Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multi-colored shades of betalains: recent advances in betacyanin chemistry
Agnieszka
Kumorkiewicz-Jamro
 *,
Tomasz
Świergosz
*,
Tomasz
Świergosz
 ,
Katarzyna
Sutor
,
Katarzyna
Sutor
 ,
Aneta
Spórna-Kucab
,
Aneta
Spórna-Kucab
 and
Sławomir
Wybraniec
and
Sławomir
Wybraniec
 *
*
Department of Chemical Technology and Environmental Analysis, Faculty of Chemical Engineering and Technology, Cracow University of Technology, Warszawska 24, 31-155 Cracow, Poland. E-mail: agnieszka.kumorkiewicz-jamro@pk.edu.pl; slawomir.wybraniec@pk.edu.pl
First published on 13th September 2021
Abstract
Covering: 2001 to 2021
Betacyanins cover a class of remarkable natural red-violet plant pigments with prospective chemical and biological properties for wide-ranging applications in food, pharmaceuticals, and the cosmetic industry. Betacyanins, forming the betalain pigment group together with yellow betaxanthins, have gained much attention due to the increasing social awareness of the positive impact of natural products on human health. Betalains are commercially recognized as natural food colorants with preliminarily ascertained, but to be further investigated, health-promoting properties. In addition, they exhibit a remarkable structural diversity based on glycosylated and acylated varieties. The main research directions for natural plant pigments are focused on their structure elucidation, methods of their separation and analysis, biological activities, bioavailability, factors affecting their stability, industrial applications as a plant-based food, natural colorants, drugs, and cosmetics as well as methods for high-yield production and stabilization. This review covers period of the last two decades of betacyanin research. In the first part of the review, we present an updated classification of all known betacyanins and their derivatives identified by chemical means as well as by mass spectrometric and NMR techniques. In the second part, we review the current research reports focused on the chemical properties of the pigments (decarboxylation, oxidation, conjugation, and chlorination reactions as well as the acyl group migration phenomenon) and describe the semi-synthesis of natural and artificial fluorescent betalamic acid conjugates, showing various prospective research directions.
1 Introduction
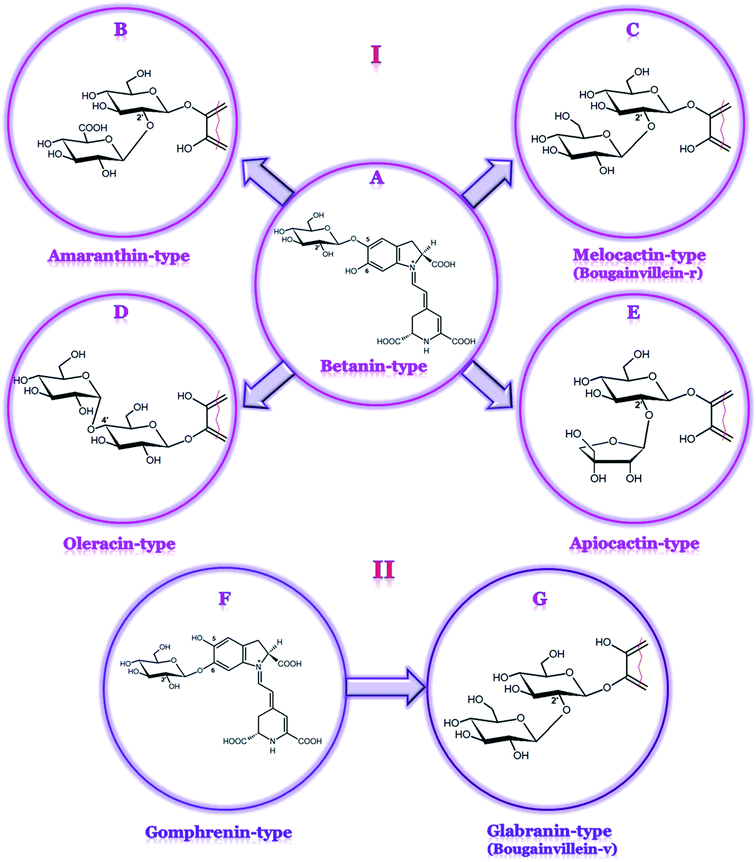
Betacyanins are unique, water-soluble, red-violet indoline- and dihydropyridine-derived nitrogen-containing natural plant pigments that occur in most families of the Caryophyllales order. Betacyanins, together with yellow-orange betaxanthins, form a group of betalain pigments.1 Simultaneously with anthocyanins, carotenoids, and chlorophylls, betalains are one of the most common plant pigments found in nature.2,3 These compounds share betalamic acid as the main chromogenic structural unit condensed with cyclo-DOPA, forming betanidin or glycosylated cyclo-DOPA in other betacyanins as well as different amino acids or amines in betaxanthins.4,5So far, betacyanins (Table 1) have been assigned to four structural groups comprising betanin-type, gomphrenin-type, amaranthin-type, and Bougainvillea-r-I-type,6,7 the latter was recently renamed as melocactin-type.8 However, for a convenient view on a wide spectrum of pigments, an expanded division with three additional betacyanin groups is proposed based on the sugar linkage: oleracin-type9 (a very early result, not indepedently confirmed), apiocactin-type,10–12 and Bougainvillea-v-type (here, we propose to name it as glabranin-type). In particular, the latter group of the pigments with the structures based on gomphrenin is important in the division system not only because of its less common 6-O-glycosylation pattern but also because of its unique and extremely complex profile of ca. 146 betacyanins tentatively detected in only one plant species, Bougainvillea glabra Choisy,11 including nine definitively identified betacyanins by LC-ESI-MS and NMR.13
| No. | Name | Trivial name/proposed new namea | m/z [M + H]+ | Chem. | LC-MS | NMR | Plant sources(Chem.)/(LC-MS)/(NMR) | Ref. (Chem.)/(LC-MS)/(NMR) |
|---|---|---|---|---|---|---|---|---|
| 1 | Betanidin 5-O-β-glucoside | Betanin | 551 | + | + | + | B. vulgaris/G. globosa/B. vulgaris | 47 and 48/24/24 |
| 2 | Betanidin | Betanidin | 389 | + | + | + | B. vulgaris/B. glabra/B. vulgaris | 49/50/50 |
| 3 | 2-Decarboxy-betanin | 507 | − | + | + | B. vulgaris | 51 | |
| 4 | 14,15-Dehydro-betanin | Neobetanin | 549 | + | + | + | B. vulgaris | 52 and 53 |
| 5 | 6′-O-Sulfate-betanin | Prebetanin | 631 | + | + | + | 3x P. americana; B. vulgaris/B. vulgaris/ | 54/10/54 |
| 6 | 2-Decarboxy-phyllocactin | 593 | − | + | + | B. vulgaris | 51 | |
| 7 | 2-Decarboxy-betanidin | 345 | + | + | − | C. acinaciformis | 55/51 | |
| 8 | 2′-O-β-Apiosyl-betanin | Apiocactina | 683 | − | + | + | H. ocamponis | 11 |
| 9 | 5′′-O-E-Sinapoyl-apiocactin | 889 | + | + | − | 2x H. ocamponis/Melocactus spp. | 11/8, 11 | |
| 10 | 4′-O-Malonyl-betanin | 637 | + | + | − | H. ocamponis | 11 | |
| 11 | 5′′-O-E-Feruloyl-apiocactin | 859 | + | + | + | P. americana | 10 | |
| 12 | 6′-O-Malonyl-betanin | Phyllocactin | 637 | − | + | + | S. buckleyi/P. hybridus | 38/48 |
| 13 | 2′-O-β-Apiosyl-phyllocactin | 683 | − | + | + | S. buckleyi | 38 | |
| 14 | 2′-O-β-(5′′-O-E-Feruloyl)-apiosyl-phyllocactin | 945 | − | + | − | S. buckleyi | 38 | |
| 15 | 6′-O-(3′′-Hydroxy-3′′-methylglutaryl)-betanin | Hylocerenin | 695 | − | + | + | H. polyrhizus | 56 |
| 16 | Betanidin 5-O-(6′-O-E-4-coumaroyl-β-glucoside) | Lampranthin I | (697) | + | − | − | Lampranthus sp. | 57 |
| 17 | Betanidin 5-O-β-(6′-O-E-feruloyl)-glucoside | Lampranthin II | 727 | + | + | + | Lampranthus sp./G. globosa/L. sociorum | 57/24/58 |
| 18 | 3′-O-Sulfate-betanin | Rivinianin | (631) | + | − | − | R. humilis | 59 |
| 19 | Betanidin 5-O-β-sophoroside | Bougainvillein-r-I; melocactina | 713 | + | + | + | 3x Boug. Mrs. Butt’/Melocactus spp./ | 32/8, 11/11 |
| 20 | (Caffeoyl and/or coumaroyl)-bougainvillein-r I | Bougainvillein-r-II, III, IV, V | − | + | − | − | Boug. Mrs. Butt | 32 |
| 21 | Betanidin 5-O-(2′-O-β-glucuronosyl)-glucoside | Amaranthin | 727 | + | + | + | A. tricolor/Amaranthus sp./C. cristata | 60/27/61 |
| 22 | 2′′-O-E-4-Coumaroyl-amaranthin | Celosianin I; argentianina | 873 | − | + | − | C. cristata | 25 and 27 |
| 23 | 2′′-O-E-Feruloyl-amaranthin | Celosianin II; celosianina | 903 | − | + | + | C. cristata | 27 and 58/58 |
| 24 | 6′-O-Malonyl-amaranthin | Celoscristatin | 813 | + | + | + | C. cristata | 29 |
| 25 | 4′-O-Malonyl-amaranthin | 813 | − | + | − | C. cristata | 29 | |
| 26 | 6′-O-(3′′-Hydroxy-3′′-methylglutaryl)-amaranthin | Iresinin I | 871 | + | + | + | I. herbstii | 61/27/61 |
| 27 | 4′-O-Malonyl-bougainvillein-r I | 799 | − | + | − | Mammillaria spp. | 36 | |
| 28 | 6′-O-Malonyl-bougainvillein-r I | Mammillarinin | 799 | − | + | + | Mammillaria spp. | 36 |
| 29 | 2-Decarboxy-mammillarinin | 755 | + | + | − | Mammillaria spp. | 36 | |
| 30 | 17-Decarboxy-mammillarinin | 755 | − | + | − | Mammillaria spp. | 36 | |
| 31 | 17-Decarboxy-bougainvillein-r I | 669 | − | + | − | Mammillaria spp. | 36 | |
| 32 | Feruloyl-bougainvillein-r I | 889 | − | + | − | M. crystallinum L.; B. vulgaris (Swiss chard) | 62 and 63 | |
| 33 | (Dehydrated phyllocactin) | 619 | − | + | − | U. tuberosus | 64 | |
| 35 | Betanidin 6-O-β-D-sophoroside | Bougainvillein-v; glabranina | 713 | − | + | + | B. glabra v. sander. | 13/33 |
| 36 | 6′′-O-Rhamnosyl-glabranin | 859 | + | − | − | B. glabra v. sander. | 65 | |
| 37 | 6′-O-E-Caffeoyl-glabranin | Cafglabranina | 875 | − | + | + | B. glabra | 13 |
| 38 | 6′-O-E-4-Coumaroyl-glabranin | Coumglabranina | 859 | − | + | + | B. glabra | 13 |
| 39 | 6′′-O-E-4-Coumaroyl-glabranin | 859 | − | + | + | B. glabra | 13 | |
| 40 | 6′,6′′-Di-O-E-4-coumaroyl-glabranin | Bicoumglabranina | 1005 | − | + | + | B. glabra | 13 |
| 41 | 2′′-O-[(6′′-O-E-4-Coumaroyl)]-glucosyl-cafglabranin | 1183 | − | + | + | B. glabra | 13 | |
| 42 | 2′′-O-Glucosyl-bicoumglabranin | 1167 | − | + | + | B. glabra | 13 | |
| 43 | 2′′-O-[(6′′-O-E-4-Coumaroyl)]-sophorosyl-cafglabranin | 1345 | − | + | + | B. glabra | 13 | |
| 44 | Feruloyl-dihexosyl-betanidin | 889 | − | + | − | M. amoenus | 8 | |
| 45 | Sinapoyl-dihexosyl-betanidin | 919 | − | + | − | M. amoenus | 8 | |
| 47 | Citryl-(caffeoyl or 4-coumaroyl)-amaranthin | Suaedin | − | + | − | − | S. fruticosa | 66 |
| 48 | 2′′-O-E-Sinapoyl-amaranthin | Lindenina | 933 | − | + | − | G. globosa; I. lindenii | 25, 30 and 67 |
| 49 | Betanidin 6-O-β-glucoside | Gomphrenin I; gomphrenina | 551 | + | + | + | G. globosa; B. alba | 68/24/24, 69 |
| 50 | 6′-O-E-4-Coumaroyl-gomphrenin I | Gomphrenin II; globosina | 697 | + | + | + | G. globosa | 68/24, 25/24 |
| 51 | 6′-O-E-Feruloyl-gomphrenin I | Gomphrenin III; basellina | 727 | + | + | + | G. globosa | 68/24, 25/24 |
| 52 | 6′-O-E-Sinapoyl-gomphrenin I | Gomphrenin IV; gandolina | 757 | − | + | − | G. globosa | 25 and 26 |
| 53 | E-Isomer of gomphrenin II | 697 | − | + | − | G. globosa | 30 | |
| 54 | E-Isomer of gomphrenin III | 727 | − | + | − | G. globosa | 30 | |
| 55 | Betanidin 5-O-β-(E-feruloyl)-cellobioside | Oleracin I | (889) | + | − | − | P. oleracea | 9 and 60 |
| Number of betacyanins identified by a given method | 22 | 50 | 31 | |||||
The schematic division of betacyanins into seven main structural groups based on their chemical structures is shown in Fig. 1. Betacyanins assigned to particular types differ in the attachment of the glucosyl moiety to the oxygen atom in the ortho position of cyclo-DOPA as well as the position of the substitution with additional glycosyl or glucuronosyl moieties.6 The esterification of sugar moieties with organic acids such as ferulic, p-coumaric, caffeic, sinapic, and malonic acids is very common and leads to the formation of various acylated derivatives. The 15R diastereomers (the isoforms, epimers) of betacyanins are found in plants at much lower concentrations than the 15S forms and are regarded rather as artifacts due to epimerization, which takes place during the preparation of extracted plant samples.14 The first studies on isomerization (as well as decarboxylation) mechanism in betacyanins were performed by Dunkelblum et al.15 In more recent reports, the chromatographic differences in the 15S/15R diastereomers of decarboxylated betacyanins16,17 as well as in the acyl migration products18 were investigated.
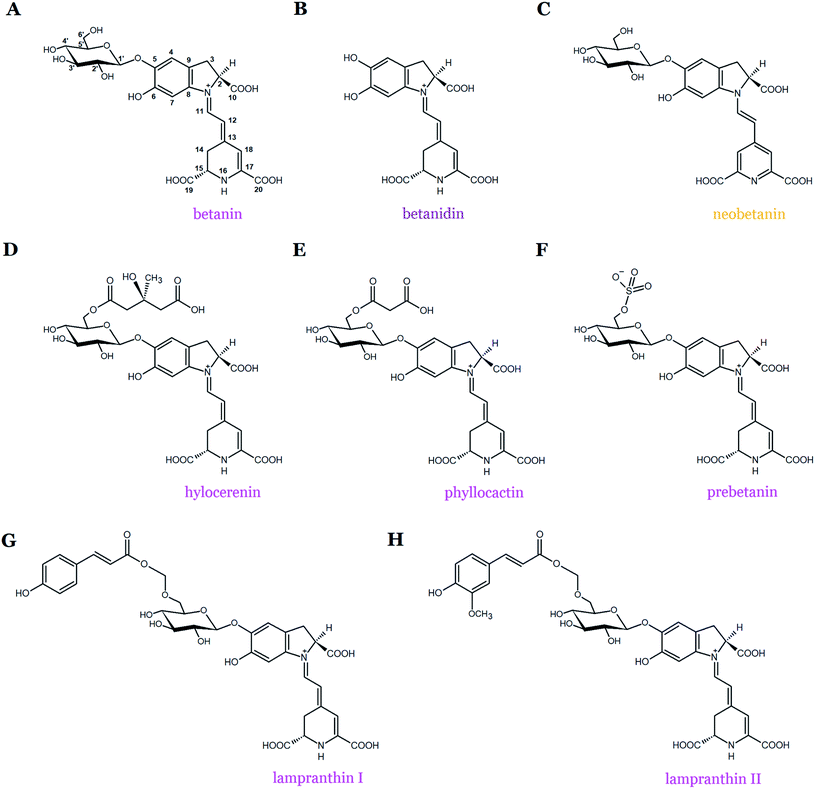
Betanin (betanidin 5-O-β-D-glucopyranoside) is the main representative and most common betacyanin pigment found in the plant kingdom (Fig. 1 and 2A). In addition, betanin isolated from Beta vulgaris L. (beetroot, red beet) is the most studied pigment in the context of the antioxidant properties of betacyanins. Structurally, betanin and most of its derivatives are composed of aglycone (betanidin) linked by the β-glucosidic bond with the glucose unit at the C-5 carbon atom.19,20 The chemical structures of additional compounds belonging to betanin-type betacyanins are shown in Fig. 2. Such compounds share a common betanidin backbone in their structures with an additional glucose moiety (betanin) along with (3-methyl-3-hydroxy-methyl)glutaryl-, malonyl-, E-4-coumaroyl-, and E-feruloyl-moieties as well as the half sulphate ester in hylocerenin, phyllocactin, lampranthin I, lampranthin II, and prebetanin, respectively, but can be also partially oxidized (neobetanin) (Fig. 2).
