CoP2/Fe-CoP2 yolk–shell nanoboxes as efficient electrocatalysts for the oxygen evolution reaction†
Vinoth
Ganesan
 ,
Jihye
Son
and
Jinkwon
Kim
,
Jihye
Son
and
Jinkwon
Kim
 *
*
Department of Chemistry, Kongju National University, 56 Gongjudaehak-ro, Gongju-si, Chungnam-do 32588, Republic of Korea. E-mail: jkim@kongju.ac.kr; Fax: +82-41-850-8613; Tel: +82-41-850-8496
First published on 1st February 2021
Abstract
The development of an efficient electrocatalyst is an important requirement for water splitting systems to produce clean and sustainable hydrogen fuel. Herein, we synthesized CoP2/Fe-CoP2 yolk–shell nanoboxes (YSBs) as efficient electrocatalysts for the oxygen evolution reaction (OER). Initially, zeolitic imidazolate framework-67/CoFe-Prussian blue analogue (ZIF-67/CoFe-PBA) YSBs were prepared by the reaction of ZIF-67 and [Fe(CN)6]3− ions in the presence of a small amount of water as an etching agent. The size of the CoP2 yolk depends on the amount of water. The heteronanostructure composed of the CoP2 yolk and the FexCo1−xP2 shell with a cubic shape was obtained by phosphidation of ZIF-67/CoFe-PBA YSBs. Benefiting from the unique structure and chemical composition, the CoP2/Fe-CoP2 YSB electrocatalyst has a large specific surface area of 114 m2 g−1 and shows superior electrocatalytic performances for the OER such as a low overpotential of 266 mV, a small Tafel slope value of 68.1 mV dec−1, and excellent cyclic stability.
1. Introduction
Electrochemical water splitting is a sustainable and environmentally friendly approach to producing clean hydrogen fuel.1 The development of active electrocatalysts for the oxygen evolution reaction (OER) is critical to promoting commercial applications of hydrogen fuel cells. The OER, the half reaction of the water splitting reaction at the anode, is a four-electron transfer reaction, which requires high energy to overcome the kinetic barrier of the reaction. Therefore, in the past several decades, many efforts have been devoted to develop an efficient OER catalyst to improve electrode kinetics and stability in acid and alkaline solutions.2–4 RuO2 and IrO2 catalysts are known to be the most active catalysts for the OER.5,6 However, their high cost and low natural abundance limit their commercial use.7 Alternatively, transition metal oxides, chalcogenides, carbides, nitrides and phosphides have been explored to replace the precious metal based OER catalysts.8–13Transition metal phosphides have recently emerged as Earth-abundant catalysts for the overall water splitting process.14 In particular, cobalt phosphides with various stoichiometric ratios (CoP, Co2P, CoP2) have attracted much attention in the research field of the OER.15–17 According to the recent theoretical and experimental studies, the catalytic activity of CoP2 is superior to those of CoP and Co2P.18 The positively charged cobalt ions serve as hydroxyl receptors and the negatively charged P atoms facilitate the desorption of O2 molecules. Wang et al. investigated for the first time the electrocatalytic OER performance of CoP2 nanoparticles on reduced graphene oxide sheets.19 Furthermore, the use of bimetallic phosphides obtained by the doping of other transition metal atoms improves the electrocatalytic performance by optimizing the electronic structure and surface energy of the catalysts.20
Morphology control is another effective way to further improve the electrochemical performances of the catalysts.21–25 Among hollow nanostructures, the yolk–shell structure has been found to be one of the most efficient structures for electrocatalysts.26 The large specific surface area of hollow yolk–shell structures can provide more active sites for redox reactions. Yin et al. reported the synthesis of NixCo1−xP yolk–shell spheres showing high activity and stability for overall water splitting.27 Metal–organic frameworks (MOFs) can provide a variety of possibilities to prepare unique hollow materials with well-defined interior voids, a large surface area, a low density, and abundant active sites.28,29 He et al. reported MOF-derived synthesis of carbon-incorporated nickel–cobalt mixed metal phosphide (NiCoP/C) nanoboxes by a reaction of ZIF-67 nanocubes with Ni(NO3)2.30 Furthermore, Prussian blue analogues (PBAs) have emerged as useful precursors for the synthesis of hollow nanostructures for energy storage and conversion applications.31–34
Herein, we demonstrate the rational design and synthesis of CoP2/Fe-CoP2 yolk–shell nanoboxes by the formation of the cobalt iron ferricyanide (CoFe-PBA) structure on sacrificial ZIF-67. CoP2/Fe-CoP2 YSBs exhibited excellent electrocatalytic activity for the OER, in terms of low overpotential and low Tafel slope values with long time cyclic stability.
