Hydration effects and negative dielectric constant of nano-confined water between cation intercalated MXenes
Abstract
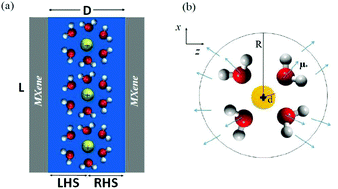
Using electrochemical methods a profound enhancement of the capacitance of electric double layer capacitor electrodes was reported when water molecules are strongly confined into the two-dimensional slits of titanium carbide MXene nanosheets [A. Sugahara et al., Nat. Commun., 2019, 10, 850]. We study the effects of hydration on the dielectric properties of nanoconfined water and supercapacitance properties of the cation intercalated MXene. A model for the electric double layer capacitor is constructed where water molecules are strongly confined in two-dimensional slits of MXene. We report an abnormal dielectric constant and polarization of nano-confined water between MXene layers. We found that by decreasing the ionic radius of the intercalated cations and in a critical hydration shell radius the capacitance of the system increases significantly (≃200 F g−1) which can be interpreted as a negative permittivity. This study builds a bridge between the fundamental understanding of the dielectric properties of nanoconfined water and the capability of using MXene films for supercapacitor technology, and in doing so provides a solid theoretical support for recent experiments.


 Please wait while we load your content...
Please wait while we load your content...