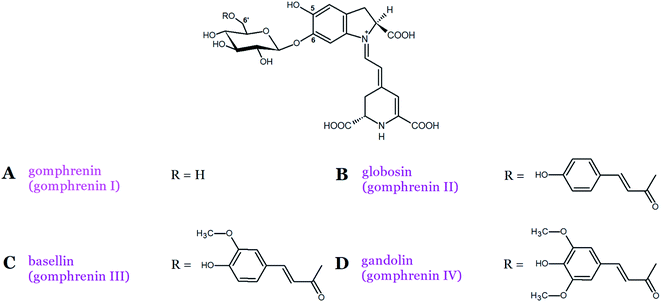
Gomphrenin (betanidin 6-O-β-D-glucopyranoside), which is the isomeric pigment to betanin, is found at high concentrations in Basella alba L. fruits as well as in the leaves of its variety Basella alba var. ‘Rubra’ L.21,22 According to our recent recommendation, we propose to rename gomphrenin I as “gomphrenin” in order to simplify the naming of its derivatives, especially generated by gomphrenin decarboxylation and oxidation.16 In contrast to betanins, gomphrenins are characterized by the presence of a glucosyl moiety attached at the carbon C-6 (Fig. 1 and 3A).21,22 Furthermore, the acylation of gomphrenin enables the formation of its derivatives such as E-4-coumaroyl-gomphrenin (former gomphrenin II, globosin), E-feruloyl-gomphrenin (former gomphrenin III, basellin), and E-sinapoyl-gomphrenin (gomphrenin IV, gandolin) (Table 1, Fig. 3B–D).12 Gomphrenin pigments are of interest because their glucosylation position at the carbon C-6 should promote an interaction of the acylated residues with the carboxyl groups by increasing the intramolecular stabilization (by intramolecular stacking) of globosin and basellin23 or other types of multiple acylated sophorosyl residues observed in glabranins. Betacyanins were also detected in red and purple Gomphrena globosa L. inflorescences. The most dominant betacyanins present in red G. globosa cultivars are amaranthin and celosianin but in violet species, gomphrenin and especially the acylated derivatives, globosin and basellin, are present.24,25 In addition, other betacyanins were initially identified, namely, Z-4-coumaroyl-gomphrenin and Z-feruloyl-gomphrenin isomers as well as gandolin.25,26
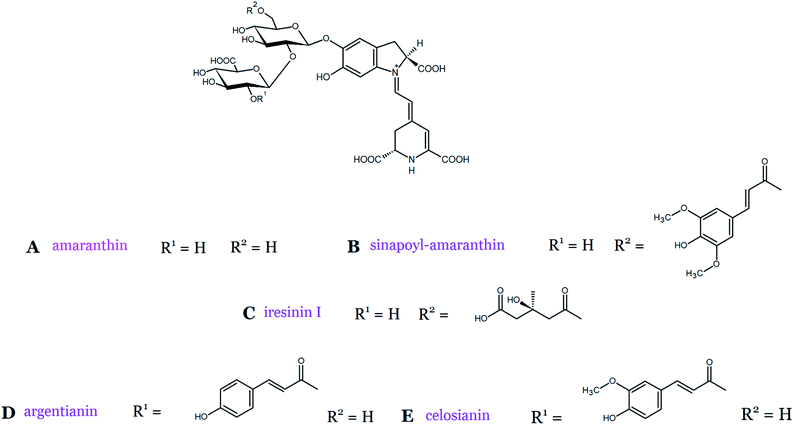
Plant pigments belonging to the amaranthin-type group have a characteristic glucuronosylglucosyl moiety attached to the carbon C-5 in their structure. The most common example of these pigments is amaranthin [betanidin 5-O-β-(2′-O-β-glucuronosyl)glucoside] (Fig. 4A) isolated from plants of the Amaranthaceae family.27,28 The other frequently detected pigments in the plants are betanin and 6′-O-malonyl-amaranthin.29 Furthermore, sinapoyl-amaranthin has been tentatively identified in G. globosa petals.30 Iresinin I [6′-O-(3′′-hydroxy-3′′-methyl)glutaryl-2′-O-glucuronosyl-betanin] and amaranthin are the most abundant pigments detected in Iresine herbstii Hook. ex Lindl leaves (Table 1).27 Acylated amaranthin-based pigments, argentianin and celosianin, were also found in Celosia species31 and the purple leaves of I. herbstii (Fig. 4).25
Most of the pigments from the two Bougainvillea groups have sophorosyl moieties linked to carbons C-5 or C-6 of the basic skeleton of betanidin, expanded further by glucosyl, rhamnosyl, apiosyl, or xylosyl moieties.13,26,32–35 A complex mixture of betacyanins that differs in acyl-oligoglycoside units and exists mostly in the 6-O-glycosylated forms of betanidin was initially identified in the purple bracts of B. glabra.13 However, due to the complex mixture of a huge number of betacyanins present in B. glabra,34 its profile has not been completely characterized. The examples of bougainvillein-type betacyanins are bougainvillein-r-I (melocactin-type) and bougainvillein-v (glabranin-type) (Table 1, Fig. 5A and B). Similar betacyanins were recently found within Melocactus species.8 The most abundant pigment, melocactin, previously named ‘bougainvillein-r-I’, was identified as betanidin 5-O-β-sophoroside (Fig. 5A). The presence of feruloylated and sinapoylated melocactins as well as melocactin's malonylated derivative, mammillarinin, was also noted (Fig. 5C).8 In some Mammillaria species, mammillarinin [betanidin 5-O-(6′-O-malonyl)-β-sophoroside] was reported as the dominant pigment.36
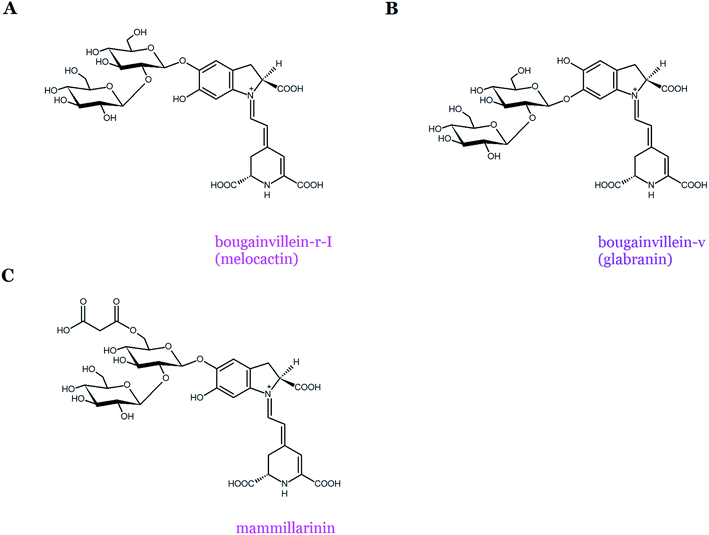 | ||
| Fig. 5 Chemical structures of betacyanins belonging to both the bougainvillein groups: melocactin-type (bougainvillein-r-I (A) and mammillarinin (C)) and glabranin-type (bougainvillein-v) (B). | ||
Two red-violet acylated betacyanins, oleracins I and II, have been found in Portulaca oleracea L. Upon hydrolysis in alkaline conditions, oleracins gave ferulic acid and two newly detected pigments that were identified as 5-O-β-cellobiosides of betanidin and isobetanidin (Fig. 1E).9 Five other yellow oleracins were also detected in P. oleracea; however, in contrast to oleracins I and II, they did not possess betalamic acid in their structures.37
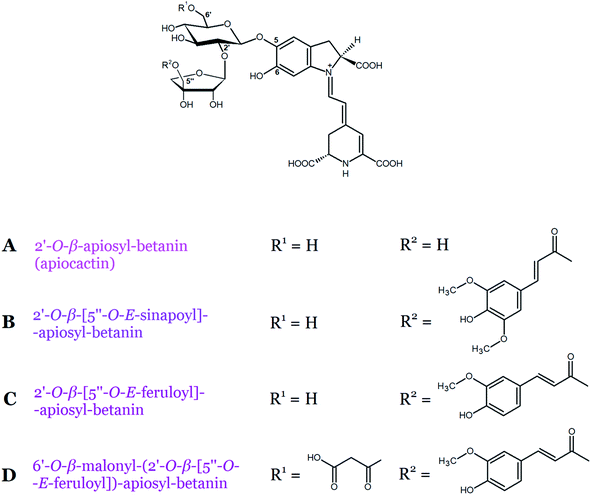
Betacyanins belonging to the apiocactin-type group contain the rare branched pentose, apiose, bound to the carbon C-2′ of betanin. Pigments such as 2′-O-β-apiosyl-betanin (apiocactin) and/or its acylated derivatives (Table 1, Fig. 6A–C) have been found in fruits of pokeberry and its corresponding suspension and callus cultures (Phytolacca americana L.), Christmas cactus flowers (Schlumbergera x buckleyi), and Hylocereus species.10,11,38 The chemical structure of the basic apiocactin-type betacyanin present in Hylocereus species was confirmed as 2′-O-β-apiosyl-betanin by Wybraniec et al.11 In addition, in the same study, acylated compounds with sinapoyl and feruloyl residues attached to C-5′′ of the apiose moiety were identified as 5′′-O-E-sinapoyl-apiocactin and 5′′-O-E-feruloyl-apiocactin.11 Similarly, 5′′-O-E-feruloyl-apiocactin was identified earlier in P. americana cultures by Schliemann et al.10 Another betacyanin, 2′-O-β-(5′′-O-E-feruloyl)-apiosyl-phyllocactin, which is the first betacyanin example containing both an aliphatic and an aromatic acyl residue, was tentatively detected in Christmas cactus flower extracts together with other apiocactin derivatives38 and later in Hylocereus species.11
2 Characterization of betacyanins
2.1 Sources of betacyanin pigments
There are several known edible sources of betacyanins (Table 1). These include red and yellow beetroots (B. vulgaris ssp. vulgaris),1B. alba fruits,39G. globosa flowers,13,30 grainy amaranth (Amaranthus sp.),27 grains of Chenopodium quinoa,40 leaves of A. hortensis var. rubra,41 and fruits/flowers of cacti genera (Opuntia, Hylocereus, Mammillaria, Melocactus, and Myrtillocactus spec.).8,36,42–45 Pokeberry (Phytolacca americana L.) is another source of betacyanins but it has been forbidden as a food colorant due to the presence of toxic saponins and lectins.46Compounds tentatively identified as betacyanin-like are also found in some higher fungi such as Amanita muscaria (fly agaric).70,71 The less common edible sources are Ulluco tubers (Ullucus tuberosus)64,72 as well as fruits and berries of pigeonberry (Rivina humilis).73
Table 1 presents a detailed summary of the identified betacyanins in different plant sources along with the methods of their identification. Most of the chemical methods were applied in the early decades of betalain research based on the typical reactions of hydrolysis and derivatization, such as permethylation and partial degradation, resulting in the generation of diagnostic products, which can be identified and can confirm certain parts of the chemical structure of a starting compound. The chemical structures of 31 naturally occurring betacyanins were definitively identified by NMR methods, whereas a huge group of over 187 betacyanins (including pigments from their richest source, B. glabra) were detected by LC-MS techniques and still await the final confirmation. In addition, structures of 18 betacyanin derivatives were also confirmed by NMR and 61 tentatively were identified by LC-MS methods (Fig. 7).