2. Experimental
2.1. Materials
Co(NO3)2·6H2O (98%), 2-methylimidazole (99%), cetyltrimethylammonium bromide (CTAB) (98%), K3[Fe(CN)6] (99%), Nafion solution (5 wt%) and NaH2PO2 (99.99%) (Sigma-Aldrich). Ethanol and propanol (Samchun Chemicals).2.2. Synthesis of ZIF-67/CoFe-PBA YSBs
ZIF-67 nanocube (NC) particles were prepared according to a previous report.35 A solution of 44 mg of ZIF-67 NCs in 30 mL of ethanol was sonicated for 10 minutes and mixed with a solution of 100 mg of K3[Fe(CN)6] in 10 mL of water at 50 °C. After stirring for 1 hour, the solid product was separated, washed several times with ethanol, and dried under reduced pressure at room temperature. To prepare ZIF-67/CoFe-PBA NBs, 15 mL of water was used in the reaction mixture.2.3. Synthesis of CoP2/Fe-CoP2 YSBs
The solid sample of ZIF-67/CoFe-PBA YSBs (30 mg) was heated at 300 °C with a heating rate of 2 °C min−1 in the presence of NaH2PO2 (150 mg) under an Ar gas flow for 2 h to afford the product. The same process was applied to prepare Fe-CoP2 nanoboxes (NBs).2.4. Characterization
X-ray diffraction (XRD) measurements were performed to characterize the crystal structure of the product (Rigaku, X-MAX 2000-PC). The morphology and composition of the synthesized nanocrystals were characterized by field emission scanning electron microscopy (FESEM, Hitachi HF-4800), transmission electron microscopy (TEM), and high-resolution TEM (JEM-ARM200F, JEOL, acceleration voltage: 200 kV). The surface area and pore size distribution were determined using an ASAP-2420 (Micromeritics, USA). X-ray photoelectron spectroscopy (XPS) was conducted to characterize the chemical and valency states of the products.2.5. Electrochemical measurements
Electrochemical investigation of the products was conducted in a three electrode configuration in 1 M KOH using PGSTAT 302N Autolab (Metrohm) – the working electrode (glassy carbon of 3 mm diameter), reference electrode (Ag/AgCl; saturated KCl) and counter electrode (graphite rod). 2 mg of catalyst was dispersed in 200 μL solution (isopropanol: 190 μL and 5 wt% Nafion: 10 μL) and sonicated for 20 min. Then, 2 μL of the catalyst ink was loaded on the glassy carbon and then dried at ambient temperature. The average loading of the catalyst was about 0.21 mg cm−2. The stability test was performed at a constant overpotential (vs. RHE). Cyclic voltammetry (CV, scan rate: 10 mV s−1) was conducted to measure the double-layer capacitance (Cdl). Impedance analyses were performed in the frequency range of 0.1 Hz to 100 Hz (ZIVA-MP2A).3. Results and discussion
The synthesis procedure of CoP2/Fe-CoP2 YSBs is schematically illustrated in Scheme 1. ZIF-67 nanocrystals with the average size of 500 nm were synthesized by a surfactant assisted hydrothermal method (Fig. S1†).35 Cobalt ferricyanide (CoFe-PBA) was formed on the sacrificial ZIF-67 NCs by the reaction of Fe(CN)63− anions and Co2+ cations released from ZIF-67 NCs to form the Co-ZIF/CoFe-PBA yolk–shell structure. CoP2/Fe-CoP2 YSBs were obtained by direct phosphidation under an anaerobic atmosphere.Fig. 1 shows the FESEM and TEM images of ZIF-67/CoFe-PBA YSBs with the average size of ∼500 nm. Fig. 1b shows the yolk–shell particle composed of a solid core and an outer shell comprising small nanograins. According to the elemental mapping analysis, the Co element is uniformly distributed in both the yolk and shell, whereas Fe is observed only in the outer shell of the nanobox (Fig. 1c). Fig. S2† shows the XRD pattern of ZIF-67/CoFe-PBA YSBs. Along with the XRD peaks of ZIF-67, two characteristic peaks were also observed at 25.0° and 35.5° due to the (002) and (400) planes of Co–Fe PBA, which proves the formation of the hybrid MOF structure.
