lin and Y. Lin, Bone Res., 2015, 3, 15029 CrossRef CAS PubMed.
- M. Barry, H. Pearce, L. Cross, M. Tatullo and A. K. Gaharwar, Curr. Osteoporos. Rep., 2016, 14, 87–94 CrossRef PubMed.
- Q. Wang, J. Yan, J. Yang and B. Li, Mater. Today, 2016, 19, 451–463 CrossRef CAS.
- H. Yi, F. U. Rehman, C. Zhao, B. Liu and N. He, Bone Res., 2016, 4, 16050 CrossRef CAS PubMed.
- H. Qu, H. Fu, Z. Han and Y. Sun, RSC Adv., 2019, 9, 26252–26262 RSC.
- M. Sanz, C. Dahlin, D. Apatzidou, Z. Artzi, D. Bozic, E. Calciolari, H. De Bruyn, H. Dommisch, N. Donos, P. Eickholz, J. E. Ellingsen, H. J. Haugen, D. Herrera, F. Lambert, P. Layrolle, E. Montero, K. Mustafa, O. Omar and H. Schliephake, J. Clin. Periodontol., 2019, 46, 82–91 CrossRef PubMed.
- N. H. Hart, S. Nimphius, T. Rantalainen, A. Ireland, A. Siafarikas and R. U. Newton, J. Musculoskeletal Neuronal Interact., 2017, 17, 114–139 CAS.
- C. Gao, S. Peng, P. Feng and C. Shuai, Bone Res., 2017, 5, 17059 CrossRef CAS PubMed.
- J.-F. Stoltz, J. Magdalou, D. George, Y. Chen, Y. Li, N. De Isla, X. He and Y. Remond, J. Cell. Immunother., 2018, 4, 10–12 CrossRef.
- M. Doblaré, J. Garcıa and M. Gómez, Eng. Fract. Mech., 2004, 71, 1809–1840 CrossRef.
- W. Suchanek and M. Yoshimura, J. Mater. Res., 1998, 13, 94–117 CrossRef CAS.
- K. Muller, E. Bugnicourt, M. Latorre, M. Jorda, Y. Echegoyen Sanz, J. M. Lagaron, O. Miesbauer, A. Bianchin, S. Hankin, U. Bolz, G. Perez, M. Jesdinszki, M. Lindner, Z. Scheuerer, S. Castello and M. Schmid, Nanomaterials, 2017, 7, 74 CrossRef PubMed.
- E. S. Ahn, N. J. Gleason, A. Nakahira and J. Y. Ying, Nano Lett., 2001, 1, 149–153 CrossRef CAS.
- E. S. Ahn, N. J. Gleason and J. Y. Ying, J. Am. Ceram. Soc., 2005, 88, 3374–3379 CrossRef CAS.
- S. K. Misra, D. Mohn, T. J. Brunner, W. J. Stark, S. E. Philip, I. Roy, V. Salih, J. C. Knowles and A. R. Boccaccini, Biomaterials, 2008, 29, 1750–1761 CrossRef CAS PubMed.
- E. Nejati, H. Mirzadeh and M. Zandi, Composites, Part A, 2008, 39, 1589–1596 CrossRef.
- H. Hajiali, S. Karbasi, M. Hosseinalipour and H. Rezaie, Sci. Iran., Trans. F, 2013, 20, 2306 Search PubMed.
- H. Hajiali, S. Karbasi, M. Hosseinalipour and H. R. Rezaie, J. Mater. Sci.: Mater. Med., 2010, 21, 2125–2132 CrossRef CAS PubMed.
- W. Jie and L. Yubao, Eur. Polym. J., 2004, 40, 509–515 CrossRef.
- H. Wang, Y. Li, Y. Zuo, J. Li, S. Ma and L. Cheng, Biomaterials, 2007, 28, 3338–3348 CrossRef CAS PubMed.
- L. Roseti, V. Parisi, M. Petretta, C. Cavallo, G. Desando, I. Bartolotti and B. Grigolo, Mater. Sci. Eng., C, 2017, 78, 1246–1262 CrossRef CAS PubMed.
- M. Mortezaei, M. H. N. Famili and M. Kokabi, Compos. Sci. Technol., 2011, 71, 1039–1045 CrossRef CAS.