 | ||
| Fig. 7 The number of naturally-occurring betacyanins and semi-synthesized betacyanins derivatives identified chemically, by NMR techniques and detected by LC-MS methods. | ||
2.2 Biological properties of betacyanins
Numerous health-promoting activities are attributed to betacyanin pigments, including antioxidative,6,74 chemopreventive,75 and anti-inflammatory76,77 properties. Furthermore, it was noted that betacyanins protect low-density lipoproteins (LDL) against oxidative damage by reacting with the LDL polar groups.78 In addition, betanin present in red beets prevents DNA from damage in lymphocytes and hepatocytes.79 Betanin also shows neuroprotective effects, improves cognitive functions and reduces oxidative stress caused by D-galactose in the brain of mice by increasing the level of antioxidant enzymes and reducing lipid peroxidation.80Some betacyanin pigments have been shown to have higher antioxidant activity compared to typical natural antioxidants such as ascorbic acid,81 rutin,82 catechin,83 β-carotene,84 and α-tocopherol.6,85 In a test of the antioxidant activity expressed in TROLOX equivalents, betacyanins extracted from red beet exhibited 1.5–2.0 times higher free radical scavenging activity than some anthocyanins such as cyanidine-3-O-glucoside and cyanidine above pH 4.86,87
The effect of the hydroxyl group position on the antioxidant activity of the betacyanins bearing the conjugated phenol moiety was studied. Three non-natural regioisomeric phenolic betacyanins (common name: o-, m-, p-OH-pBeets) were semi-synthesized by coupling betalamic acid with appropriate aminophenols in water according to a procedure described by Schliemann and co-authors88 and their antioxidant activity was studied.89,90 The results showed that the meta isomer exhibits greater antiradical activity than most of the betalains, flavonoids, and anthocyanins. This result may be explained by the fact that the phenolic moiety is not conjugated with the diazapolymethine system but both groups are prone to further oxidation and can stabilize the radicals by resonance. In addition, the N–H imine bond present in the meta regioisomeric structure is the preferred site for oxidation, whereas 1e− oxidation of the phenolic groups in p- and o-OH-pBeet results in the formation of semi-quinone and leads to lower values of the potential energy (Ep) compared to m-OH-pBeet.89,90
Betacyanins have been shown to actively participate in free radical scavenging and consequently, their consumption may lead to chemoprevention and delay of the development of cancer tissues.75 Purified betanin shows strong inhibition of melanoma cancer cell proliferation91 and excellent growth inhibition of MCF-7 (breast), HCT-116 (colon), AGS (stomach), SF-268 (CNS), and NCI-H460 (lungs) cancer cell lines with IC50 values of 162, 142, 158, 164, and 147 μg mL−1, respectively.92 It has also been found that betanin induces dose- and time-dependent apoptosis of cells in the human chronic myelogenous leukemia (K562) cell line.93
In vitro experiments revealed that betalains extracted from B. vulgaris juice show pro-apoptotic effects on activated neutrophils and inhibit the neutrophil oxidative metabolism.94 In addition, pilot clinical trials have shown that short-term treatment with red beet extract improves joint function in people with knee joint discomfort.76,95
2.3 Applications of betacyanin pigments
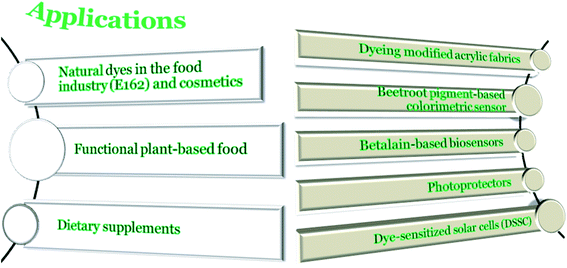
In Fig. 8, betacyanin applications are schematically presented. Natural plant extracts containing betacyanin pigments are increasingly used in food technology as a safe alternative to synthetic food colorants. According to Title 21 of the Code of Federal Regulations part 73.40 of the Food and Drug Administration (FDA), USA, and under the E-162 code in the European Union, beetroot powder rich in betanin is permitted as a natural red food colorant.3 Food preparations obtained in different industrial conditions can significantly vary in their stability and coloration due to the presence and the composition of betacyanin degradation products. Betalains are stable at pH ranging from 3 to 7 and they are suitable for dyeing low acidic and neutral foods. In addition, they may be stabilized by ascorbic acid. In contrast, due to instability at pH over 3, the application of anthocyanins in the coloration of such foods is not possible. Furthermore, the use of ascorbic acid also facilitates the degradation of anthocyanins. For this reason, the utilization of betalain pigments instead of anthocyanins for coloring food with a high amount of vitamin C or vitamin C-supplemented products seems to be more favorable.96,97 Beetroot extracts are utilized to emphasize the redness of dairy products such as tomato soups, sauces, pastes, desserts, jams, sweets, and jelly beans. They are also used to protect meat from discoloration and to extend its shelf-life.98 However, due to the unpleasant flavor of beetroot extracts due to their geosmin and pyrazine derivatives content, a membrane process during juice concentration is needed to be applied for commercial red beet application.14Due to health-related benefits, betalains are also regarded as natural dietary supplements.99 The effect of food supplements containing betalain-rich extracts (a betalain-rich supplement of red beetroot and a betacyanin-rich supplement of Opuntia stricta) on different atherosclerotic risk factors were tested among coronary artery disease patients. The results showed that the levels of glucose, total cholesterol, homocysteine, triglyceride, and low-density lipoprotein (LDL) of 48 male patients were decreased. Furthermore, betalain-rich supplements taken at a safe dose of 50 mg betalain/betacyanins for 2 week interventions reduced the systolic and diastolic blood pressures.100 In the past 20 years, a number of food and nutraceutical preparations have been developed from quinoa (Chenopodium quinoa Willd.) as well. Several clinical studies have demonstrated that quinoa supplementation exerts prominent effects on the cardiovascular, gastrointestinal, and metabolic health of humans.101
Natural plant pigments were also tested in the context of solar cell applications as a cheaper, faster, low energy, and environment-friendly alternative to dyes based on ruthenium complexes.102,103 Betanin, 2,17-bidecarboxy-betanin, vulgaxanthin I, extracted from red beetroot extract and betanidin-6-O-(6′,6′′-di-O-E-4-coumaroyl)-β-sophoroside from Bougainvillea were also tested toward the application as dye-sensitized solar cells (DSSC). The construction of betalain-based DSSC is possible due to the carboxylic groups in betalains, which serve as anchors after the immersion of TiO2 electrodes in betalain aqueous solutions at pH ≈ 3. The quantum yield of electron injection in betacyanin-based and betaxanthin-based DSSC is about 50% and 70%, respectively. In the case of betaxanthin-based DSSC, the total sunlight conversion efficiency was however smaller because of the blue-shifted absorption band.104 The electron injection from betanin to TiO2 is a two-electron, one-proton process.105,106
Enhanced light-harvesting and the photoconversion efficiency of nanocrystalline TiO2 sensitized with betanin was observed and revealed the self-assembly of betanin on the surface. Due to the fact that an aggregated betanin sensitizer improved the performance in DSSC, the mechanism of improved electron injection and collection in DSSCs with more aggregation as compared to monomeric betanin need to be regarded.107
Betanin is capable of injecting up to two electrons per photon absorbed into the ZnO conduction band in less than 15 ps.108 Betanin was also used for the light-harvesting process in ultra-stable ZnO nanocrystals modified with a carboxylate oligoethylene glycol shell system studied for utilization in H2 production under visible light irradiation.109
The photophysical properties of betanin in aqueous and alcoholic solutions were determined and formation of betanin electronically excited species was studied. Betanin has a short S1 state lifetime (π, π*) (6.4 ps in water), mainly determined by the efficient S1 → S0 radiationless relaxation, which probably requires a strong geometry change. Other processes, such as photoproduct formation or S1 → T1 intersystem crossing have been reported as virtually absent. In the absence of triplet excited state formation, the fast light-to-heat conversion was observed, supporting the consideration that betanin is a photoprotector in vivo.110,111
Due to the presence of the phenolic moiety within the betanin structure, which may react with 1O2, the colorimetric detection of reactive oxygen species is possible. Efficient singlet oxygen quenching by betanin in deuterated water was reported. The capacity of betanin to quench 1O2 supports the photo-protective role of betanin in vivo.112
In another study, a red beetroot pigment-based colorimetric sensor for detecting copper ions in drinking water was developed. In addition, an application for quantitative and visual colorimetric analysis was designed for the Android system smartphone. When Cu2+ concentration in drinking water increased, the red beet pigment solution gradually changed from bright purple to orange-red as it formed a complex by chelating Cu2+. The linear range of this detection system ranged from 4 to 20 μM and the limit of detection was 0.84 μM.113
Betanin can also compete with synthetic dyes in terms of the depth and color stability. The possibility of dying of modified acrylic fabrics with betanin was investigated. It was found that the optimal conditions during 45 min dyeing of acrylic fabrics with betanin were 50 °C and pH 5. The modified materials were resistant to color loss under the influence of water and the addition of cobalt(II) sulphate(VI) (CoSO4) ensured light resistance.114
Attempts are being made to develop cheap, green, and easy-to-use betalain-based biosensors. A good example is a need for the early detection of Bacillus species. The highly stable and virulent B. anthracis has been considered as a dangerous bioterrorism agent after the anthrax attack took place in 2001.115 Another threat is pathogenic B. cereus that grows on food.116 In order to detect the Bacillus species early, research on the application of betanin as a ligand in the new europium(III) complex, sensitive to calcium dipicolinate, CaDPA (the major component of the bacterial spore core) was conducted. In the presence of Eu(III) ions, betanin was converted into a water-soluble, non-luminescent orange complex. The addition of CaDPA changes the orange color of the [Eu(betanin)+] aqueous solution to magenta. The limit of detection of CaDPA is about 2.06 × 10−6 mol L−1. In addition, the complex is sensitive to CaDPA but not to other structurally similar aromatic, pyridinic, and acidic ligands.117,118
Beetroot extracts are under consideration as a mild alternative to organometallic reductants, such as NaBH4 and N2H4, and as stabilizing (growth-limiting) agents. Beetroot extract was exploited for the bottom-up synthesis of metal nanoparticles, leading to broad size and shape distributions.119
3 Chemistry of betacyanins and betalamic acid derivatives
3.1 Overview of betacyanin chemical reactions
Taking into account the pro-health nature of betacyanin pigments, their diversity, structural characteristics, and light-absorption properties, there is a growing need to supplement and broaden the knowledge on their chemistry. Understanding the reactions that betacyanins undergo may contribute to the determination of their fate and distribution in the human body, increase their stability, the development of betalain-based biosensors, screening assays and tailor-made probes, the preparation of betalain-based potential medicinal substances or cosmetics. In the following part of this contribution, we sum up the current knowledge obtained during the last decade on the chemical properties of betacyanins as well as new perspectives of the semi-synthesis of betalamic acid derivatives with extended chromophoric systems. The list of betacyanin derivatives semi-synthesized from plant sources and betacyanin degradation or chemical conversion products, with their structural identification by LC-MS and NMR methods, is presented in Table 2.| No. | Name | Trivial name or abbreviation | m/z [M + H]+ | LC-MS | NMR | Plant sources (Chem.)/(LC-MS)/(NMR) | References (Chem.)/(LC-MS)/(NMR) |
|---|---|---|---|---|---|---|---|
| 56 | 2-Decarboxy-betanin | 2-dBt | 507 | + | + | B. vulgaris/H. polyrhizus | 126/127 |
| 57 | 15-Decarboxy-betanin | 15-dBt | 507 | + | − | B. vulgaris | 122 |
| 58 | 17-Decarboxy-betanin | 17-dBt | 507 | + | + | B. vulgaris/H. polyrhizus | 122/127 |
| 59 | 2,17-Bidecarboxy-betanin | 2,17-dBt | 463 | + | + | B. vulgaris/H. polyrhizus | 126/127 |
| 60 | 2,15,17-Tridecarboxy-betanin | 2,15,17-dBt | 419 | + | − | B. vulgaris | 126 |
| 61 | 2-Decarboxy-neobetanin | 2-dNBt | 505 | + | − | B. vulgaris | 126 |
| 62 | 2,17-Bidecarboxy-neobetanin | 2,17-dNBt | 461 | + | − | B. vulgaris | 126 |
| 63 | 2,15,17-Tridecarboxy-neobetanin | 2,15,17-dNBt | 417 | + | − | B. vulgaris | 126 |
| 64 | 2,17-Bidecarboxy-xanbetanin | 2,17-dXBt | 461 | + | − | B. vulgaris | 52 |
| 65 | 2-Decarboxy-xanneobetanin | 2-dXNBt | 503 | + | + | B. vulgaris | 52 |
| 66 | 2,17-Bidecarboxy-xanneobetanin | 2,17-dXNBt | 459 | + | + | B. vulgaris | 52 |
| 67 | 2,15,17-Tridecarboxy-xanneobetanin | 2,15,17-dXNBt | 415 | + | + | B. vulgaris | 52 |
| 68 | 18-Chloro-betanin | Bt-Cl | 585 | + | + | B. vulgaris | 156 |
| 69 | 18-Chloro-17-decarboxy-betanin | 17-dBt-Cl | 541 | + | + | B. vulgaris | 156 |
| 70 | 18-Chloro-2,17-bidecarboxy-betanin | 2,17-dBt-Cl | 497 | + | + | B. vulgaris | 156 |
| 71 | 18-Chloro-2-decarboxy-betanin | 2-dBt-Cl | 541 | + | − | B. vulgaris | 156 |
| 72 | 18-Chloro-15-decarboxy-betanin | 15-dBt-Cl | 541 | + | − | B. vulgaris | 156 |
| 73 | Cysteinyl-2-decarboxy-xanbetanin | 2-dXBt-Cys | 624 | + | − | B. vulgaris | 149 |
| 74 | 2-Decarboxy-xanbetanin | 2-dXBt | 505 | + | − | B. vulgaris | 149 |
| 75 | N-Methyl-phenyl-betalain | mepBeet | 301 | + | + | B. vulgaris | 165 |
| 76 | N-Aryl-phenyl-betalains | dipBeet | 363 | + | + | B. vulgaris | 165 |
| 77 | 2,4-Dimethylpyrrole-betalain | BeetBlue | 289 | + | + | B. vulgaris | 166 |
| 78 | 7-Amino-4-methylcoumarin-betalain | cBeet120 | 369 | + | + | B. vulgaris | 167 |
| 79 | 7-Amino-4-trifluoromethylcoumarin-betalain | cBeet151 | 423 | + | + | B. vulgaris | 167 |
| 80 | 7-Amino-4-methylcoumarin-betalain | BtC | 369 | + | − | B. vulgaris | 168 |
| 81 | 17-Decarboxy-neobetanin | 17-dNBt | 505 | + | − | H. polyrhizus | 125 |
| 82 | 2-Decarboxy-phyllocactin | 2-dPc | 593 | + | + | H. polyrhizus | 125/127 |
| 83 | 17-Decarboxy-phyllocactin | 17-dPc | 593 | + | + | H. polyrhizus | 125/127 |
| 84 | 2,17-Bidecarboxy-phyllocactin | 2,17-dPc | 549 | + | + | H. polyrhizus | 125/127 |
| 85 | 2,15,17-Tridecarboxy-phyllocactin | 2,15,17-dPc | 505 | + | − | H. polyrhizus | 125 |
| 86 | 2-Decarboxy-hylocerenin | 2-dHc | 651 | + | + | H. polyrhizus | 125/127 |
| 87 | 17-Decarboxy-hylocerenin | 17-dHc | 651 | + | − | H. polyrhizus | 125 |
| 88 | 2,17-Bidecarboxy-hylocerenin | 2,17-dHc | 607 | + | + | H. polyrhizus | 125/127 |
| 89 | 2,15,17-Tridecarboxy-hylocerenin | 2,15,17-dHc | 563 | + | − | H. polyrhizus | 125 |
| 90 | 2-Decarboxy-neophyllocactin | 2-dNPc | 591 | + | − | H. polyrhizus | 125 |
| 91 | 17-Decarboxy-neophyllocactin | 17-dNPc | 591 | + | − | H. polyrhizus | 125 |
| 92 | 2,17-Bidecarboxy-neophyllocactin | 2,17-dNPc | 547 | + | − | H. polyrhizus | 125 |
| 93 | 2,15,17-Tridecarboxy-neophyllocactin | 2,15,17-dNPc | 503 | + | − | H. polyrhizus | 125 |
| 94 | 2-Decarboxy-neohylocerenin | 2-dNHc | 649 | + | − | H. polyrhizus | 125 |
| 95 | 17-Decarboxy-neohylocerenin | 17-dNHc | 649 | + | − | H. polyrhizus | 125 |
| 96 | 2,17-Bidecarboxy-neohylocerenin | 2,17-dNHc | 605 | + | − | H. polyrhizus | 125 |
| 97 | 2,15,17-Tridecarboxy-neohylocerenin | 2,15,17-dNHc | 561 | + | − | H. polyrhizus | 125 |
| 98 | 2-Decarboxy-xanbetanidin | 2-dXBd | 343 | + | − | B. alba | 140 |
| 99 | 2,17-Bidecarboxy-xanbetanidin | 2,17-dXBd | 299 | + | − | B. alba | 140 |
| 100 | 2-Decarboxy-xangomphrenin; | 2-dXGp | 505 | + | − | B. alba | 140 |
| 101 | 2,17-Bidecarboxy-xangomphrenin | 2,17-dXGp | 461 | + | − | B. alba | 140 |
| 102 | 2-Decarboxy-xanneobetanidin | 2-dXNBd | 341 | + | − | B. alba | 140 |
| 103 | 2,17-Bidecarboxy-xanneobetanidin | 2,17-dXNBd | 297 | + | − | B. alba | 140 |
| 104 | 2-Decarboxy-xanneogomphrenin | 2-dXNGp | 503 | + | − | B. alba | 140 |
| 105 | 2,17-Bidecarboxy-xanneogomphrenin | 2,17-dXNGp | 459 | + | − | B. alba | 140 |
| 106 | 2,15,17-Tridecarboxy-xanneogomphrenin | 2,15,17-dXNGp | 415 | + | − | B. alba | 140 |
| 107 | Cysteinyl-betanidin | Cys-Bd | 508 | + | − | B. alba | 149 |
| 108 | Cysteaminyl-betanidin | CSH-Bd | 464 | + | − | B. alba | 149 |
| 109 | N-Acetyl-cysteinyl-betanidin | NAC-Bd | 550 | + | − | B. alba | 149 |
| 110 | Dithiothreitol-betanidin | DTT-Bd | 541 | + | − | B. alba | 149 |
| 111 | Cysteinyl-gomphrenin | Cys-Gp | 670 | + | − | B. alba | 149 |
| 112 | Cysteaminyl-gomphrenin | CSH-Gp | 626 | + | − | B. alba | 149 |
| 113 | Dithiothreitol-gomphrenin | DTT-Gp | 703 | + | − | B. alba | 149 |
| 114 | N-Acetyl-cysteinyl-gomphrenin | NAC-Gp | 712 | + | + | B. alba | 149 |
| 115 | Glutathionyl-betanidin | GSH-Bd | 694 | + | + | B. alba | 140 |
| 116 | Glutathionyl-gomphrenin | GSH-Gp | 856 | + | − | B. alba | 140 |
| Number of betacyanin derivatives identified by a given method | 18 | 6 | |||||
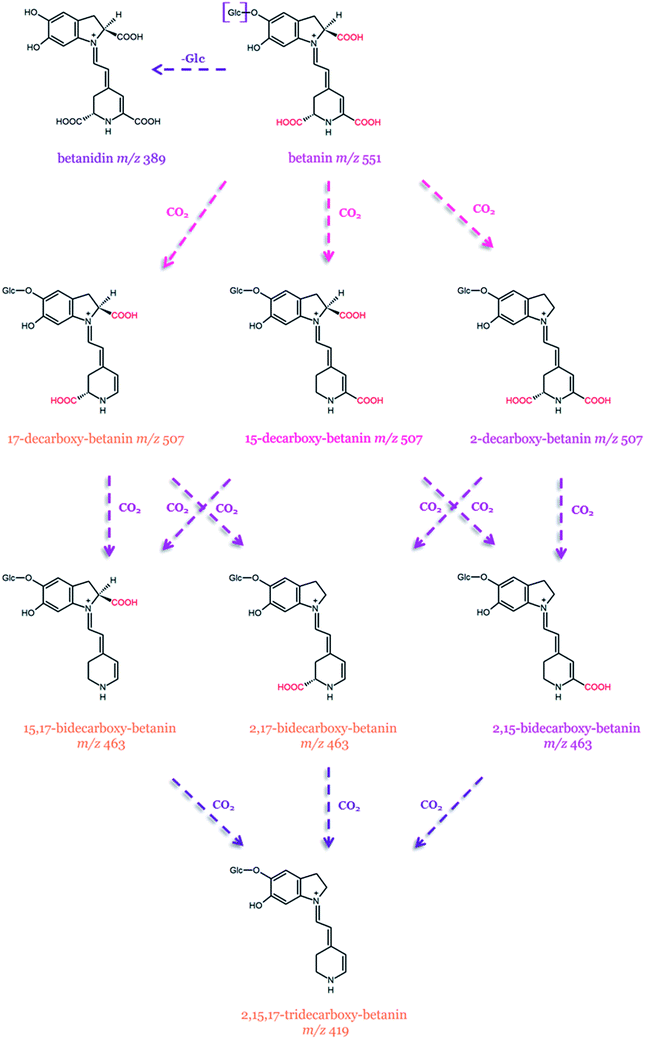 | ||
| Fig. 9 Possible pathways of betanin transformation by decarboxylation, dehydrogenation, and deglucosylation.129 The m/z values are from the [M + H]+ ions. | ||
The first heat-degradation structural studies on betanin from B. vulgaris, as well as betacyanins isolated from H. polyrhizus (betanin, phyllocactin, and hylocerenin), were performed by Herbach et al.122–124 and Wybraniec et al.125–127 Betanin was proven to be the most stable pigment, while the stability of phyllocactin and hylocerenin was seemingly enhanced due to the formation of red degradation products, exhibiting improved color retention in comparison to their precursors. Betanin degradation proceeded through hydrolytic cleavage, while hylocerenin dominated in decarboxylation and dehydrogenation. The degradation of phyllocactin involves the decarboxylation of the malonic acid moiety and the formation of betanin by demalonylation along with subsequent betanin degradation. After prolonged heating, dehydrogenation at C2–C3 carbons was also noticed.123–125,127
Decarboxylated and dehydrogenated betanin derivatives occurring at the highest concentrations in a betacyanin-rich red beetroot extract (RBE) were also characterized (Table 2).128 The main pigments in the RBE are betanin and its isoform with 17-decarboxy-betanin/-isobetanin, 15-decarboxy-betanin, and small quantities of 2-decarboxy-betanin/-isobetanin as well as 2,17-bidecarboxy-betanin/-isobetanin.128 Furthermore, mixtures of mono-, bi-, and tridecarboxylated betanins together with their corresponding neo-derivatives obtained from the RBE were identified as the heating degradation products.129 In addition, new isomeric decarboxylated derivatives, namely, 2,15-bidecarboxy-betanin and 15,17-bidecarboxy-betanin, were detected for the first time. These newly detected derivatives were also formed during heating experiments on red beetroot extract. Furthermore, the presence of 2,15,17-tridecarboxy-betanin was also acknowledged.129 Presumably, the presence of these additional betanin derivatives results from enhanced decarboxylation, which is an integral process during RBE preparation. It was also reported that the higher concentration of acetic acid during the thermal treatment of RBE contributes to a selective formation of 2-decarboxy-betanin/-isobetanin. The latter pigments are present in the RBE at a very low level. However, according to the report, the most decisive factors are the concentrations of the substrate and acetic acid. Higher RBE concentration favors the formation of 2,17-bidecarboxy-betanin/-isobetanin over 2,15-bidecarboxy-betanin.129
An effective method for the separation of newly formed derivatives was also proposed. A bunch of decarboxylated and dehydrogenated betacyanins obtained during mild thermal treatment of B. vulgaris juice was subjected to high-performance counter-current chromatographic (HPCCC) preparative fractionations.130 The mixtures composed of betacyanins with different polarity and physicochemical properties were separated in highly polar solvent systems containing a high concentration of ammonium sulfate and ion-pairs, aqueous-organic solvent systems including ion-pair reagents (trifluoroacetic acid, heptafluorobutyric acid). The application of both the solvent systems enabled the separation and purification of 2-decarboxy-betanin, 2,17-bidecarboxy-betanin, their corresponding isoforms, and neobetanin from a mixture composed mainly of betacyanins, neobetanin, and their decarboxylated derivatives (Table 2), as well as 17-decarboxy-neobetanin, 2,15,17-tridecarboxy-2,3-dehydro-neobetanin, 2,17-bidecarboxy-2,3-dehydro-neobetanin, 2,15,17-tridecarboxy-neobetanin, and 2-decarboxy-neobetanin from another mixture consisting of decarboxy- and dehydrobetacyanins. The results also show that the utilization of heptafluorobutyric acid as an ion-pair reagent is more suitable than a polar solvent system with trifluoroacetic acid because it contributes to the formation of more hydrophobic betacyanin ion-pairs.130 The separation of betacyanins from a processed B. vulgaris juice (betanin, isobetanin, neobetanin, and decarboxylated betacyanins) was also demonstrated in a food-grade, gradient solvent system consisting of sodium chloride, butanol, water, as well as different volumes of phosphoric acid and/or ethanol. The quality of isolation was dependent on the ethanol and acid concentrations with the lowest volume gradient, providing the best separation conditions. Betacyanins were eluted in the organic, upper mobile phase, which has a low salt content compared to the aqueous lower phase.131
The first study on the thermal decomposition of gomphrenin pigments present in the fruit juice of B. alba was carried out and the chromatographic profiles, as well as fragmentation pathways of decarboxylation and dehydrogenation products of gomphrenin (Table 2), were determined by LC-DAD-ESI-MS/MS and LC-MS-IT-TOF.16 The short-term treatment of B. alba juice revealed the profiles of mono- and bi-decarboxylated gomphrenins analogous to betacyanins from heated beetroot juice.