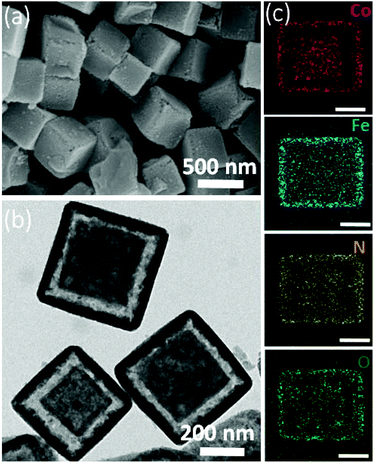 | ||
| Fig. 1 (a) SEM and (b) TEM images of ZIF-67/CoFe-PBA YSBs. (c) Corresponding mapping images (scale bar: 200 nm). | ||
Water promotes the ion exchange reaction of ZIF-67 NCs and K3[Fe(CN)6]. The morphology of YSBs can be controlled by changing the water/ethanol ratio. In a condition of 5 mL water and 25 mL ethanol, the size of ZIF-67 crystals reduces slightly and the CoFe-PBA shell begins to appear (Fig. 2a). Increasing the amount of water to 10 mL makes the ZIF-67 cube smaller and results in a cubic yolk–shell structure (Fig. 2b). The ZIF-67 yolk disappears by adding more water (Fig. 2c). Water dissolves ZIF-67 crystals and the released Co2+ cations react with [Fe(CN)6]3− anions to form a cubic shell, i.e., the etching of ZIF-67 crystals and the formation of Co–Fe PBA occur simultaneously. The shrinkage and collapse of the nanobox structure were observed with 20 mL of water (Fig. 2d).
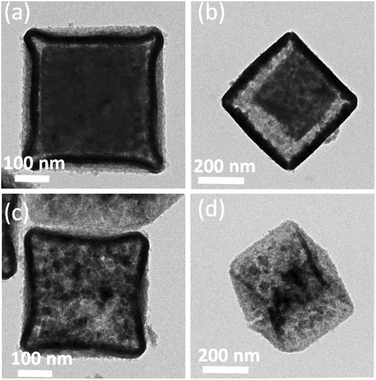 | ||
| Fig. 2 TEM images observed under different reaction conditions. (a) 5 mL (b) 10 mL (c) 15 mL and (d) 20 mL of water. | ||
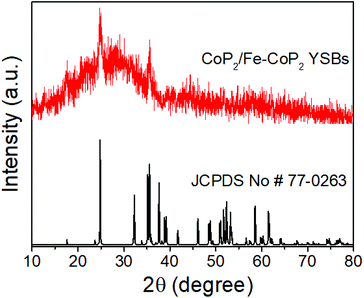
CoP2/Fe-CoP2 YSBs were synthesized by a direct phosphidation process. Fig. 3 shows the XRD pattern of the product and the main diffraction peaks can be assigned to monoclinic CoP2 (JCPDS no. #77-0263).
The shape and size of the yolk–shell structure were retained without noticeable changes even after phosphidation with the average size of 500 nm (Fig. 4a). Broken particles clearly show that the outer shell of a particle is comprised of tiny nano-sized particles (Fig. 4b). Fig. 4c and d show the TEM images of yolk–shell particles, which are in full consistency with the SEM results. The lattice fringe spacing of 0.25 nm corresponds to the (200) plane of CoP2 (Fig. 4e). STEM mapping analysis reveals that Co, Fe, and P elements are evenly distributed. Co and P are found throughout the particle, but Fe is found at the outer shell (Fig. 4f). The elemental composition of Fe/Co/P was observed to be 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.73
0.73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, being consistent with the stoichiometry of transition metal diphosphides (MP2) (Fig. S3†). Furthermore, the relative amounts of CoP2 and Fe-CoP2 of CoP2/Fe-CoP2 YSBs could be estimated by measuring the dimensions of a single hybrid particle. The calculated mass percentages of Fe-CoP2 and CoP2 are about 61% and 39%, respectively, suggesting that the atomic ratio of Fe and Co is about 1
2, being consistent with the stoichiometry of transition metal diphosphides (MP2) (Fig. S3†). Furthermore, the relative amounts of CoP2 and Fe-CoP2 of CoP2/Fe-CoP2 YSBs could be estimated by measuring the dimensions of a single hybrid particle. The calculated mass percentages of Fe-CoP2 and CoP2 are about 61% and 39%, respectively, suggesting that the atomic ratio of Fe and Co is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.26.
