Expanding the NMR toolkit for biological solids: oxygen-17 enriched Fmoc-amino acids†
Abstract
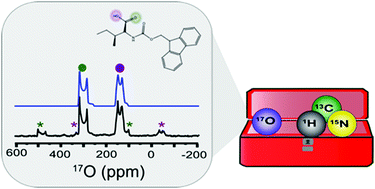
We report the solid-state 17O NMR parameters for five previously uncharacterized N-α-fluoren-9-yl-methoxycarbonyl-O-t-butyl (Fmoc) protected amino acids. These molecules are critical to constructing synthetic biological systems, like peptides, and provide an avenue for introducing 17O as an NMR probe nucleus. A multiple-turnover reaction was used to efficiently 17O label the carboxylic acid moieties of Fmoc-L-isoleucine, Fmoc-L-tryptophan, Fmoc-L-proline, Fmoc-L-tyrosine and Fmoc-L-threonine. Magic-angle spinning (MAS) and non-spinning NMR spectra were obtained at two magnetic field strengths (14.1 and 21.1 T) and the quadrupolar and chemical shift parameters for the carbonyl and hydroxyl sites were determined. Computed NMR parameters using density functional theory (DFT) were found to be in good agreement with experimental results, supporting the identification of minor unprotonated species present. This work continues to highlight 17O as a sensitive probe nucleus of its local environment and reinforces the importance of developing solid-state 17O NMR techniques to expand the analytical NMR toolkit for exploring biologically relevant molecules.
- This article is part of the themed collection: NJC Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...