Prolonged heating of B. alba extract acidified with acetic acid led to the formation of dehydrogenated derivatives as a result of gomphrenin derivative oxidation. The presence of neogomphrenin was detected at a low concentration level in the heated juice, which is opposite to RBE rich in neobetanin. In addition, in contrast to the results of the RBE heating experiments,129 the isomeric 2,15- and 15,17-decarboxylated derivatives were not detected in the heated samples. The above experiments indicated that the chromatographic differences between gomphrenin and betanin derivatives arose from the position of glucosylation in betanidin at carbon atoms C-5 or C-6.16
Sawicki and Wiczkowski investigated the impact of boiling and spontaneous fermentation on the betalain profile and content in beetroot. The boiling and fermentation of beetroot decreased the content of the betalains by 51–61% and 61–88%, respectively. Processes occurring during boiling and spontaneous fermentation such as heating, softening, leaching, and acidification together with the matrix effect may be responsible for the changes. In addition, the microbial activity and matrix softening occurring during fermentation caused a release of betalains, which is responsible for the potent antioxidant capacity of the formed beet juice.132
Sawicki et al. determined the impact of three technological processes (fermentation, boiling, and microwave-vacuuming treatment) and in vitro digestion on betalain profiles. The content of betalains in the products was reduced by 42–70%. Microwave-vacuuming treatment has contributed to the degradation of betanin at the lowest level and may be regarded as the best treatment method. In addition, the content of betalains released from the beetroot products after in vitro digestion reached the range of 0.001–0.10%, and the number of compounds identified in the digestion phases was decreased.133
Betanidin is the basic structure of all the betacyanins and the only one with the catechol moiety (5,6-dihydroxyl moiety). Studies show that during the oxidation of betanidin, the pigment is most likely converted into three tautomeric quinoid derivatives (Fig. 10). Interconvertions, which occur slowly at pH 4–7 and quickly in a more acidic environment, are likely to result in the formation of dehydrogenated and decarboxylated derivatives. Interconversions between the three tautomeric quinoid forms of oxidized betanidin are based on the proven oxidation pathways of DOPA and dopamine.137,138
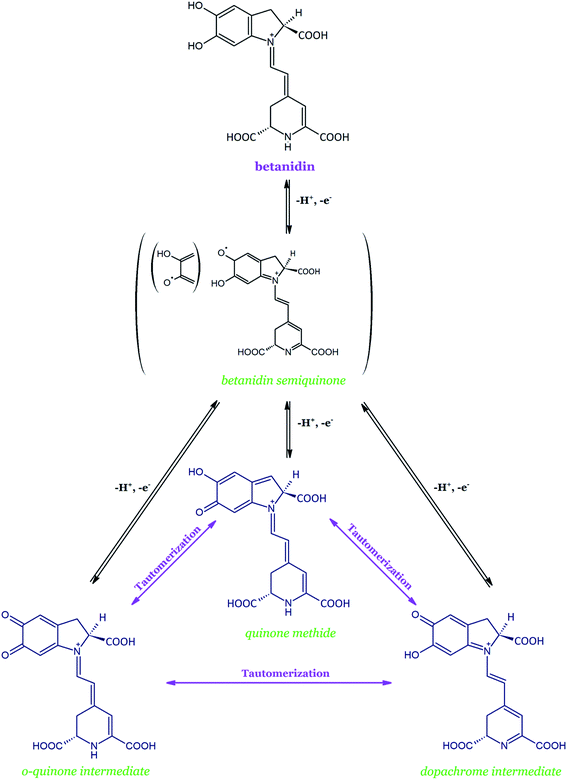 | ||
| Fig. 10 Scheme of the initial transformations of betanidin during its oxidation and with an indication of the intramolecular conversions between three tautomeric forms based on.134 | ||
The initial oxidation of betanidin should generate the transient radical cation, which rapidly loses a proton and forms a neutral phenoxy radical; it is equivalent to the donation of a hydrogen atom by betanidin or to the loss of a proton with further electron transfer. In the next step, the loss of a second proton and electron results in the formation of a two-electron oxidized form, i.e., one of the three quinoid tautomers. Presumably, not only the betanidin o-quinone with its characteristic absorption spectrum (λmax at 550 and 400 nm) is formed on the first oxidation stage but it is assumed that the two other quinoid derivatives are also possible: dopamine derivative and/or quinone methide. The discussion of the balance between the three tautomeric forms of quinoid remains open.135 As a result, 2,17-bidecarboxy-xanbetanidin (synonym of 2,17-bidecarboxy-2,3-dehydro-betanidin) and 2-decarboxy-xanbetanidin (synonym of 2-decarboxy-2,3-dehydro-betanidin) are postulated as the principal oxidation products formed (Table 2). The recently proposed xan is derived from the hypsochromic shift toward the yellow color of the resulting compounds.
The further oxidation of 2,17-bidecarboxy-xanbetanidin again involves the formation of one of the two possible quinoid forms as the oxidized intermediates, e.g., the dopachromic derivative of the indolic system. Subsequent rearrangement of the conjugated system of the originated structures into the neo-forms (14,15-dehydrogenated derivatives) is possible which finally leads to 2,17-bidecarboxy-xanneobetanidin (2,17-bidecarboxy-2,3-dehydro-neobetanidin), i.e., a compound doubly dehydrogenated at the positions C-2,3 and C-14,15.134–136
In contrast to betanidin oxidation, no compound that could be regarded as the betanin oxidation intermediate (quinone methide) was detected.139 This could be a consequence of its instability and fast rearrangement combined with decarboxylation. The main compound formed during the first step of betanin oxidation is 2-decarboxy-xanbetanin (Table 2), which exhibits a characteristic protonated molecular ion [M + H]+ at m/z 505 and an absorption maximum at λmax = 446 nm (Fig. 11). It is not excluded, however, that the generated 2-decarboxy-xanbetanin rearranges to a more stable structure of 2-decarboxy-neobetanin (synonym of 2-decarboxy-14,15-dehydro-betanin). According to the alternative pathway proposed by Wybraniec et al.,135 the formation of 2-decarboxy-neobetanin can result from a rearrangement of the quinone methide generated from 2-decarboxy-betanin. This alternate pathway does not include the presumably more stable product, 2-decarboxy-xanbetanin. Further, the decarboxylation of 2-decarboxy-xanbetanin presumably results in the formation of 2,17-bidecarboxy-xanbetanin. The most hydrophobic product of betanin degradation/oxidation is 2-decarboxy-xanneobetanin, characterized by a protonated molecular ion at m/z 503 and maximum absorption at λmax 422 nm, followed by 2,15,17-tridecarboxy-xanneobetanin.
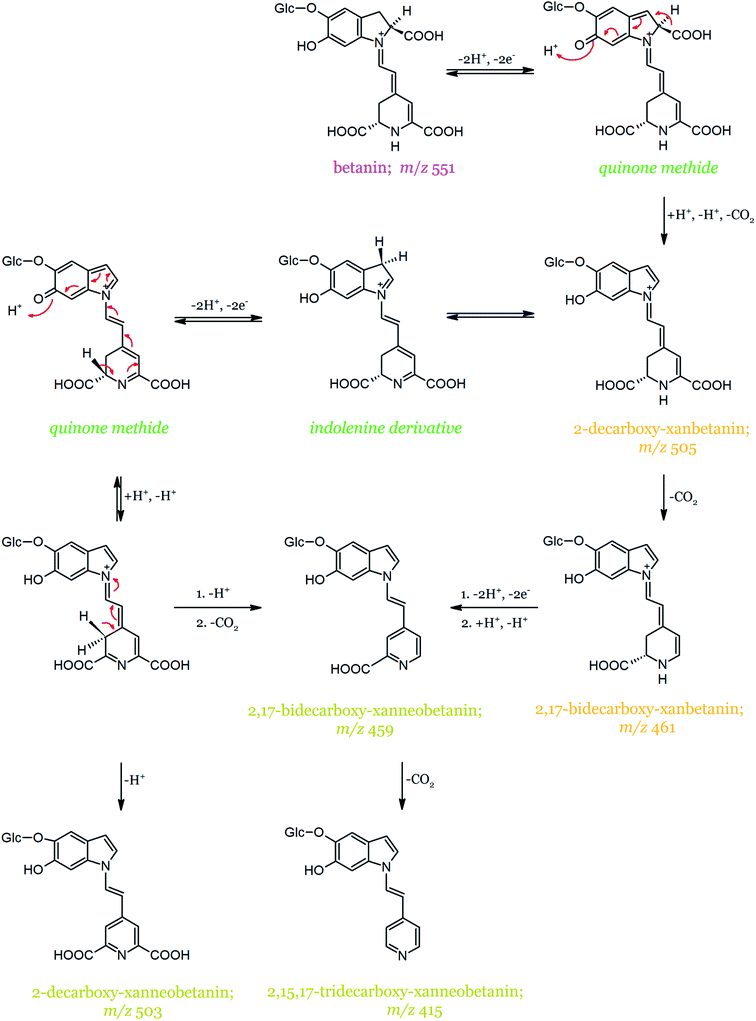 | ||
| Fig. 11 Proposed mechanism of betanin oxidation proceeding through the quinone methide intermediate based on.134,136 | ||
In Fig. 12, the schematic formation of dehydrogenated betanin derivatives is presented along with 2,3-dehydro- and 14,15-dehydro-positions marked on particular pigment structures, together with their abbreviations (xan- and neo-, respectively).52
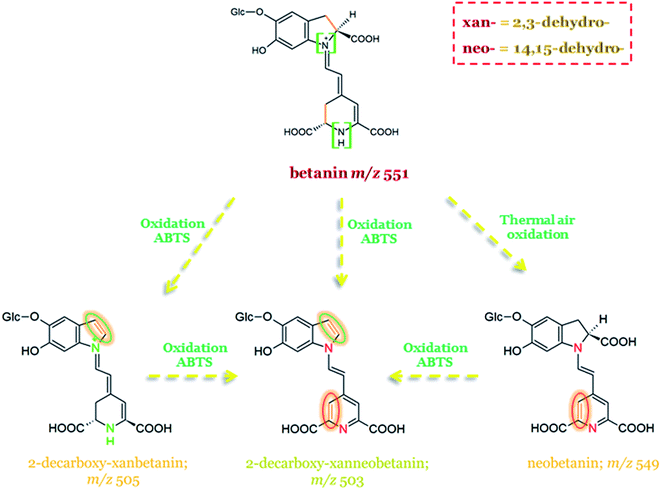 | ||
| Fig. 12 Possible pathways of betanin oxidation with dehydrogenation positions marked on dehydrogenated derivative structures.52,136 | ||
In the case of gomphrenin oxidation by the ABTS cation radical, except for its decarboxylated and dehydrogenated derivatives, the formation of betanidin as well as betanidin derivatives was detected. It is assumed that the rearrangement of dopachromic derivative affects the hydrolysis of the glucosidic bond. This is opposite to betanin, which is not deglucosylated during oxidation in the same reaction conditions. Therefore, it is important to notice that the position of betanidin glucosylation at carbon C-5 or C-6 has a significant influence on the differences in the reactivity of both the 5-O- and 6-O-glucosides.140
It was reported that metal cations, e.g., copper, aluminum, tin, and iron, accelerate the degradation of betanin even at trace amounts. It is worth noting that the release of metals from food packaging materials may also catalyze the unwanted betacyanin degradation.141 The results of high-resolution mass spectrometry experiments (HRMS) and NMR structural studies on key dehydrogenation products generated during the oxidation of betanin, neobetanin, and decarboxylated derivatives by ABTS cation radical as well as by Cu2+ complexation confirmed that Cu2+ catalyzed oxidation of betanin and 2-decarboxy-betanin leads to the formation of neo-derivatives (14,15-dehydrogenated betanins). It has been proven for the first time that betanin oxidation is possible in the dihydropyridine ring and this can be achieved presumably by omitting the stage of quinone methide formation in the dihydroindole system (Fig. 13). The additional results obtained for Cu2+-catalyzed oxidation of 17-decarboxy-betanin and 2,17-bidecarboxy-betanin suggest the possibility of formation of xan-derivatives, similar to the results obtained for oxidation by the ABTS cation radical. In this case, the factor determining the oxidation direction is presumably the absence of the carboxyl moiety at carbon C-17, clearly changing the geometry of the complex formed with Cu2+. In addition, the exclusive effect of 2,3-dehydrogenation in 17-decarboxy-betanin is probably supported by the concurrent occurrence of decarboxylation at carbon C-2.52
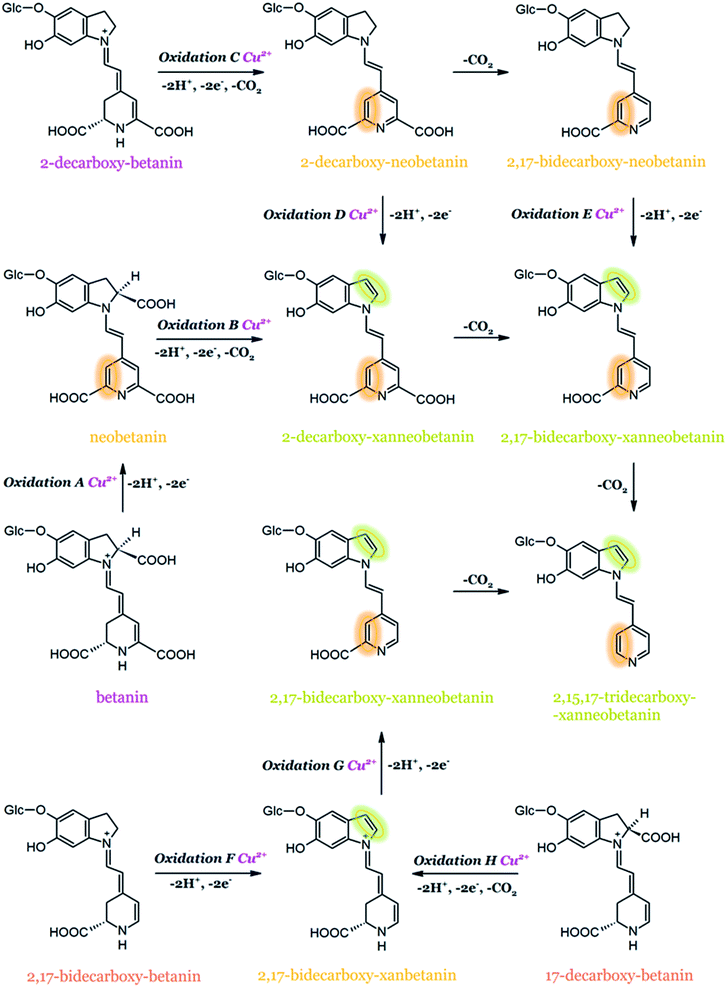 | ||
| Fig. 13 Proposed general oxidation mechanism for betanin and its derivatives, superimposing the effect of Cu2+-catalyzed oxidation with an indication of marked 2,3- and 14,15-dehydrogenation positions based on.52 | ||
The first studies on the possibility of conjugation of betacyanin reactive quinoids with sulfhydryl scavengers such as glutathione (GSH), cysteine (Cys), N-acetylcysteine (NAC), cysteamine (CSH), and DL-ditiothreitol (DTT) were performed recently (Table 2, Fig. 14).140,149 The formation of glutathionylated quinoids, following betanidin and gomphrenin oxidation, confirmed the presence of quinoid forms in the products of pigment oxidation. The chemical structure of glutathionylated betanidin was confirmed by NMR studies. It was also reported that glutathione reacts with quinones at the C-4 carbon of the betanidin ring. On the contrary, the conjugation of the betanin quinoid intermediate (quinone methide) with glutathione was not observed, presumably because of a steric hindrance at the C-7 carbon of betanin, which presumably confirms that the quinoid form generated during betanin oxidation is a quinone methide.140