1.26.
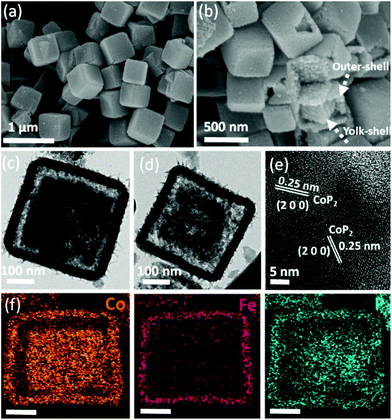 | ||
| Fig. 4 (a and b) SEM and (c and d) TEM images of CoP2/Fe-CoP2 YSBs. (e) Corresponding HR-TEM image and (f) STEM mapping images (scale bar: 100 nm). | ||
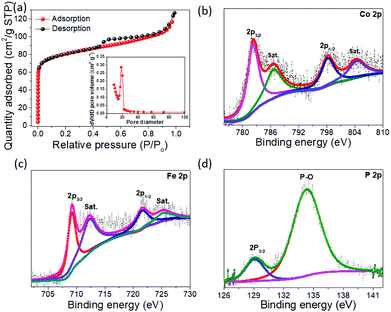
The Brunauer–Emmett–Teller (BET) specific surface area was found to be 114 m2 g−1 with a pore size distribution of 11–19 nm, indicating that the yolk–shell particles have a large surface area and are of mesoporous nature (Fig. 5a). The chemical valence states of the elements were characterized by XPS analyses. The spin–orbit doublets of Co 2p3/2 and Co 2p1/2 observed at 781.2 and 794.1 eV, respectively, in the Co 2P spectrum (Fig. 5b) can be consistent with those of the spectrum of FeCoP2 nanowires.36 Additional doublets at 786.2 and 803.8 eV are attributed to the oxidized Co.36,37 The Fe 2p spectrum (Fig. 5c) exhibits two distinct peaks located at 708.9 and 722.1 eV, reflecting the binding energies of Fe2+ 2p3/2 and Fe2+ 2p1/2, respectively. The second doublets at 711.5 eV and 724.5 eV are due to Fe3+.36,37Fig. 5d shows the P 2p spectrum consisting of two characteristic peaks at 129.1 eV for P 2p3/2 and a relatively broader peak at 133.7 eV for the P–O species due to surface oxidation.38
The TEM images of Fe-CoP2 NBs are shown in Fig. 6. The surface of the nanoboxes is composed of small nanograins. The obtained lattice fringe value of 0.25 nm corresponds to the (2 0 0) plane of CoP2 (Fig. 6b). The XRD pattern and elemental composition of Fe-CoP2 NBs are shown in Fig. S4 and S5.† The obtained diffraction peaks are well matched with monoclinic CoP2 (JCPDS no. #77-0263). The elemental composition of Fe/Co/P of the Fe-CoP2 nanobox is almost similar to that of CoP2/Fe-CoP2 YSBs (Fig. S5†). In addition, the elemental mapping image (Fig. 6c) shows that Co and P elements are relatively evenly distributed on the entire surface of the image, whereas Fe accumulated on the edge of the image. This suggests that the dissolved Co cations from the core part were deposited on the inner surface of the hollow box. Therefore, the total elemental ratio of Fe/Co/P does not seem to depend on the structural change during the reaction. Furthermore, CoP2 nanocubes were synthesized by the phosphidation of ZIF-67 NCs for the comparison of the eletrocatalytic activity with that of yolk–shell particles. The XRD pattern and FESEM image of CoP2 nanocubes are provided in Fig. S6.†
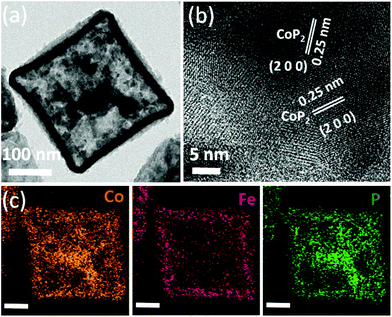 | ||
| Fig. 6 (a) TEM image and (b) HR-TEM image of Fe-CoP2 NBs. (c) Corresponding mapping images (scale bar: 100 nm). | ||