- Y. Wang, Y. Zhang, H. Zhao, X. Li, Y. Huang, L. S. Schadler, W. Chen and L. C. Brinson, Compos. Sci. Technol., 2018, 162, 146–155 CrossRef CAS.
- S. A. Ferreira, M. S. Motwani, P. A. Faull, A. J. Seymour, T. T. L. Yu, M. Enayati, D. K. Taheem, C. Salzlechner, T. Haghighi, E. M. Kania, O. P. Oommen, T. Ahmed, S. Loaiza, K. Parzych, F. Dazzi, O. P. Varghese, F. Festy, A. E. Grigoriadis, H. W. Auner, A. P. Snijders, L. Bozec and E. Gentleman, Nat. Commun., 2018, 9, 4049 CrossRef PubMed.
- A. Elosegui-Artola, E. Bazellières, M. D. Allen, I. Andreu, R. Oria, R. Sunyer, J. J. Gomm, J. F. Marshall, J. L. Jones and X. Trepat, Nat. Mater., 2014, 13, 631 CrossRef CAS PubMed.
- J. Hao, Y. Zhang, D. Jing, Y. Shen, G. Tang, S. Huang and Z. Zhao, Acta Biomater., 2015, 20, 1–9 CrossRef PubMed.
- D. N. Heo, N. J. Castro, S.-J. Lee, H. Noh, W. Zhu and L. G. Zhang, Nanoscale, 2017, 9, 5055–5062 RSC.
- Z. Fan, J. Wang, Z. Wang, H. Ran, Y. Li, L. Niu, P. Gong, B. Liu and S. Yang, Carbon, 2014, 66, 407–416 CrossRef CAS.
- J. Wu, C. Ruan, Y. Ma, Y. Wang and Y. Luo, J. Mater. Sci. Technol., 2018, 34, 503–507 CrossRef.
- T. W. Sun, Y. J. Zhu, F. Chen and Y. G. Zhang, Chem. – Asian J., 2017, 12, 655–664 CrossRef CAS PubMed.
- A. Berner, J. Henkel, M. A. Woodruff, R. Steck, M. Nerlich, M. A. Schuetz and D. W. Hutmacher, Stem Cells Transl. Med., 2015, 4, 503–512 CrossRef CAS PubMed.
- Y. M. Kolambkar, K. M. Dupont, J. D. Boerckel, N. Huebsch, D. J. Mooney, D. W. Hutmacher and R. E. Guldberg, Biomaterials, 2011, 32, 65–74 CrossRef CAS PubMed.
- P. Lohmann, A. Willuweit, A. Neffe, S. Geisler, T. Gebauer, S. Beer, H. Coenen, H. Fischer, B. Hermanns-Sachweh and A. Lendlein, Biomaterials, 2017, 113, 158–169 CrossRef CAS PubMed.
- J. C. Reichert, A. Cipitria, D. R. Epari, S. Saifzadeh, P. Krishnakanth, A. Berner, M. A. Woodruff, H. Schell, M. Mehta and M. A. Schuetz, Sci. Transl. Med., 2012, 4, 141ra193–141ra193 Search PubMed.
- A. T. Nguyen, S. R. Sathe and E. K. Yim, J. Phys.: Condens. Matter, 2016, 28, 183001 CrossRef PubMed.
- M. J. Dalby, N. Gadegaard and R. O. C. Oreffo, Nat. Mater., 2014, 13, 558 CrossRef CAS PubMed.
- H. Donnelly, M. J. Dalby, M. Salmeron-Sanchez and P. E. Sweeten, Nanomedicine, 2018, 14, 2455–2464 CrossRef CAS PubMed.
- S. A. Wickström and C. M. Niessen, Curr. Opin. Cell Biol., 2018, 54, 89–97 CrossRef PubMed.
- T. J. Webster, C. Ergun, R. H. Doremus, R. W. Siegel and R. Bizios, J. Biomed. Mater. Res., 2000, 51, 475–483 CrossRef CAS PubMed.
- K. Nune and R. Misra, Mater. Technol., 2016, 31, 772–781 CrossRef CAS.
- E. Palin, H. Liu and T. J. Webster, Nanotechnology, 2005, 16, 1828 CrossRef CAS.
- H. H. Xu, M. D. Weir and C. G. Simon, Dent. Mater., 2008, 24, 1212–1222 CrossRef CAS PubMed.
- M. E. Taygun and A. Boccaccini, in Bioactive Glasses, Elsevier, 2018, pp. 235–283 Search PubMed.