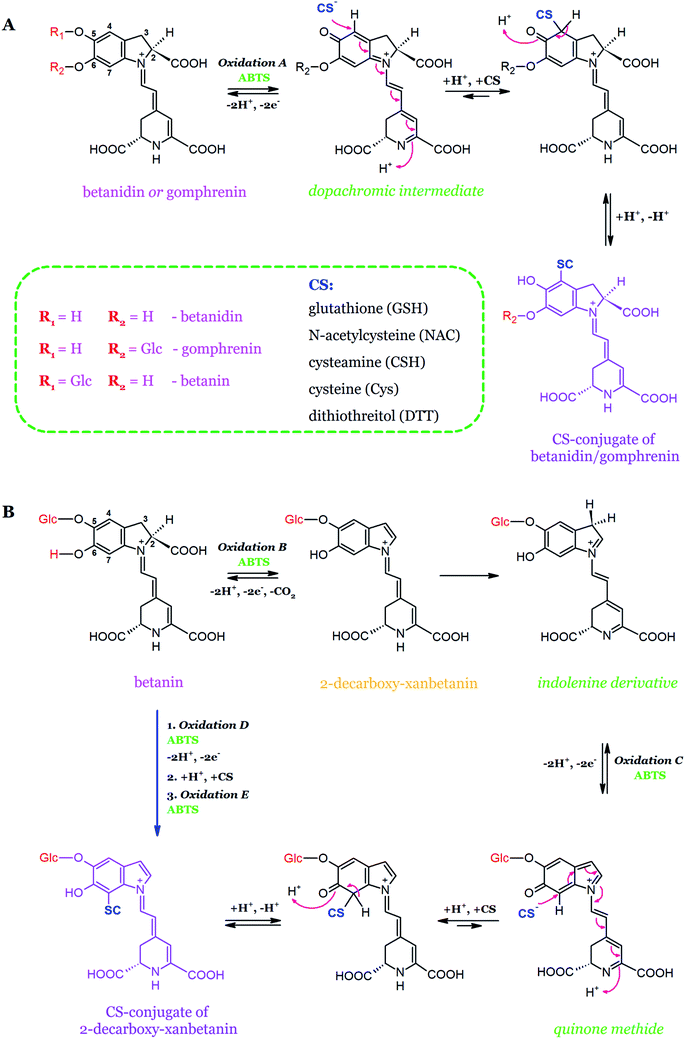 | ||
| Fig. 14 Schematic representation of the conjugation mechanism of betanidin and gomphrenin (A) with sulfhydryl radical scavengers as well as the oxidation of betanin to 2-decarboxy-xanbetanin (B) through the quinone methide intermediate with the following conjugation based on.149 | ||
In order to determine the possibility of attaching thiols to the C-7 carbon of the betanin quinoid, additional research was performed using low molecular weight thiols (Cys, CSH, NAC, DTT). Furthermore, since the conjugation of betacyanins with the thiol groups may generate new molecules with the modified chemical and biological properties, the conjugation reactions with quinones derived from betanidin and gomphrenin were also performed (Fig. 14). The chemical structure of N-acetylcysteinyl-gomphrenin, generated with the highest yield, was determined by the NMR method. The dopachrome derivative from gomphrenin oxidation undergoes conjugation at the C-4 carbon atom. In addition, cysteine conjugate with the oxidized betanin derivative was produced for the first time. The results suggest that the formation of quinone methide during the oxidation of betanin and the production of dopachrome derivative during the oxidation of gomphrenin is highly possible.149
The results confirm so far that gomphrenin is able to form conjugates with a higher yield than betanin but the ability to form stable conjugates with dehydrogenated betanin derivatives produced during the oxidation of betanin or its intermediate conjugates may also favor this pigment as a valuable food protection component.149 From the perspective of the food industry, this aspect appears to be relevant as the biological activity of gomphrenin and betanin in combination with the possibility of adduct formation between the thiols and betacyanin quinoids may contribute to the increased quality and durability of food products such as meat.146 From another perspective, adduct research can significantly contribute to the development of detection methods for betacyanin quinoids formed in the complex food matrices as well as to research the bioavailability and distribution of betacyanins in humans. The sulfhydryl part of the molecules may modulate their ability to penetrate the biological membranes as well as their absorption and metabolism. Many attempts have been made in the food chemistry to inhibit the formation of oxidative cross-linking proteins, affecting the quality, water content, and red color of meat products. In this respect, CS-conjugates of betacyanins may play a significant role in blocking the thiol groups in food proteins, contributing to improving the food quality.150–152
Whether betacyanins act as effective antioxidants without adverse pro-oxidative effects depends on how quickly their oxidized derivatives gradually degrade through decarboxylation and dehydrogenation as well as through other transformations, including hydrolysis and the final polymerization of betalamic acid and cyclo-DOPA to inactive forms.134,136 So far, no research on the toxicity and stability of quinones produced from betacyanins has been conducted. The literature data are lacking on the pro-oxidative properties of betalains. In addition, there are questions whether betalains are susceptible to undesired metabolic activation and if the blocking of the sites prone to the formation of reactive forms in molecules is required for betalain application as potential therapeutic agents.
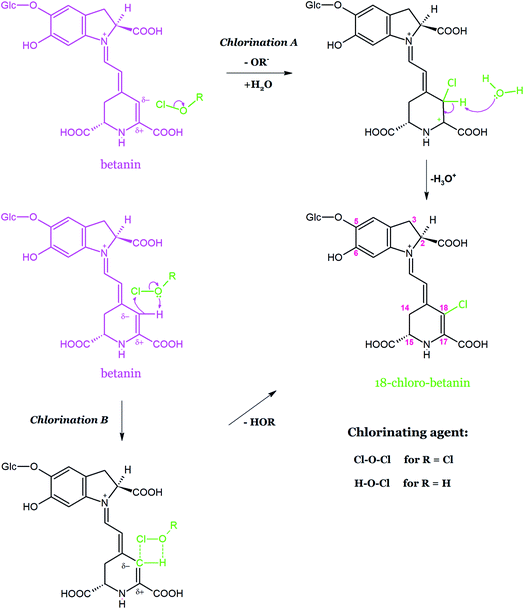
For neobetanin, no chlorinated products in the reaction mixtures were detected. The fact that the pyridinic ring within the neobetanin structure cannot be chlorinated to form the detectable pigment products indicates that only the unsaturated bond is attacked, preferably at C-18, due to its partial negative charge.154 Monochloro-betanin and monochloro-betanidin were formed with the highest yield at pH 3–5 while at higher Cl− concentrations, the efficiency was dramatically decreased, suggesting that the generated Cl2 is not the chlorinating agent in the presence of sodium hypochlorite. The low activity of Cl2 during betanin and betanidin chlorination compared to HOCl and/or Cl2O was explained by a special position (C-18) of the attack by HOCl and/or Cl2O. In the case of the MPO/H2O2/Cl− system, the highest efficiency of monochloro-betanin/-betanidin generation was observed at pH 5.
In another study,155 additional chlorination experiments were extended to decarboxylated betacyanins (Table 2). The comparison of the chromatographic profiles of the chlorinated decarboxylated betanins and betanidins generated under the activity of hypochlorous acid revealed two different directions of retention changes in relation to the corresponding precursors. The chlorination of betacyanins decarboxylated at C-17 (17-, 2,17-bi-, and 2,15,17-tri-decarboxy derivatives) resulted in higher retention times in comparison to the corresponding substrates. In contrast, the non-decarboxylated betacyanins as well as their 2- and 15-decarboxy-derivatives exhibited lower chromatographic retention after chlorination.155
The identification of the chlorinated betacyanins was completed by a further study on the chlorination mechanism and the position of the electrophilic substitution in betacyanins by high-resolution mass spectrometry and further structural analyses by NMR techniques,156 which confirmed that the chlorination position in betanin and decarboxylated betanins occurs within the dihydropyridinic moiety at the C-18 carbon.156
According to an electrophilic mechanism based upon the leaving group ability from Cl+ in HOCl (–OH−) and in Cl2O (–OCl−), the reaction paths between betanin and HOCl/Cl2O were proposed (Fig. 15).157 The mechanism was supported by the NMR elucidation results as well as by the inactivity of neobetanin toward chlorination.154 Chlorination version A shows the position of Cl+ attack on C-18 carbon within the betanin structure. The failure in the chlorination of betanin with Cl2, which is an active oxidizing agent, confirmed that Cl+ leaving from HOCl or Cl2O can be the chlorinating factor. Also, the hypothesis that H2OCl+ may be regarded as a chlorinating factor carrying Cl+ is intriguing but has not been confirmed for decades.158
In the chlorination version B, a cyclic intermediate159 is formed during the electrophilic attack of the whole HOCl or Cl2O molecules as a result of the polarization of Cl–O bond along with the stabilizing effect of hydrogen bonding. The oxidation of C-18 carbon in betanin did not take place because of its negative charge, making the nucleophilic attack of oxygen159 impossible.
Since fluorescent probes have been promising analytical tools for the rapid and specific detection of HOCl/OCl−, the HCSe and HCS probes were tested in the determination of the betalains' anti-hypochlorite activity. The comparison of the in vitro anti-hypochlorite activities of a betalain-rich red beetroot extract (RBE) with its pure betalainic pigments revealed that the extract had the highest anti-hypochlorite activity, far exceeding the activity of all of the betalainic derivatives and selected reference antioxidants.160 In addition, the pilot clinical studies showed that the short-term treatment with RBE improved the function and comfort of knee joints in individuals with knee distress resulting from increased hypochlorous acid formation.76
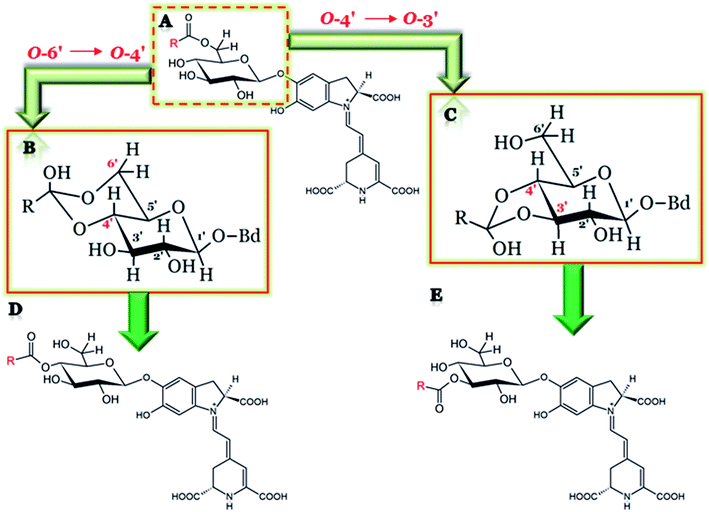
A series of new isomers of various 6′-O-acylated betacyanins as well as decarboxylated betacyanins formed by intramolecular pH-dependent acyl migration between adjacent hydroxy groups on polyhydroxy compounds in aqueous solutions was chromatographically characterized.18 The migration rate was markedly accelerated under alkaline conditions at pH 10.5, however, always favoring the 6′-O-position. The phenomenon of acyl migration was less apparent at pH below 7.0. Partial rearrangement may result in the formation of 3′-O- and 4′-O-acylated compounds. In malonylated betacyanins and 17-decarboxy-betacyanins, the 3′-O-acylated forms were presumably the most polar isomers and were eluted first in RP-HPLC, whereas the 4′-O-forms were characterized by retention higher than the 6′-O forms. In contrast, in 2-decarboxy- and 2,17-bidecarboxy-betacyanins, both 3′-O- and 4′-O-acyl-betacyanins were eluted before the 6′-O-acylated forms. The study on the acyl migration effect in 4′-O-malonyl-betanin revealed a strong tendency to reverse acyl migration (4′ → 6′) and also partial rearrangement (4′ → 3′), leading to the monoester regioisomeric distribution in the proportion 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 [%] in relation to 6′-O-, 4′-O-, and 3′-O-, respectively.
6 [%] in relation to 6′-O-, 4′-O-, and 3′-O-, respectively.