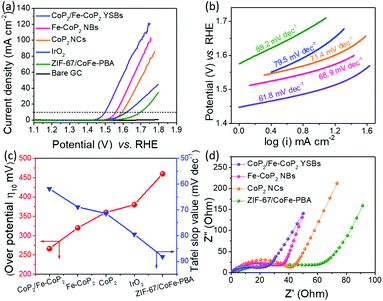
The polarization curve in Fig. 7a shows that CoP2/Fe-CoP2 YSBs exhibit a low overpotential (η10) of 266 mV, whereas Fe-CoP2 NBs need a slightly high overpotential (η10) of 320 mV. These values are much lower than those of the IrO2 benchmark catalyst (380 mV), CoP2 NCs (360 mV), and ZIF-67/CoFe-PBA YSBs (460 mV). However, the yolk–shell nanobox catalyst exhibits a high current density of 50 mA cm−2 and 100 mA cm−2 at an overpotential (η10) of 350 and 410 mV, respectively, which are higher than other catalysts. Additionally, the overpotential of CoP2/Fe-CoP2 YSBs is also significantly lower than the reported values of other catalysts, including NiCoP/C nanoboxes (300 mV),30 Co0.6Fe0.4P (298 mV),32 AlCoP (330 mV),39 CoP/carbon dots (400 mV),40 (Co0.54Fe0.46)2P (370 mV),41 Ni0.6Co1.4P nanocages (300 mV),42 Fe–Co–P nanoboxes (269 mV),43 and hollow FeCo2P (320 mV).44 As shown in Fig. 7b, the measured Tafel slope of CoP2/Fe-CoP2 YSBs (61.8 mV dec−1) is lower than those of Fe-CoP2 NBs (68.9 mV dec−1), CoP2 NCs (71.4 mV dec−1), IrO2 (79.5 mV dec−1) and ZIF-67/CoFe-PBA YSBs (88.2 mV dec−1), implying that the CoP2/Fe-CoP2 YSB catalyst has faster OER kinetics. Fig. 7c shows the overpotential and the corresponding Tafel slope values of the catalysts. EIS measurement was carried out to demonstrate the charge transfer resistance (Rct) of the catalysts (Fig. 7d). CoP2/Fe-CoP2 YSBs exhibited a lower Rct value (18.3 Ω) than Fe-CoP2 NBs (33.1 Ω), CoP2 NCs (40.1 Ω), and ZIF-67/CoFe-PBA YSBs (57.8 Ω). The higher electrical conductivity of CoP2/Fe-CoP2 YSBs might be due to the hybrid yolk–shell structure, which promotes efficient charge transfer, prevents aggregation during the electrochemical reaction, and generates strong couple effects that result in enhanced electrocatalytic activity.32,45
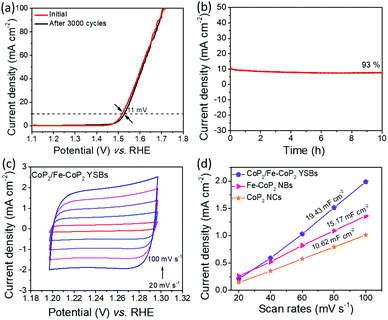
The durability test was performed for CoP2/Fe-CoP2 YSBs using CV at a fixed scan rate of 10 mV s−1 (Fig. 8a). A slight overpotential increase (11 mV) was observed for CoP2/Fe-CoP2 YSBs, whereas Fe-CoP2 NBs showed an overpotential increase of 17 mV after 3000 cycles (Fig. S7†). The chronoamperometry test was conducted at a static potential of 1.50 V (vs. RHE) (Fig. 8b). The current density of the CoP2/Fe-CoP2 YSB catalyst could be maintained even after electrolysis for 10 hours. The SEM image revealed that the yolk–shell structure was preserved well after the durability test (Fig. S8†), implying the robust nature of the catalysts.