- H. Hajiali, M. Hosseinalipour, S. Karbasi and M. A. Shokrgozar, Int. J. Artif. Organs, 2012, 35, 1015–1024 CrossRef CAS PubMed.
- Y. Cai and R. Tang, J. Mater. Chem., 2008, 18, 3775–3787 RSC.
- M. Mačković, A. Hoppe, R. Detsch, D. Mohn, W. J. Stark, E. Spiecker and A. R. Boccaccini, J. Nanopart. Res., 2012, 14, 966 CrossRef.
- H. Eslami, M. Solati-Hashjin and M. Tahriri, Mater. Sci. Eng., C, 2009, 29, 1387–1398 CrossRef CAS.
- M. Yu, B. Lei, C. B. Gao, J. Yan and P. X. Ma, Nano Res., 2017, 10, 49–63 CrossRef CAS.
- H. D. Kim, H. L. Jang, H.-Y. Ahn, H. K. Lee, J. Park, E.-s. Lee, E. A. Lee, Y.-H. Jeong, D.-G. Kim and K. T. Nam, Biomaterials, 2017, 112, 31–43 CrossRef CAS PubMed.
- T. Tanaka, M. Hirose, N. Kotobuki, H. Ohgushi, T. Furuzono and J. Sato, Mater. Sci. Eng., C, 2007, 27, 817–823 CrossRef CAS.
- D. Guan, Z. Chen, C. Huang and Y. Lin, Appl. Surf. Sci., 2008, 255, 324–327 CrossRef CAS.
- J. R. Xavier, T. Thakur, P. Desai, M. K. Jaiswal, N. Sears, E. Cosgriff-Hernandez, R. Kaunas and A. K. Gaharwar, ACS Nano, 2015, 9, 3109–3118 CrossRef CAS PubMed.
- D. Zhou, C. Qi, Y. X. Chen, Y. J. Zhu, T. W. Sun, F. Chen and C. Q. Zhang, Int. J. Nanomedicine, 2017, 12, 2673–2687 CrossRef CAS PubMed.
- M. Ibrahim, J. Schoelermann, K. Mustafa and M. R. Cimpan, J. Biomed. Mater. Res., Part A, 2018, 106, 2582–2593 CrossRef CAS PubMed.
- S. Sharifi, S. Behzadi, S. Laurent, M. L. Forrest, P. Stroeve and M. Mahmoudi, Chem. Soc. Rev., 2012, 41, 2323–2343 RSC.
- A. Sukhanova, S. Bozrova, P. Sokolov, M. Berestovoy, A. Karaulov and I. Nabiev, Nanoscale Res. Lett., 2018, 13, 44 CrossRef PubMed.
- O. J. Osborne, S. Lin, C. H. Chang, Z. Ji, X. Yu, X. Wang, S. Lin, T. Xia and A. E. Nel, ACS Nano, 2015, 9, 9573–9584 CrossRef CAS PubMed.
- J. A. Champion and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4930–4934 CrossRef CAS PubMed.
- M. Baalousha and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6134–6142 CrossRef CAS PubMed.
- J. Lee, G. D. Lilly, R. C. Doty, P. Podsiadlo and N. A. Kotov, Small, 2009, 5, 1213–1221 CAS.
- J. Dong, H. Kojima, T. Uemura, M. Kikuchi, T. Tateishi and J. Tanaka, J. Biomed. Mater. Res., Part A, 2001, 57, 208–216 CrossRef CAS.
- S. Dasgupta, K. Maji and S. K. Nandi, Mater. Sci. Eng., C, 2019, 94, 713–728 CrossRef CAS PubMed.
- S. Kuttappan, D. Mathew, J.-i. Jo, R. Tanaka, D. Menon, T. Ishimoto, T. Nakano, S. V. Nair, M. B. Nair and Y. Tabata, Acta Biomater., 2018, 78, 36–47 CrossRef CAS PubMed.
- Z. Julier, A. J. Park, P. S. Briquez and M. M. Martino, Acta Biomater., 2017, 53, 13–28 CrossRef CAS PubMed.
- R. Lagier and C.-A. Baud, Pathol., Res. Pract., 2003, 199, 329–335 CrossRef CAS PubMed.
- N. Reznikov, J. Steele, P. Fratzl and M. Stevens, Nat. Rev. Mater., 2016, 1, 16041 CrossRef CAS.