A new malonyl betacyanin, 6′-O-malonyl-amaranthin (celoscristatin), as well as 4′-O-malonyl-amaranthin, formed as a result of the malonyl group migration in celoscristatin, were also identified in Celosia cristata Linn. callus culture.29
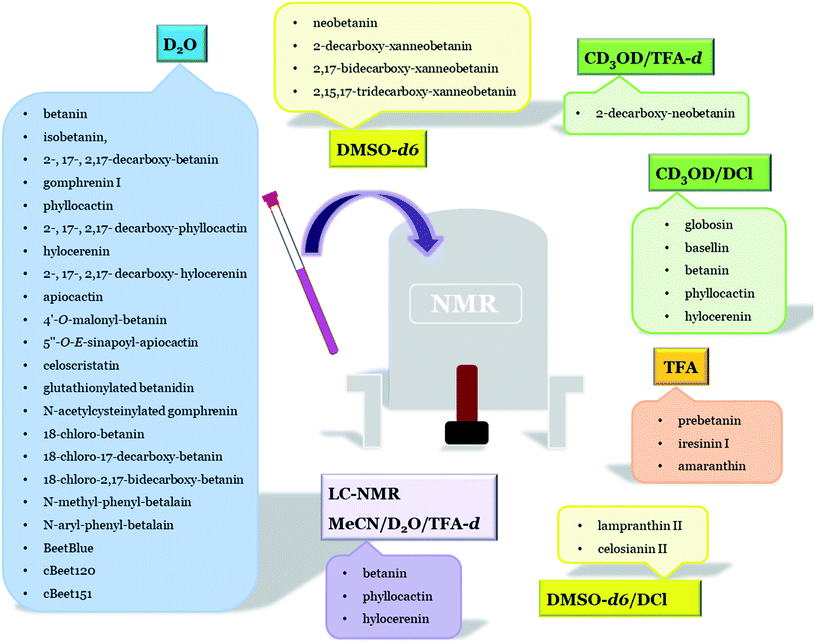
The key products formed after betanin decomposition were identified and their structures were confirmed by NMR analysis. The structural elucidation of 2-, 17-, and 2,17-decarboxy-betacyanins generated during the decarboxylation of betanin, phyllocactin, and hylocerenin isolated from H. polyrhizus fruits was performed by Wybraniec et al.127 A variety of coupled spin systems of all the analyzed compounds were successfully distinguished and assigned by 1H NMR, 1D TOCSY, gCOSY, and NOESY spectra in D2O. Due to gHSQC correlations, the 13C chemical shifts for all the carbons directly bound to the protons were unambiguously assigned, while the gHMBC (the 1H–13C long-range) correlations enabled the establishment of the chemical shifts of the quaternary carbons. In addition, the first 1H NMR spectra of 2-decarboxy-hylocerenin together with all the studied 17-decarboxy- and 2,17-bidecarboxy-betacyanins were received. Furthermore, the 13C NMR spectra of the decarboxylated betacyanins were obtained for the first time.127
The NMR spectra of amaranthin and iresinin I were recorded in trifluoroacetic acid.61 The structures of the two other acylated betacyanins and lampranthin II from the petals of Lampranthus peersii and L. sociorum as well as celosianin (previously named as celosianin II) from the cell suspension cultures of Chenopodium rubrum were elucidated as 6′-O-E-feruloyl-betanin and 2′′-O-E-feruloyl-β-(1′′,2′)-glucuronosyl-betanin, respectively, by 1H NMR. All 1D (normal and NOE difference) and 2D spectra (COSY) were registered in acidic DMSO-d6 containing traces of DCl.58 A low field shift of the protons H-6′A and H-6′B of the glucosylic moiety in lampranthin II caused by acylation indicated an attachment of the feruloyl moiety at the C-6′ carbon. For celosianin II, the NOE difference spectra indicated that the β-glucuronosyl moiety was bound to C-2′ of the β-glucosyl moiety. The presence of the feruloyl moiety attached at C-2′′ of β-glucuronosyl was evident from the low field shift of H-2′′.58
In addition to betanin and phyllocactin in H. polyrhizus fruits, another characteristic pigment, hylocerenin [6′-O-(3′′-hydroxy-3′′-methyl-glutaryl)-betanin], was also characterized by 1D and 2D (COSY) NMR techniques in deuterated methanol (CD3OD) containing traces of deuterium chloride (DCl).56 Other unknown pigments, 2′-O-β-apiofuranosyl-betanin and 2′-O-β-apiofuranosyl-phyllocactin, were also elucidated by 1H and 13C NMR techniques in non-acidified D2O.11 The structure of a new malonyl derivative, celoscristatin (6′-O-malonyl-amaranthin), isolated from C. cristata callus culture was confirmed by NMR analysis in D2O.29
In order to determine the oxidation mechanism of betanin, structures of tentatively identified key dehydrogenation products of betanin, decarboxylated betanin, and neobetanin oxidation by the ABTS cation radical were elucidated by NMR.52 The 1H and 2D (COSY, TOCSY, HSQC, HMBC, and NOESY) measurements were performed in DMSO-d6 for all the studied compounds except for 2-decarboxy-neobetanin, which was analyzed in CD3OD acidified with 0.01% deuterated TFA. The structures of five neo- and xanneo-derivatives, namely, neobetanin, 2-decarboxy-neobetanin, 2-decarboxy-xanneobetanin, 2,17-bidecarboxy-xanneobetanin, and 2,15,17-tridecarboxy-xanneobetanin, were confirmed.52
The structures of gomphrenin as well as its acylated derivatives, globosin and basellin, (gomphrenin I, II, and III, respectively) were elucidated by NMR spectroscopy by Heuer et al.24 The 1H and 2D COSY NMR spectra were recorded in CD3OD containing traces of DCl. The presence of acyl (4-coumaroyl- and feruloyl-) moieties at the C-6′ carbon in globosin and basellin, respectively, was indicated by a low field shift of the corresponding protons H-6′A and H-6′B. Non-typical 1H chemical shift differences between gomphrenin I and globosin and basellin suggested intramolecular stacking occurring in the latter two compounds.24 In another attempt, gomphrenin was identified by means of 1H, selective NOESY, and TOCSY as well as 2D NMR (COSY, NOESY, HSQC, and HMBC) techniques in D2O.69 By NOESY-correlation spectral analysis, it was demonstrated that the glucopyranosyl group is linked to the phenolic group at the C-6 carbon of gomphrenin, which differentiated it from betanin.
The structures of glutathionylated conjugate of betanidin140 and N-acetylcysteinylated gomphrenin149 were established by NMR analysis, confirming the conjugation position at the C-4 carbon, thus indicating the presence of a dopachromic intermediate during gomphrenin oxidation. All 1H and 2D NMR (COSY, HSQC, HMBC, TOCSY, and NOESY) analyses were accomplished in non-acidified D2O. The attachment position of the glutathionyl moiety was primarily indicated by the strong long-range correlation of the H-12a/b′ protons to quaternary C-4 and C-9 carbons as well as a weak correlation to C-8. The lack of the H-4 signal in the 1H spectrum unambiguously confirmed this position.140,149 In the case of N-acetylcysteinylated gomphrenin, the attachment position of the N-acetyl-cysteinyl moiety was initially suggested by a strong, long-range correlation of H-6a/b′′ protons to quaternary carbon C-4. In addition, the lack of the H-4 signal in the 1H spectrum definitely confirmed this position. Furthermore, the CS-conjugation position in gomphrenin was supported by the downfield chemical shift of the H-6a/b′′ proton signal compared to the signal obtained for free N-acetyl-cysteine.149
In order to prove the position of chlorination in betanin and its decarboxylated derivatives, structural studies were also performed.156 The structure elucidation of 18-chloro-betanin, 18-chloro-17-decarboxy-betanin, and 18-chloro-2,17-bidecarboxy-betanin confirmed that the chlorination position in betanin occurs within the dihydropyridine moiety at carbon C-18. The results of 1H NMR, COSY, and TOCSY analyses obtained for 18-chloro-betanin in non-acidified D2O enabled to distinguish and characterize several diagnostic, conjugated spin systems characteristic for betanin (H-2, H-3ab; H-11, H-12; H-14ab, H-15) in D2O. The dihydroindolic system was indicated by the HSQC correlations of H-2, H-3ab, H-4, and H-7 with their respective carbons. In the dihydropyridine system, the correlations of H-14ab and H-15 with their respective carbons in the HSQC spectra were visible.156
The structural studies of the newly semi-synthesized pseudo-natural pigments based on betanin hydrolysis were also reported. The structures of various compounds semi-synthesized by coupling betalamic acid with N-methyl anilines, N-aryl anilines, and blue solid named BeetBlue as well as 7-amino-4-methylcoumarin- and 7-amino-4-trifluoromethylcoumarin-betacyanins (cBeet120 and cBeet151) were accomplished by NMR analysis in D2O.165–167
4 Chemistry of betacyanins and their semi-synthetic derivatives based on betanin hydrolysis
4.1 Hydrolysis of betanin
Betanin is susceptible to acid- and base-catalyzed hydrolysis, leading to the formation of colorless cyclo-DOPA-5-O-β-D-glucoside and fairly stable bright yellow betalamic acid (Fig. 18). Recently, new light was shed on betanin hydrolysis mechanism.169 The impact of temperature and pH on the rate of betanin hydrolysis was examined. The mechanism of betanin decomposition was studied in the phosphate solution at pH ranging from 2 to 11 and temperature of 60–85 °C. It was postulated that the fully protonated betanin form is reactive toward the nucleophilic attack of water and the intramolecular hydrogen bond between the carboxylic group at C-2 carbon and N-1 nitrogen stabilizes the transition state, leading to the hydrated betanin. The N-1 nitrogen atom was expected to be protonated in the acidic media and to form a leaving group. The protonation of the N-16 nitrogen atom enhances the electrophilicity of C-11, C-13, and C-17 carbons and catalyzes betanin hydrolysis, finally contributing to the opening of the 2,6-dicarboxy-1,2,3,4-tetrahydropyridine ring of betalamic acid. It was indicated that the hydrogen phosphate ion may act as a general base at pH 8.2, even at a low buffer concentration. The formation of cyclo-DOPA and 2,6-dicarboxy-4-methylpyridine as the products of betanin degradation in an alkaline environment at 130 °C identified by IR spectroscopy was reported in the pioneering work of Wyler and Dreiding on betanin purification by crystallization.47,170 In contrast to that study, 2,6-dicarboxy-4-methylpyridine was not detected.169 However, the presence of cyclo-DOPA, betalamic acid, and decarboxylation/oxidation products was observed.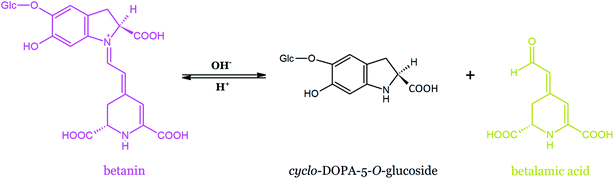 | ||
| Fig. 18 Hydrolysis of betanin leading to the formation of betalamic acid and cyclo-DOPA-5-O-β-D-glucoside. | ||
Based on theoretical calculations, the double protonation of the nitrogen atom within the tetrahydropyridine ring cannot be excluded from what was observed for the amino dicarboxylic acids of simpler structures.169
Next to hydrolysis, betacyanins are also sensitive to water-catalyzed isomerization. A study on the hydrolytic stability of betanin Bn, indicaxanthin BtP, and an artificial betalainic coumarin BtC soluble hydrogen bond donating 2,2,2-trifluoroethanol (TFE) was conducted.171 The hydrolytic stability in water was as follows: BtP > Bn > BtC. Improved hydrolytic stability of the abovementioned betalains in hydroalcoholic solutions was noticed with an increase in the TFE co-solvent. The hydrolysis rate of betalainic coumarin in water was 700-fold greater than in TFE, whereas the use of TFE as a co-solvent reduced the hydrolysis of betanin and indicaxanthin by 20 and 100-times, respectively. The proportion of water and TFE in the co-solvent mixture determined the hydrogen-bond donating capacity, polarity, and ionization power. However, the most important factor of the enhanced hydrolytic stability of the betacyanins is probably due to the low nucleophilicity of TFE. In addition, the fluorescence quantum yields of betaxanthins were also increased with the presence of TFE.171
4.2 One step semi-synthesis of betalains from betalamic acid-derivatized support
So far, all the proposed methods of the de novo synthesis of betalains achieve very low efficiency; therefore, the best way to obtain individual betalain derivatives is to isolate them from the biological material and purify. However, this makes the whole process highly time-consuming due to the plant matrix that is rich in various compounds, especially polysaccharides. The process of betalain formation was simplified using a novel betalamic acid-derivatized support (Fig. 19).172 Betalamic acid was produced during betanin hydrolysis from B. vulgaris extract in the presence of cross-linked polystyrene resin at room temperature and nitrogen atmosphere. After the release of betalamic acid and neutralization with acetic acid, the reaction of imine formation between the aldehyde group of betalamic acid and the free primary amine groups present in the matrix surface was performed, resulting in the immobilization of betalamic acid in the resin. The derivatization of the resin with betalamic acid was indicated by the resin color change from pale to intense yellow. After washing and drying at room temperature, the novel solid support was generated and characterized by SEM. The material exhibited the spectroscopic properties of a pseudobetaxanthin. Then, the resin from the betalamic acid-derivatized support was tested toward the production of betalains. As model amines, individual dopamine, tyramine, indoline, and pyrrolidine were added to the support turning the color of the solutions from colorless into yellow in the case of dopamine, tyramine, and pyrrolidine, as well as into purple in the case of indoline. Then, a Schiff's condensation between the amine and betalamic acid anchored in the support provided the dopamine-, tyramine-, pyrrolidine-, and indoline-betaxanthins.172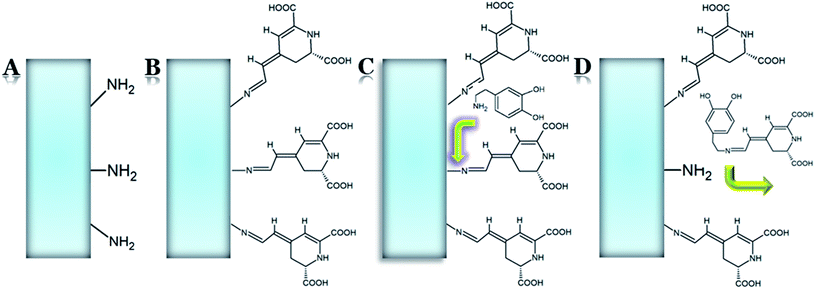 | ||
| Fig. 19 Betalamic acid-derivatized support and the surface reaction leading to pigment isolation, based on dopamine-derived betaxanthin: (A) starting material containing primary amine groups; (B) betalamic acid-derivatized support; (C) amine attack to betalamic acid; (D) synthesis and concomitant release of miraxanthin V.172 | ||
4.3 Semi-synthetic betalain pigments with extended conjugated systems
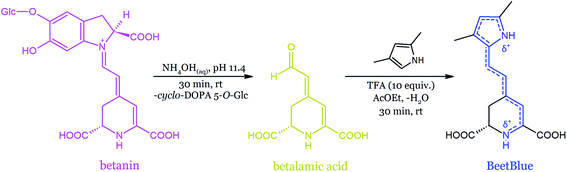 | ||
| Fig. 20 Semi-synthesis of a blue dye from betanin derived from beetroot juice.166 | ||
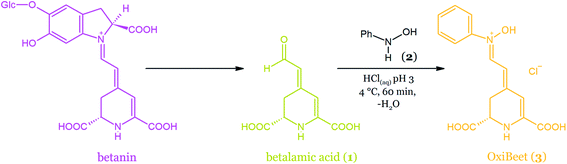 | ||
| Fig. 21 Semi-synthesis of OxiBeet pigment (3) by the acid-catalyzed coupling of betalamic acid (1) and N-phenylhydroxylamine 2 in water. | ||
This bio-based molecule exhibits low reduction potential and even higher radical scavenging activity than typical antioxidants such as ascorbic acid and gallic acid. Furthermore, it is non-cytotoxic toward human hepatic cell line HepaRG up to 1 mM concentrations. This pseudo-natural product is resistant to hydrolysis under neutral and slightly alkaline conditions and it may also be converted into a persistent N-oxide 1,7-diazaheptamethinium radical cation in an aqueous solutions via autoxidation. These findings open up new perspectives for utilizing betalain pigments and betalain derivatives as therapeutics against various human diseases.