As shown in Fig. S9,†ex situ XPS measurement was conducted to investigate the surface chemical composition of CoP2/Fe-CoP2 after the OER. The high resolution Co 2p XPS spectrum shows that the peaks at 780.3 and 795.6 eV are related to Co3+ and high resolution Fe 2p peaks at 709.5 and 722.6 eV are attributed to Fe3+.46–48 The binding energy of P 2p at 129.1 eV disappeared, which suggests the oxidation of the P centre on the surface of the catalyst. Therefore, the surface of the catalyst is oxidized into metal oxides or hydroxides during the OER under strong alkaline conditions, consistent with the previous reports.43,49
The electrochemically active surface area (ECSA) was determined using CV measurements for CoP2/Fe-CoP2 YSBs, Fe-CoP2 NBs and CoP2 NCs (Fig. 8c, S10a and S10b†). The anodic current densities measured at a fixed potential of 1.35 V (vs. RHE) are plotted with respect to scan rates in Fig. 8d. The capacitance of 19.43 mF cm−2 for CoP2/Fe-CoP2 YSBs is higher than those of Fe-CoP2 NBs (15.17 mF cm−2) and CoP2 NCs (10.62 mF cm−2), implying that the CoP2/Fe-CoP2 YSB catalyst shows a high double-layer capacitance and a high electrochemically active surface area because of the yolk–shell structure.
The superior electrocatalytic activity might be ascribed to the unique yolk–shell structure with a favourable chemical composition. CoP2/Fe-CoP2 YSBs composed of CoP2 particles (core) and Fe-CoP2 nanoboxes (shell) show a significantly lower overpotential than their ingredients. The presence of Fe, a unique yolk–shell structure, and the hollow nature of the catalyst are believed to contribute to enhancing the electrocatalytic activity for the OER. Particularly, a large electrochemical surface area and abundant electroactive sites of the yolk–shell structure can effectively shorten the diffusion length of ions and expose more active sites than solid counterparts.50–52 In addition, the integrated nanostructure prevents the aggregation during continuous gas evolution.53
3. Conclusions
In summary, we synthesized CoP2/Fe-CoP2 YSBs using hybrid MOFs as a promising electrocatalyst for the OER. The anion-exchange reaction and subsequent phosphidation process resulted in the formation of CoP2/Fe-CoP2 YSBs. The formation process of yolk–shell structures was observed systematically by TEM under different reaction conditions. Owing to the large surface area and many active sites, the CoP2/Fe-CoP2 YSB catalyst not only provides superior electrocatalytic OER performances such as low overpotential and small Tafel slope values, but also shows superior cyclic stability. This synthetic approach can be extended to produce a variety of metal phosphides with a hollow structure for energy storage and conversion applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2019R1F1A1059831).References
- H. Wang, H. W. Lee, Y. Dend, Z. Li, P. C. Hsu, Y. Li, D. Lin and Y. Cui, Nat. Commun., 2015, 6, 1 Search PubMed.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- N. B. Halck, V. Petrykin, P. Krtil and J. Rossmeisl, Phys. Chem. Chem. Phys., 2014, 16, 13682–13688 RSC.
- M. Tahira, L. Pana, F. Idrees, X. Zhang, L. Wang, J.-J. Zou and Z. L. Wang, Nano Energy, 2017, 37, 136–157 CrossRef.
- Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 399–404 CrossRef CAS.
- T. Reier, M. Oezaslan and P. Strasser, ACS Catal., 2012, 2, 1765–1772 CrossRef CAS.
- Z. Cai, X. Bu, P. Wang, J. C. Ho, J. Yang and X. Wang, J. Mater. Chem. A, 2019, 7, 5069–5089 RSC.
- E. Fabbri, A. Habereder, K. Waltar, R. Kötz and T. J. Schmidt, Catal. Sci. Technol., 2014, 4, 3800–3821 RSC.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS.
- U. D. Silva, J. Masud, N. Zhang, Y. Hong, W. P. R. Liyanage, M. A. Zaeem and M. Nath, J. Mater. Chem. A, 2018, 6, 7608–7622 RSC.