- X. Bai, M. Gao, S. Syed, J. Zhuang, X. Xu and X.-Q. Zhang, Bioact. Mater., 2018, 3, 401–417 CrossRef PubMed.
- H. J. Yan, T. Casalini, G. Hulsart-Billström, S. Wang, O. P. Oommen, M. Salvalaglio, S. Larsson, J. Hilborn and O. P. Varghese, Biomaterials, 2018, 161, 190–202 CrossRef CAS PubMed.
- D. Gothard, E. L. Smith, J. M. Kanczler, C. R. Black, J. A. Wells, C. A. Roberts, L. J. White, O. Qutachi, H. Peto and H. Rashidi, PLoS One, 2015, 10, e0145080 CrossRef PubMed.
- F. Schmieder, J. Ströbel, M. Rösler, S. Grünzner, B. Hohenstein, U. Klotzbach and F. Sonntag, Curr. Dir. Biomed. Eng., 2016, 2, 105–108 Search PubMed.
- B. Nelson, E. Petersen, B. Marquis, D. Atha, J. Elliott, D. Cleveland, S. Watson, I. Tseng, A. Dillon, M. Theodore and I. Jackman, Nanotoxicology, 2013, 7, 21–29 CrossRef CAS PubMed.
- T. Albrektsson and C. Johansson, Eur. Spine J., 2001, 10(Suppl 2), S96–101 Search PubMed.
- L. Ghasemi-Mobarakeh, D. Kolahreez, S. Ramakrishna and D. Williams, Curr. Opin. Biomed. Eng., 2019, 10, 45–50 CrossRef.
- E. García-Gareta, M. J. Coathup and G. W. Blunn, Bone, 2015, 81, 112–121 CrossRef PubMed.
- X. Wu, X. Liu, J. Wei, J. Ma, F. Deng and S. Wei, Int. J. Nanomed., 2012, 7, 1215 CAS.
- J. C. Fricain, S. Schlaubitz, C. Le Visage, I. Arnault, S. M. Derkaoui, R. Siadous, S. Catros, C. Lalande, R. Bareille and M. Renard, Biomaterials, 2013, 34, 2947–2959 CrossRef CAS PubMed.
- J. Rogowska-Tylman, J. Locs, I. Salma, B. Woźniak, M. Pilmane, V. Zalite, J. Wojnarowicz, A. Kędzierska-Sar, T. Chudoba, K. Szlązak, A. Chlanda, W. Święszkowski, A. Gedanken and W. Łojkowski, Mater. Sci. Eng., C, 2019, 99, 669–684 CrossRef CAS PubMed.
- M. A. Yassin, K. Mustafa, Z. Xing, Y. Sun, K. E. Fasmer, T. Waag, A. Krueger, D. Steinmuller-Nethl, A. Finne-Wistrand and K. N. Leknes, Macromol. Biosci., 2017, 17, 1600427 CrossRef PubMed.
- M. Gong, C. Huang, Y. Huang, G. Li, C. Chi, J. Ye, W. Xie, R. Shi and L. Zhang, Nanomedicine, 2019, 17, 124–136 CrossRef CAS PubMed.
- C. Schlickewei, T. O. Klatte, Y. Wildermuth, G. Laaff, J. M. Rueger, J. Ruesing, S. Chernousova, W. Lehmann and M. Epple, J. Mater. Sci.: Mater. Med., 2019, 30, 15 CrossRef PubMed.
- M. L. Etheridge, S. A. Campbell, A. G. Erdman, C. L. Haynes, S. M. Wolf and J. McCullough, Nanomedicine, 2013, 9, 1–14 CrossRef CAS PubMed.
- C. L. Ventola, P & T, 2012, 37, 582–591 Search PubMed.
- 510(K) Summary, https://www.accessdata.fda.gov/cdrh_docs/pdf3/K032288.pdf, 2003.
- 510(K) Summary, https://www.accessdata.fda.gov/cdrh_docs/pdf/K083033.pdf, 2008.
- M. J. Uzeda, R. F. de Brito Resende, S. C. Sartoretto, A. Alves, J. M. Granjeiro and M. D. Calasans-Maia, Clin. Implant Dent. Relat. Res., 2017, 19, 802–811 CrossRef PubMed.