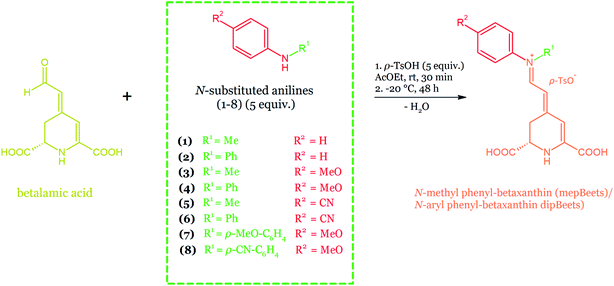 | ||
| Fig. 22 Semi-synthesis of the mepBeets and the dipBeets via the acid-catalyzed coupling of betalamic acid (1) with N-substituted anilines (2) in ethyl acetate.165 | ||
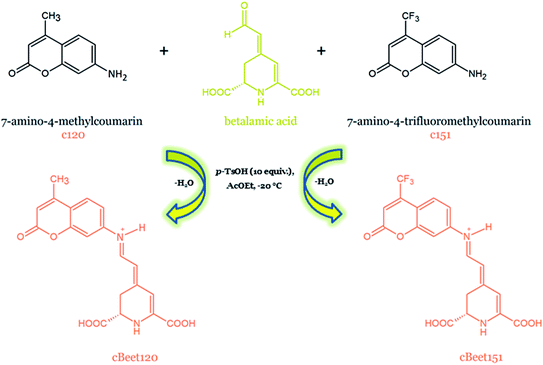 | ||
| Fig. 23 Semi-synthesis of artificial coumarinic betalain pigments with an extended conjugated system.167 | ||
In another study, an artificial coumarinic betalain (BtC) semi-synthesized via the aldimine coupling of 7-amino-4-methylcoumarin to betalamic acid was used for the fabrication of the fluorescent probe for the live-cell imaging of erythrocytes infected with Plasmodium falciparum responsible for malaria disease in humans.168 In order to test the capacity of BtC for labeling living Plasmodium-infected red blood cells, erythrocytes with parasites were incubated with BtC and examined by fluorescence microscopy. BtC was accumulated within the infected cells selectively and fluorescence imaging microscopy has shown that only the parasite was stained. On the contrary, no stain was observed in the infected erythrocytes incubated with an indicaxanthin (control probe) and uninfected cells.168
5 Conclusions
During the last two decades, the research on betalains, more specifically on betacyanins, has experienced significant acceleration due to their health-protective properties as well as applications in the food industry. Before 2000, there were no reports on any pro-health activities of betacyanins; from 2020 onwards, this is the main direction of projects and research on betacyanins. Furthermore, studies on the oxidation mechanism of betacyanins are highly relevant because pigments with different chemical structures seem to have different influence on their oxidation pathways. Considering the fairly simple semi-synthetic methods for additional betacyanin derivatives summarized in this review as well as the first promising results of various studies, the perspectives for further investigations on the bioactivity of betacyanins are inexhaustible. For the time being, the main obstacle that limits the research is the lack of highly efficient and inexpensive methods of betacyanin isolation and purification as well as methods of de novo synthesis. The constantly growing number of potential applications of betalains as natural colorants of food, fabrics, and dietary supplements but also their utilization in sensors, photoprotectors, and solar cells set promising directions of the investigations on these still not fully-explored plant pigments in the near future. In spite of the most recognizable betanin-based betacyanins present in a variety of betalainic plant sources, gomphrenin-like pigments also represent a promising group of potentially more stable and more active compounds at once. There are still many unexplored plants with presumably new interesting betacyanin profiles. The chemical structures of only 31 betalain pigments isolated from the plant sources were definitively identified by the NMR method, which is relatively small in comparison to the other groups of plant pigments. Furthermore, a huge group of over 187 betacyanins (including pigments from their richest source, B. glabra) detected by LC-MS techniques still awaits the final confirmation of their structures. Particular attention should also be paid to the chemical modifications of betacyanins that result in the generation of an increasing number of new derivatives with completely unknown bioactivities. The aspects discussed in this review show that the field of betalain chemistry is a goldmine and ideas for further research are still limitless.6 Conflicts of interest
The authors declare no competing financial interest.7 Acknowledgements
This research was financed by Polish National Science Centre for the years 2020–2023 (Project No. UMO-2019/33/N/NZ9/01590) as well as for the years 2018–2021 (Project No. UMO-2017/27/B/NZ9/02831). The authors are grateful to Dr Willibald Schliemann for his invaluable suggestions and comments.8 References
- N. Chhikara, K. Kushwaha, P. Sharma, Y. Gat and A. Panghal, Food Chem., 2019, 272, 192–200 CrossRef CAS PubMed.
- W. S. Choo, in Bioactive Molecules in Food. Reference Series in Phytochemistry, ed. J.-M. Mérillon and K. G. Ramawat, Springer, Cham, 2018, pp. 1–28 Search PubMed.
- P. Rahimi, S. Abedimanesh, S. A. Mesbah-Namin and A. Ostadrahimi, Crit. Rev. Food Sci. Nutr., 2019, 59, 2949–2978 CrossRef CAS PubMed.
- F. Gandía-Herrero, J. Escribano and F. García-Carmona, Crit. Rev. Food Sci. Nutr., 2016, 56, 937–945 CrossRef PubMed.
- F. C. Stintzing and R. Carle, in Food Colorants: Chemical and Functional Properties, CRC Press, Boca Raton, 2008, pp. 87–99 Search PubMed.
- I. B. Slimen, T. Najar and M. Abderrabba, J. Agric. Food Chem., 2017, 65, 675–689 CrossRef PubMed.
- G. Polturak and A. Aharoni, Molecular Plant, 2018, 11, 7–22 CrossRef CAS PubMed.
- K. Sutor and S. Wybraniec, J. Agric. Food Chem., 2020, 68, 11459–11467 CrossRef CAS PubMed.
- F. Imperato, Phytochemistry, 1975, 14, 2091–2092 CrossRef CAS.
- W. Schliemann, R. W. Joy IV, A. Komamine, J. W. Metzger, M. Nimtz, V. Wray and D. Strack, Phytochemistry, 1996, 42, 1039–1046 CrossRef CAS PubMed.
- S. Wybraniec, B. Nowak-Wydra, K. Mitka, P. Kowalski and Y. Mizrahi, Phytochemistry, 2007, 68, 251–259 CrossRef CAS PubMed.
- M. I. Khan and P. Giridhar, Phytochemistry, 2015, 117, 267–295 CrossRef CAS PubMed.
- S. Heuer, S. Richter, J. W. Metzger, V. Wray, M. Nimtzt and D. Strack, Phytochemistry, 1994, 37, 761–767 CrossRef CAS PubMed.
- F. C. Stintzing and R. Carle, Trends Food Sci. Technol., 2007, 18, 514–525 CrossRef CAS.
- E. Dunkelblum, H. E. Miller and A. S. Dreiding, Helv. Chim. Acta, 1972, 55, 642–648 CrossRef CAS PubMed.
- A. Kumorkiewicz and S. Wybraniec, J. Agric. Food Chem., 2017, 65, 7500–7508 CrossRef CAS PubMed.
- S. Wybraniec, Anal. Bioanal. Chem., 2007, 389, 1611–1621 CrossRef CAS PubMed.
- S. Wybraniec, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 861, 40–47 CrossRef CAS PubMed.
- T. Esatbeyoglu, A. E. Wagner, V. B. Schini-Kerth and G. Rimbach, Mol. Nutr. Food Res., 2015, 59, 36–47 CrossRef CAS PubMed.
- F. Gandía-Herrero, J. Escribano and F. García-Carmona, Planta, 2010, 232, 449–460 CrossRef PubMed.
- S. S. Kumar, P. Manoj, P. Giridhar, R. Shrivastava and M. Bharadwaj, J. Funct. Foods, 2015, 15, 509–515 CrossRef CAS.
- D. Strack, T. Vogt and W. Schliemann, Phytochemistry, 2003, 62, 247–269 CrossRef CAS.
- W. Schliemann and D. Strack, Phytochemistry, 1998, 49, 585–588 CrossRef CAS.
- S. Heuer, V. Wray, J. W. Metzger and D. Strack, Phytochemistry, 1992, 31, 1801–1807 CrossRef CAS.
- A. Spórna-Kucab, N. Wróbel, A. Kumorkiewicz-Jamro and S. Wybraniec, J. Chromatogr. A, 2020, 1626, 461370–461379 CrossRef PubMed.
- F. Kugler, S. Graneis, F. C. Stintzing and R. Carle, Z. Naturforsch., C: J. Biosci., 2007, 62, 311–318 CrossRef CAS PubMed.
- Y. Cai, M. Sun and H. Corke, J. Agric. Food Chem., 2001, 49, 1971–1978 CrossRef CAS PubMed.
- A. Spórna-Kucab, A. Kumorkiewicz, N. Szmyr, E. Szneler and S. Wybraniec, J. Sep. Sci., 2019, 42, 1676–1685 CrossRef PubMed.
- K. Lystvan, A. Kumorkiewicz, E. Szneler and S. Wybraniec, J. Agric. Food Chem., 2018, 66, 3870–3879 CrossRef CAS PubMed.
- F. Kugler, F. C. Stintzing and R. Carle, Anal. Bioanal. Chem., 2007, 387, 637–648 CrossRef CAS PubMed.
- A. Spórna-Kucab, A. Milo, A. Kumorkiewicz and S. Wybraniec, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1073, 96–103 CrossRef PubMed.
- M. Piattelli and F. Imperato, Phytochemistry, 1970, 9, 455–458 CrossRef CAS.
- M. Piattelli and F. Imperato, Phytochemistry, 1970, 9, 2557–2560 CrossRef CAS.
- G. Jerz, S. Wybraniec, N. Gebers and P. Winterhalter, J. Chromatogr. A, 2010, 1217, 4544–4554 CrossRef CAS PubMed.
- S. Wybraniec, G. Jerz, N. Gebers and P. Winterhalter, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 538–550 CrossRef CAS PubMed.
- S. Wybraniec and B. Nowak-Wydra, J. Agric. Food Chem., 2007, 55, 8138–8143 CrossRef CAS.
- L. Xiang, D. Xing, W. Wang, R. Wang, Y. Ding and L. Du, Phytochemistry, 2005, 66, 2595–2601 CrossRef CAS PubMed.
- N. Kobayashi, J. Schmidt, M. Nimtz, V. Wray and W. Schliemann, Phytochemistry, 2000, 54, 419–426 CrossRef CAS.
- S. S. Kumar, P. Manoj, N. P. Shetty, M. Prakash and P. Giridhar, J. Food Sci. Technol., 2015, 52, 4994–5002 CrossRef CAS PubMed.
- D. E. Jarvis, Y. S. Ho, D. J. Lightfoot, S. M. Schmöckel, B. Li, T. J. A. Borm, H. Ohyanagi, K. Mineta, C. T. Michell, N. Saber, N. M. Kharbatia, R. R. Rupper, A. R. Sharp, N. Dally, B. A. Boughton, Y. H. Woo, G. Gao, E. G. W. M. Schijlen, X. Guo, A. A. Momin, S. Negrão, S. Al-Babili, C. Gehring, U. Roessner, C. Jung, K. Murphy, S. T. Arold, T. Gojobori, C. G. Van Der Linden, E. N. Van Loo, E. N. Jellen, P. J. Maughan and M. Tester, Nature, 2017, 542, 307–312 CrossRef CAS PubMed.
- K. H. Wright, O. A. Pike, D. J. Fairbanks and C. S. Huber, J. Food Sci., 2002, 67, 1383–1385 CrossRef CAS.
- R. Reynoso, F. A. Garcia, D. Morales and E. Gonzalez De Mejia, J. Agric. Food Chem., 1997, 45, 2884–2889 CrossRef CAS.
- E. Castellanos-Santiago and E. M. Yahia, J. Agric. Food Chem., 2008, 56, 5758–5764 CrossRef CAS PubMed.
- S. Wybraniec, P. Stalica, A. Spórna and Y. Mizrahi, J. Agric. Food Chem., 2010, 58, 5347–5354 CrossRef CAS PubMed.
- A. Reyes-Martínez, M. Antunes-Ricardo, J. Gutiérrez-Uribe and M. del S. Santos-Díaz, Appl. Microbiol. Biotechnol., 2019, 103, 2583–2595 CrossRef.
- A. Gengatharan, G. A. Dykes and W. S. Choo, LWT--Food Sci. Technol., 2015, 64, 645–649 CrossRef CAS.
- H. Wyler and A. S. Dreiding, Helv. Chim. Acta, 1957, 40, 191–192 CrossRef CAS.
- M. Piattelli and L. Minale, Phytochemistry, 1964, 3, 307–311 CrossRef CAS.
- H. Wyler and A. S. Dreiding, Helv. Chim. Acta, 1959, 42, 1699–1702 CrossRef CAS.
- H. Wyler and A. S. Dreiding, Helv. Chim. Acta, 1984, 67, 1793–1800 CrossRef CAS.
- N. Kobayashi, J. Schmidt, V. Wray and W. Schliemann, Phytochemistry, 2001, 56, 429–436 CrossRef CAS PubMed.
- A. Kumorkiewicz, N. Szmyr, Ł. Popenda, Z. Pietrzkowski and S. Wybraniec, J. Agric. Food Chem., 2019, 67, 7455–7465 CrossRef CAS PubMed.
- D. Alard, V. Wray, L. Grotjahn, H. Reznik and D. Strack, Phytochemistry, 1985, 24, 2383–2385 CrossRef CAS.
- H. Wyler, H. Rösler, M. Mercier and A. S. Dreiding, Helv. Chim. Acta, 1967, 50, 545–561 CrossRef CAS.
- M. Piattelli and G. Impellizzeri, Phytochemistry, 1970, 9, 2553–2556 CrossRef CAS.
- S. Wybraniec, I. Platzner, S. Geresh, H. E. Gottlieb, M. Haimberg, M. Mogilnitzki and Y. Mizrahi, Phytochemistry, 2001, 58, 1209–1212 CrossRef CAS PubMed.
- M. Piattelli and G. Impellizzeri, Phytochemistry, 1969, 8, 1595–1596 CrossRef CAS.
- D. Strack, M. Bokern, N. Marxen and V. Wray, Phytochemistry, 1988, 27, 3529–3531 CrossRef CAS.
- F. Imperato, Phytochemistry, 1975, 14, 2526–2527 Search PubMed.
- M. Piattelli and L. Minale, Phytochemistry, 1964, 3, 547–557 CrossRef CAS.
- L. Minale, M. Piattelli, S. De Stefano and R. A. Nicolaus, Phytochemistry, 1966, 5, 1037–1052 CrossRef CAS.
- T. Vogt, M. Ibdah, J. Schmidt, V. Wray, M. Nimtz and D. Strack, Phytochemistry, 1999, 52, 583–592 CrossRef CAS.
- F. Kugler, F. C. Stintzing and R. Carle, J. Agric. Food Chem., 2004, 52, 2975–2981 CrossRef CAS PubMed.
- N. Mosquera, M. J. Cejudo-Bastante, F. J. Heredia and N. Hurtado, Plant Foods Hum. Nutr., 2020, 75, 434–440 CrossRef CAS PubMed.
- F. Imperato, Phytochemistry, 1975, 14, 2526 Search PubMed.
- M. Piattelli and F. Imperato, Phytochemistry, 1971, 10, 3133–3134 CrossRef CAS.
- G. Jerz, N. Gebers, D. Szot, M. Szaleniec, P. Winterhalter and S. Wybraniec, J. Chromatogr. A, 2014, 1344, 42–50 CrossRef CAS PubMed.
- L. Minale, M. Piattelli and S. De Stefano, Phytochemistry, 1967, 6, 703–709 CrossRef CAS.
- J.-Y. Wu, W.-C. Chen, Y.-Y. Wu, J.-T. Chen, L.-G. Chen and R. Y.-Y. Chiou, J. Agric. Sci. Appl., 2013, 2, 8–12 Search PubMed.
- F. Stintzing and W. Schliemann, Z. Naturforsch., C: J. Biosci., 2007, 62, 779–785 CrossRef CAS PubMed.
- F. Delgado-Vargas, A. R. Jimenez and O. Paredes-Lopez, Crit. Rev. Food Sci. Nutr., 2010, 40, 173–289 CrossRef PubMed.
- J. Svenson, B. M. Smallfield, N. I. Joyce, C. E. Sansom and N. B. Perry, J. Agric. Food Chem., 2008, 56, 7730–7737 CrossRef CAS PubMed.
- E. A. Hussain, Z. Sadiq and M. Zia-Ul-Haq, in Betalains: Biomolecular Aspects, Springer, Cham, 2018, pp. 15–32 Search PubMed.
- E. L. Bastos and W. Schliemann, in Plant Antioxidants and Health, Reference Series in Phytochemistry, ed. H. M. Ekiert, K. G. Ramawat and J. Arora, Springer, Cham, 2021, pp. 1–44 Search PubMed.
- G. J. Kapadia, H. Tokuda, T. Konoshima and H. Nishino, Cancer Lett., 1996, 100, 211–214 CrossRef CAS PubMed.
- Z. Pietrzkowski, B. Nemzer, A. Spórna, P. Stalica, W. Tresher, R. Keller, R. Jimenez, T. Michałowski and S. Wybraniec, New Medicine, 2010, 1, 12–17 Search PubMed.
- M. Allegra, L. Tesoriere and M. Livrea, Free Radical Res., 2007, 41, 335–341 CrossRef CAS PubMed.
- L. Tesoriere, D. Butera, M. Allegra, M. Fazzari and M. A. Livrea, J. Agric. Food Chem., 2005, 53, 1266–1270 CrossRef CAS PubMed.
- J. H. Lee, C. W. Son, M. Y. Kim, M. H. Kim, H. R. Kim, E. S. Kwak, S. Kim and M. R. Kim, Nutr. Res. Pract., 2009, 3, 114–121 CrossRef CAS PubMed.
- C. Q. Wang and G. Q. Yang, Phytomedicine, 2010, 17, 527–532 CrossRef CAS PubMed.
- F. Gandía-Herrero, J. Escribano and F. García-Carmona, J. Nat. Prod., 2009, 72, 1142–1146 CrossRef PubMed.
- Y. Cai, M. Sun and H. Corke, J. Agric. Food Chem., 2003, 51, 2288–2294 CrossRef CAS PubMed.
- J. Kanner, S. Harel and R. Granit, J. Agric. Food Chem., 2001, 49, 5178–5185 CrossRef CAS PubMed.
- J. Zhang, X. Hou, H. Ahmad, H. Zhang, L. Zhang and T. Wang, Food Chem., 2014, 145, 57–65 CrossRef CAS PubMed.
- L. Tesoriere, M. Allegra, C. Gentile and M. A. Livrea, Free Radical Res., 2009, 43, 706–717 CrossRef CAS PubMed.
- A. Gliszczyńska-Świgło, H. Szymusiak and P. Malinowska, Food Addit. Contam., 2006, 23, 1079–1087 CrossRef PubMed.
- T. Borkowski, H. Szymusiak, A. Gliszczyńska-Świgło, I. M. C. M. Rietjens and B. Tyrakowska, J. Agric. Food Chem., 2005, 53, 5526–5534 CrossRef CAS PubMed.