- Y.-J. Tang, C.-H. Liu, W. Huang, X.-L. Wang, L.-Z. Dong, S.-L. Li and Y.-Q. Lan, ACS Appl. Mater. Interfaces, 2017, 9, 16977–16985 CrossRef CAS.
- C. Liu, G. Bai, X. Tong, Y. Wang, B. Lv, N. Yang and X.-Y. Guo, Electrochem. Commun., 2019, 98, 87–91 CrossRef CAS.
- L. Ji, J. Wang, X. Teng, T. J. Meyer and Z. Chen, ACS Catal., 2020, 10, 412–419 CrossRef CAS.
- A. Parra-Puerto, K. L. Ng, K. Fahy, A. E. Goode, M. P. Ryan and A. Kucernak, ACS Catal., 2019, 9, 11515–11529 CrossRef CAS.
- H. Li, Q. Li, P. Wen, T. B. Williams, S. Adhikari, C. Dun, C. Lu, D. Itanze, L. Jiang, D. L. Carroll, G. L. Donati, P. M. Lundin, Y. Qiu and S. M. Geyer, Adv. Mater., 2018, 30, 1705796 CrossRef.
- Y. Bai, H. Zhang, Y. Feng, L. Fang and Y. Wang, J. Mater. Chem. A, 2016, 4, 9072–9079 RSC.
- S. Yang, M. Xie, L. Chen, W. Wei, X. Lv, Y. Xu, N. Ullah, O. C. Judith, Y. B. Adegbemiga and J. Xie, Int. J. Hydrogen Energy, 2019, 44, 4543–4552 CrossRef CAS.
- H. Li, P. Wen, D. S. Itanze, M. W. Kim, S. Adhikari, C. Lu, L. Jiang, Y. Qiu and S. M. Geyer, Adv. Mater., 2019, 31, 1900813 CrossRef.
- J. Wang, W. Yangac and J. Liu, J. Mater. Chem. A, 2016, 4, 4686–4690 RSC.
- L. Wang, H. Wu, S. Xi, S. T. Chua, F. Wang, S. J. Pennycook, Z. G. Yu, Y. Du and J. Xue, ACS Appl. Mater. Interfaces, 2019, 11, 17359–17367 CrossRef CAS.
- H. Mistry, A. S. Varela, S. Kühl, P. Strasser and B. R. Cuenya, Nat. Rev., 2016, 1, 16009 CAS.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara, J. Tang and Y. Yamauchi, Adv. Mater., 2019, 31, 1807134 CrossRef.
- S. Li, X. Hao, A. Abudulac and G. Guan, J. Mater. Chem. A, 2019, 7, 18674–18707 RSC.
- B. Wang, C. Tang, H.-F. Wang, X. Chen, R. Cao and Q. Zhang, Adv. Mater., 2019, 31, 1805658 CrossRef.
- M. Li, X. Deng, Y. Liang, K. Xiang, D. Wu, B. Zhao, H. Yang, J.-L. Luo and X.-Z. Fu, J. Energy Chem., 2020, 50, 314–323 CrossRef.
- Z. Li, M. Li, Z. Bian, Y. Kathiraser and S. Kawi, Appl. Catal., B, 2016, 188, 324–341 CrossRef CAS.
- Z. Yin, C. Zhu, C. Li, S. Zhang, X. Zhang and Y. Chen, Nanoscale, 2016, 8, 19129–19138 RSC.
- X.-C. Xie, K.-J. Huang and X. Wu, J. Mater. Chem. A, 2018, 6, 6754–6771 RSC.
- V. Ganesan, P. Ramasamy and J. Kim, Int. J. Hydrogen Energy, 2017, 42, 5985–59992 CrossRef CAS.
- P. He, X.-Y. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 3897–3900 CrossRef CAS.
- C. Xuan, J. Wang, W. Xia, Z. Peng, Z. Wu, W. Lei, K. Xia, H. L. Xin and D. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 26134–26142 CrossRef CAS.
- Y. Lian, H. Sun, X. Wang, P. Qi, Q. Mu, Y. Chen, J. Ye, X. Zhao, Z. Deng and Y. Peng, Chem. Sci., 2019, 10, 464–474 RSC.
- Y. Fang, X.-Y. Yu and X. W. Lou, Adv. Mater., 2018, 30, 1706668 CrossRef.
- J.-Y. Xie, Z.-Z. Liu, J. Li, L. Feng, M. Yang, Y. Ma, D.-P. Liu, L. Wang, Y.-M. Chai and B. Dong, J. Energy Chem., 2020, 48, 328–333 CrossRef.
- V. Ganesan, S. Lim and J. Kim, Chem. – Asian J., 2018, 13, 413–420 CrossRef CAS.
- C. Tang, L. Gan, R. Zhang, W. Lu, X. Jiang, A. M. Asiri, X. Sun, J. Wang and L. Chen, Nano Lett., 2016, 16, 6617–6621 CrossRef CAS.
- J. Han, G. Chen, X. Liu, N. Zhang, S. Liang, R. Ma and G. Qiuc, Chem. Commun., 2019, 55, 9212–9215 RSC.
- F. Li, Y. Bu, Z. Lv, J. Mahmood, G.-F. Han, I. Ahmad, G. Kim, Q. Zhong and J.-B. Baek, Small, 2017, 13, 1701167 CrossRef.
- X. Lv, Z. Hu, J. Ren, Y. Liu, Z. Wang and Z.-Y. Yuan, Inorg. Chem. Front., 2019, 6, 74–81 RSC.
- M. Zhu, Y. Zhou, Y. Sun, C. Zhu, L. Hu, J. Gao, H. Huang, Y. Liu and Z. Kang, Dalton Trans., 2018, 47, 5459–5464 RSC.
- A. Mendoza-Garcia, D. Sub and S. Sun, Nanoscale, 2016, 8, 3244–3247 RSC.
- B. Qiu, L. Cai, Y. Wang, Z. Lin, Y. Zuo, M. Wang and Y. Chai, Adv. Funct. Mater., 2018, 28, 1706008 CrossRef.
- H. Zhang, W. Zhou, J. Dong, X. F. Lu and X. W. Lou, Energy Environ. Sci., 2019, 12, 3348–3355 RSC.
- J. Wang, J. Wang, M. Zhang, S. Li, R. Liu and Z. Li, J. Alloys Compd., 2020, 821, 153463 CrossRef CAS.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS.
- L.-M. Cao, Y.-W. Hu, S.-F. Tang, A. Iljin, J.-W. Wang, Z.-M. Zhang and T.-B. Lu, Adv. Sci., 2018, 5, 1800949 CrossRef.
- Y. Lian, H. Sun, X. Wang, P. Qi, Q. Mu, Y. Chen, J. Ye, X. Zhao, Z. Deng and Y. Peng, Chem. Sci., 2019, 10, 464–474 RSC.
- J. Shi, F. Qiu, W. Yuan, M. Guo and Z.-H. Lu, Chem. Eng. J., 2021, 403, 126312 CrossRef CAS.
- C. Du, L. Yang, F. Yang, G. Cheng and W. Luo, ACS Catal., 2017, 7, 4131–4137 CrossRef CAS.
- S. Yue, S. Wang, Q. Jiao, X. Feng, K. Zhan, Y. Dai, C. Feng, H. Li, T. Feng and Y. Zhao, ChemSusChem, 2019, 12, 4461–4470 CrossRef CAS.
- R. Cai, H. Jin, D. Yang, K.-T. Lin, K. Chan, J. Sun, Z. Chen, X. Zhang and W. Tan, Nano Energy, 2020, 17, 104542 CrossRef.
- T. Yang, L. Yin, M. He, W. Wei, G. Cao, X. Ding, Y. Wang, Z. Zhao, T. Yu, H. Zhaoa and D. Zhang, Chem. Commun., 2019, 55, 14343–14346 RSC.
- G. Mei, H. Liang, B. Wei, H. Shi, F. Ming, X. Xu and Z. Wang, Electrochim. Acta, 2018, 290, 82–89 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern and SEM image of ZIF-67. The XRD pattern of ZIF-67/CoFe-PBA YSBs. The EDS spectrum of CoP2/Fe-CoP2 YSBs and Fe-CoP2 NBs. The OER stability curves of Fe-CoP2 NBs and the cyclic voltammetry curves of Fe-CoP2 NBs and CoP2 NCs. The SEM images of CoP2/Fe-CoP2 YSBs after the durability test. See DOI: 10.1039/d0nr08108f |
| This journal is © The Royal Society of Chemistry 2021 |