- T. Winkler, F. A. Sass, G. N. Duda and K. Schmidt-Bleek, Bone Joint Res., 2018, 7, 232–243 CrossRef CAS PubMed.
- M. Fassbender, C. Strobel, J. S. Rauhe, C. Bergmann, G. Schmidmaier and B. Wildemann, Eur. Cells Mater., 2011, 22, 1–11 CrossRef CAS PubMed.
- A. P. Kaushik, A. Das and Q. Cui, World J. Orthop., 2012, 3, 49–57 CrossRef PubMed.
- B. H. Schonmeyr, M. Soares, T. Avraham, N. W. Clavin, F. Gewalli and B. J. Mehrara, Tissue Eng. Part A, 2010, 16, 653–662 CrossRef PubMed.
- U. Helmrich, N. Di Maggio, S. Guven, E. Groppa, L. Melly, R. D. Largo, M. Heberer, I. Martin, A. Scherberich and A. Banfi, Biomaterials, 2013, 34, 5025–5035 CrossRef CAS PubMed.
- A. R. Altman, W. J. Tseng, C. M. J. de Bakker, A. Chandra, S. Lan, B. K. Huh, S. Luo, M. B. Leonard, L. Qin and X. S. Liu, Bone, 2015, 81, 370–379 CrossRef PubMed.
- S. Mclaughlin, J. Podrebarac, M. Ruel, E. J. Suuronen, B. McNeill and E. I. Alarcon, Front. Mater. Sci., 2016, 3, 27 Search PubMed.
- J. Hong and Y.-Q. Zhang, Nanotechnology, 2016, 27, 112001 CrossRef PubMed.
- M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS.
- J. Lee, H. Byun, S. K. Madhurakkat Perikamana, S. Lee and H. Shin, Adv. Healthcare Mater., 2019, 8, e1801106 Search PubMed.
- F. R. Rose, Q. Hou and R. O. Oreffo, J. Pharm. Pharmacol., 2004, 56, 415–427 CrossRef CAS PubMed.
- F. R. Rose and R. O. Oreffo, Biochem. Biophys. Res. Commun., 2002, 292, 1–7 CrossRef CAS PubMed.
- K. Hu and B. R. Olsen, J. Clin. Invest., 2016, 126, 509–526 CrossRef PubMed.
- A. Grosso, M. G. Burger, A. Lunger, D. J. Schaefer, A. Banfi and N. Di Maggio, Front. Bioeng. Biotechnol., 2017, 5, 68–68 CrossRef PubMed.
- G. T. Kirby, L. J. White, C. V. Rahman, H. C. Cox, O. Qutachi, F. R. Rose, D. W. Hutmacher, K. M. Shakesheff and M. A. Woodruff, Polymers, 2011, 3, 571–586 CrossRef CAS.
- W. Tang, D. Lin, Y. Yu, H. Niu, H. Guo, Y. Yuan and C. Liu, Acta Biomater., 2016, 32, 309–323 CrossRef CAS PubMed.
- V. Llopis-Hernández, M. Cantini, C. González-García, Z. A. Cheng, J. Yang, P. M. Tsimbouri, A. J. García, M. J. Dalby and M. Salmerón-Sánchez, Sci. Adv., 2016, 2, e1600188 CrossRef PubMed.
- V. Moulisová, C. Gonzalez-García, M. Cantini, A. Rodrigo-Navarro, J. Weaver, M. Costell, R. S. i Serra, M. J. Dalby, A. J. García and M. Salmerón-Sánchez, Biomaterials, 2017, 126, 61–74 CrossRef PubMed.
- Z. A. Cheng, A. Alba-Perez, C. Gonzalez-Garcia, H. Donnelly, V. Llopis-Hernandez, V. Jayawarna, P. Childs, D. W. Shields, M. Cantini, L. Ruiz-Cantu, A. Reid, J. F. C. Windmill, E. S. Addison, S. Corr, W. G. Marshall, M. J. Dalby and M. Salmeron-Sanchez, Adv. Sci., 2019, 6, 1800361 CrossRef PubMed.
- H. Kang, Y.-R. V. Shih, M. Nakasaki, H. Kabra and S. Varghese, Sci. Adv., 2016, 2, e1600691 CrossRef PubMed.
- E. O'Neill, G. Awale, L. Daneshmandi, O. Umerah and K. W. H. Lo, Drug Discovery Today, 2018, 23, 879–890 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2021 |