- W. Schliemann, N. Kobayashi and D. Strack, Plant Physiol., 1999, 119, 1217–1232 CrossRef CAS PubMed.
- L. C. P. Gonçalves, N. B. Lopes, F. A. Augusto, R. M. Pioli, C. O. Machado, B. C. Freitas-Dörr, H. B. Suffredini and E. L. Bastos, Pure Appl. Chem., 2020, 92, 243–253 Search PubMed.
- K. K. Nakashima and E. L. Bastos, Antioxidants, 2019, 8, 222–235 CrossRef CAS PubMed.
- L. C. Wu, H. W. Hsu, Y. C. Chen, C. C. Chiu, Y. I. Lin and J. A. A. Ho, Food Chem., 2006, 95, 319–327 CrossRef CAS.
- M. K. Reddy, R. L. Alexander-Lindo and M. G. Nair, J. Agric. Food Chem., 2005, 53, 9268–9273 CrossRef CAS PubMed.
- D. Sreekanth, M. K. Arunasree, K. R. Roy, T. Chandramohan Reddy, G. V. Reddy and P. Reddanna, Phytomedicine, 2007, 14, 739–746 CrossRef CAS PubMed.
- D. Cruz-Vega, M. J. Verde-Star and B. Salinas-Gonzalez, Zhongguo Zhongyao Zazhi, 2009, 22, 557–559 Search PubMed.
- T. Reyes-Izquierdo, Z. Pietrzkowski, R. Argumedo, C. Shu, B. Nemzer and S. Wybraniec, Nutr. Diet. Suppl., 2014, 6, 9–13 CrossRef.
- J. Kezi and J. H. Sumathy, Discov, 2014, 20, 51–58 Search PubMed.
- T. S. Kujala, M. S. Vienola, K. D. Klika, J. M. Loponen and K. Pihlaja, Eur. Food Res. Technol., 2002, 214, 505–510 CrossRef CAS.
- L. Martínez, I. Cilla, J. A. Beltrán and P. Roncalés, J. Sci. Food Agric., 2006, 86, 500–508 CrossRef.
- Y. Fu, J. Shi, S. Y. Xie, T. Y. Zhang, O. P. Soladoye and R. E. Aluko, J. Agric. Food Chem., 2020, 68, 11595–11611 CrossRef CAS PubMed.
- P. Rahimi, S. A. Mesbah-Namin, A. Ostadrahimi, A. Separham and M. Asghari Jafarabadi, J. Funct. Foods, 2019, 60, 103401 CrossRef CAS.
- B. L. Graf, P. Rojas-Silva, L. E. Rojo, J. Delatorre-Herrera, M. E. Baldeón and I. Raskin, Compr. Rev. Food Sci. Food Saf., 2015, 14, 431–445 CrossRef CAS PubMed.
- G. Calogero, J. H. Yum, A. Sinopoli, G. Di Marco, M. Grätzel and M. K. Nazeeruddin, Sol. Energy, 2012, 86, 1563–1575 CrossRef CAS.
- M. Shahid, S.-U. Islam and F. Mohammad, J. Cleaner Prod., 2013, 53, 310–331 CrossRef CAS.
- M. Wendel, A. Kumorkiewicz, S. Wybraniec, M. Ziółek and G. Burdziński, Dyes Pigm., 2017, 141, 306–315 CrossRef CAS.
- F. J. Knorr, D. J. Malamen, J. L. McHale, A. Marchioro and J. E. Moser, Proc. SPIE-Int. Soc. Opt. Eng., 2014, 9165, 91650–91659 Search PubMed.
- F. J. Knorr, J. L. McHale, A. E. Clark, A. Marchioro and J. E. Moser, J. Phys. Chem. C, 2015, 119, 19030–19041 CrossRef CAS.
- N. A. Treat, F. J. Knorr and J. L. McHale, J. Phys. Chem. C, 2016, 120, 9122–9131 CrossRef CAS.
- A. M. Cieślak, M. V. Pavliuk, L. D'Amario, M. Abdellah, K. Sokołowski, U. Rybinska, D. L. A. Fernandes, M. K. Leszczyński, F. Mamedov, A. M. El-Zhory, J. Föhlinger, A. Budinská, M. Wolska-Pietkiewicz, L. Hammarström, J. Lewiński and J. Sá, Nano Energy, 2016, 30, 187–192 CrossRef.
- M. V. Pavliuk, A. M. Cieślak, M. Abdellah, A. Budinská, S. Pullen, K. Sokołowski, D. L. A. Fernandes, J. Szlachetko, E. L. Bastos, S. Ott, L. Hammarström, T. Edvinsson, J. Lewiński and J. Sá, Sustainable Energy Fuelsc, 2017, 1, 69–73 RSC.
- M. Wendel, S. Nizinski, D. Prukala, M. Sikorski, S. Wybraniec and G. Burdzinski, J. Photochem. Photobiol., A, 2017, 332, 602–610 CrossRef CAS.
- M. Wendel, S. Nizinski, D. Tuwalska, K. Starzak, D. Szot, D. Prukala, M. Sikorski, S. Wybraniec and G. Burdzinski, Phys. Chem. Chem. Phys., 2015, 17, 18152–18158 RSC.
- M. Wendel, S. Nizinski, M. Gierszewski, D. Prukala, M. Sikorski, K. Starzak, S. Wybraniec and G. Burdzinski, Photochem. Photobiol. Sci., 2016, 15, 872–878 CrossRef CAS PubMed.
- Y. Cao, Y. Liu, F. Li, S. Guo, Y. Shui, H. Xue and L. Wang, Microchem. J., 2019, 150, 104176 CrossRef CAS.
- A. Guesmi, N. Ladhari, N. Ben Hamadi and F. Sakli, Ind. Crops Prod., 2012, 37, 342–346 CrossRef CAS.
- W. T. Sanderson, R. R. Stoddard, A. S. Echt, C. A. Piacitelli, D. Kim, J. Horan, M. M. Davies, R. E. McCleery, P. Muller, T. M. Schnorr, E. M. Ward and T. R. Hales, J. Appl. Microbiol., 2004, 96, 1048–1056 CrossRef CAS PubMed.
- G. W. Gould, J. Appl. Bacteriol., 1977, 42, 297–309 CrossRef CAS PubMed.
- L. C. P. Gonçalves, S. M. Da Silva, P. C. DeRose, R. A. Ando and E. L. Bastos, PLoS One, 2013, 8, e73701 CrossRef PubMed.
- M. A. Guerrero-Rubio, J. Martínez-Zapata, P. Henarejos-Escudero, F. García-Carmona and F. Gandía-Herrero, Dyes Pigm., 2020, 180, 108493 CrossRef CAS.
- D. L. A. Fernandes, C. Paun, M. V. Pavliuk, A. B. Fernandes, E. L. Bastos and J. Sá, RSC Adv., 2016, 6, 95693–95697 RSC.
- N. Martins, C. L. Roriz, P. Morales, L. Barros and I. C. F. R. Ferreira, Food Funct., 2017, 8, 1357–1372 RSC.
- H. M. C. Azeredo, Int. J. Food Sci. Technol., 2009, 44, 2365–2376 CrossRef CAS.
- K. M. Herbach, F. C. Stintzing and R. Carle, J. Food Sci., 2004, 69, 491–498 CrossRef.
- K. M. Herbach, F. C. Stintzing and R. Carle, Rapid Commun. Mass Spectrom., 2005, 19, 2603–2616 CrossRef CAS.
- K. M. Herbach, F. C. Stintzing and R. Carle, J. Agric. Food Chem., 2006, 54, 390–398 CrossRef CAS PubMed.
- S. Wybraniec and Y. Mizrahi, J. Agric. Food Chem., 2005, 53, 6704–6712 CrossRef CAS PubMed.
- S. Wybraniec, J. Agric. Food Chem., 2005, 53, 3483–3487 CrossRef CAS PubMed.
- S. Wybraniec, B. Nowak-Wydra and Y. Mizrahi, Tetrahedron Lett., 2006, 47, 1725–1728 CrossRef CAS.
- B. Nemzer, Z. Pietrzkowski, A. Spórna, P. Stalica, W. Thresher, T. Michałowski and S. Wybraniec, Food Chem., 2011, 127, 42–53 CrossRef CAS.
- A. Kumorkiewicz, K. Sutor, B. Nemzer, Z. Pietrzkowski and S. Wybraniec, Pol. J. Food Nutr. Sci., 2020, 70, 7–14 CrossRef CAS.
- A. Spórna-Kucab, S. Ignatova, I. Garrard and S. Wybraniec, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 941, 54–61 CrossRef PubMed.
- A. Spórna-Kucab, I. Garrard, S. Ignatova and S. Wybraniec, J. Chromatogr. A, 2015, 1380, 29–37 CrossRef.
- T. Sawicki and W. Wiczkowski, Food Chem., 2018, 259, 292–303 CrossRef CAS PubMed.
- T. Sawicki, C. Martinez-Villaluenga, J. Frias, W. Wiczkowski, E. Peñas, N. Bączek and H. Zieliński, J. Funct. Foods, 2019, 55, 229–237 CrossRef CAS.
- S. Wybraniec and T. Michalowski, J. Agric. Food Chem., 2011, 59, 9612–9622 CrossRef CAS PubMed.
- S. Wybraniec, P. Stalica, A. Spórna, B. Nemzer, Z. Pietrzkowski and T. Michałowski, J. Agric. Food Chem., 2011, 59, 12163–12170 CrossRef CAS PubMed.
- S. Wybraniec, K. Starzak, A. Skopińska, B. Nemzer, Z. Pietrzkowski and T. Michałowski, J. Agric. Food Chem., 2013, 61, 6465–6476 CrossRef CAS.
- M. Sugumaran and V. Semensi, J. Biol. Chem., 1991, 266, 6073–6078 CrossRef CAS.
- S. Ito, Pigm. Cell Res., 2003, 16, 230–236 CrossRef CAS PubMed.
- S. Wybraniec, K. Starzak, A. Skopińska, B. Nemzer, Z. Pietrzkowski and T. Michałowski, J. Agric. Food Chem., 2013, 61, 6465–6476 CrossRef CAS PubMed.
- A. Kumorkiewicz, E. Szneler and S. Wybraniec, J. Agric. Food Chem., 2018, 66, 12815–12826 CrossRef CAS PubMed.
- S. Wybraniec, K. Starzak, A. Skopińska, M. Szaleniec, J. Słupski, K. Mitka, P. Kowalski and T. Michałowski, Food Sci. Biotechnol., 2013, 22, 353–363 CrossRef CAS.
- O. J. Pozo, C. Gómez, J. Marcos, J. Segura and R. Ventura, Drug Test. Anal., 2012, 4, 786–797 CrossRef CAS PubMed.
- K. Cao, D. E. Stack, R. Ramanathan, M. L. Gross, E. G. Rogan and E. L. Cavalieri, Chem. Res. Toxicol., 1998, 11, 909–916 Search PubMed.
- A. J. L. Cooper, B. F. Krasnikov, Z. V. Niatsetskaya, J. T. Pinto, P. S. Callery, M. T. Villar, A. Artigues and S. A. Bruschi, Amino Acids, 2011, 41, 7–27 CrossRef CAS.
- R. Larcher, L. Tonidandel, G. Nicolini and B. Fedrizzi, Food Chem., 2013, 141, 1196–1202 CrossRef CAS PubMed.
- C. B. Tang, W. G. Zhang, C. Dai, H. X. Li, X. L. Xu and G. H. Zhou, J. Agric. Food Chem., 2015, 63, 902–911 CrossRef CAS PubMed.
- C. Chenot, R. Robiette and S. Collin, J. Agric. Food Chem., 2019, 67, 4002–4010 CrossRef CAS PubMed.
- Y. Zhai, H. Cui, K. Hayat, S. Hussain, M. U. Tahir, J. Yu, C. Jia, X. Zhang and C. T. Ho, J. Agric. Food Chem., 2019, 67, 8632–8640 CrossRef CAS PubMed.
- A. Kumorkiewicz-Jamro, Ł. Popenda and S. Wybraniec, ACS Omega, 2020, 5, 14955–14967 CrossRef CAS PubMed.
- H. Salminen, M. Estévez, R. Kivikari and M. Heinonen, Eur. Food Res. Technol., 2006, 223, 461–468 CrossRef CAS.
- M. N. Lund, M. Heinonen, C. P. Baron and M. Estévez, Mol. Nutr. Food Res., 2011, 55, 83–95 CrossRef CAS PubMed.
- A. Carreras, J. A. Mesa, M. Cascante, J. L. Torres and L. Juliá, New J. Chem., 2013, 37, 2043–2050 RSC.
- M. Allegra, P. G. Furtmüller, W. Jantschko, M. Zederbauer, L. Tesoriere, M. A. Livrea and C. Obinger, Biochem. Biophys. Res. Commun., 2005, 332, 837–844 CrossRef CAS PubMed.
- S. Wybraniec, K. Starzak and Z. Pietrzkowski, J. Agric. Food Chem., 2016, 64, 2865–2874 CrossRef CAS PubMed.
- S. Wybraniec, K. Starzak, E. Szneler and Z. Pietrzkowski, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1036, 20–32 CrossRef.
- A. Kumorkiewicz-Jamro, K. Starzak, K. Sutor, B. Nemzer, Z. Pietrzkowski, Ł. Popenda and S. Wybraniec, Molecules, 2020, 25, 378 CrossRef CAS PubMed.
- J. D. Sivey, C. E. McCullough and A. L. Roberts, Environ. Sci. Technol., 2010, 44, 3357–3362 CrossRef CAS PubMed.
- S. S. Lau, K. P. Reber and A. L. Roberts, Environ. Sci. Technol., 2019, 53, 11133–11141 CrossRef CAS PubMed.
- S. Agrawal, N. Ingle, U. Maity, R. V. Jasra and P. Munshi, ACS Omega, 2018, 3, 6692–6702 CrossRef CAS.
- K. Starzak, K. Sutor, T. Świergosz, B. Nemzer, Z. Pietrzkowski, Ł. Popenda, S. R. Liu, S. P. Wu and S. Wybraniec, Int. J. Mol. Sci., 2021, 22, 1155 CrossRef CAS PubMed.
- J. Sugihara, Adv. Carbohydr. Chem.c, 1953, 8, 1–44 CAS.
- R. M. Rowell, Carbohydr. Res., 1972, 23, 417–424 CrossRef CAS.
- A. Haines, Adv. Carbohydr. Chem. Biochem., 1981, 39, 13–70 CrossRef CAS.
- F. C. Stintzing, J. Conrad, I. Klaiber, U. Beifuss and R. Carle, Phytochemistry, 2004, 65, 415–422 CrossRef CAS PubMed.
- R. M. Pioli, R. R. Mattioli, L. C. Esteves, S. Dochev and E. L. Bastos, Dyes Pigm., 2020, 183, 108609 CrossRef CAS.
- B. C. Freitas-Dörr, C. O. Machado, A. C. Pinheiro, A. B. Fernandes, F. A. Dörr, E. Pinto, M. Lopes-Ferreira, M. Abdellah, J. Sá, L. C. Russo, F. L. Forti, L. C. P. Gonçalves and E. L. Bastos, Sci. Adv., 2020, 6, 421–424 Search PubMed.
- A. C. B. Rodrigues, I. de F. A. Mariz, E. M. S. Maçoas, R. R. Tonelli, J. M. G. Martinho, F. H. Quina and E. L. Bastos, Dyes Pigm., 2018, 150, 105–111 CrossRef CAS.
- L. C. P. Gonçalves, R. R. Tonelli, P. Bagnaresi, R. A. Mortara, A. G. Ferreira and E. L. Bastos, PLoS One, 2013, 8, e53874 CrossRef PubMed.
- L. C. Esteves, A. C. Pinheiro, R. M. Pioli, T. C. Penna, W. J. Baader, T. C. Correra and E. L. Bastos, Photochem. Photobiol., 2018, 94, 853–864 CrossRef CAS PubMed.
- T. J. Mabry, H. Wyler, G. Sassu, M. Mercier, I. Parikh and A. S. Dreiding, Helv. Chim. Acta, 1962, 23, 640–647 CrossRef.
- F. H. Bartoloni, L. C. P. Gonçalves, A. C. B. Rodrigues, F. A. Dörr, E. Pinto and E. L. Bastos, Monatsh. Chem., 2013, 144, 567–571 CrossRef CAS.
- J. Cabanes, F. Gandía-Herrero, J. Escribano, F. García-Carmona and M. Jiménez-Atiénzar, J. Agric. Food Chem., 2014, 62, 3776–3782 CrossRef CAS.
- A. C. Pinheiro, R. B. Fazzi, L. C. Esteves, C. O. Machado, F. A. Dörr, E. Pinto, Y. Hattori, J. Sa, A. M. da Costa Ferreira and E. L. Bastos, Free Radical Biol. Med., 2021, 168, 110–116 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |