Alternative mechanisms of action for the apoptotic activity of terpenoid-like chalcone derivatives†
Abstract
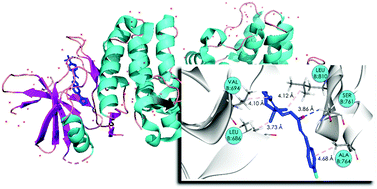
Apoptosis is a defense mechanism against pre-cancerous and infected cells. Although the applicability of β-ionone against diverse cancer cell lines has been exhaustively investigated, the apoptotic activity of terpenoid-like chalcones, as well as their mechanisms of action, is not well understood. Here we present a new terpenoid-like chalcone derivative (I) and its biological potential against HL-60 (leukemia), HCT-116 (colon), and SNB-19 (glioblastoma) cancer cell lines. Compound I showed cytotoxicity against over 90% of the tested cell lines. However, I has an IC50 slightly higher than doxorubicin, a DNA-binding cancer drug, which motivated us to investigate an alternative mechanism of action for I other than DNA-mediated. We performed an in silico structure-based pharmacophoric screening against various proteins, which indicated mitogen-activated protein kinase 5 (MAP3K5) as a potential protein target. Compound I was docked within its active site and was predicted to bind MAP3K5 with a comparable affinity to IM6, a cocrystallized ligand. Finally, we describe the reaction of a reduced glutathione adduct (GSH) with compound I and previously published derivatives bearing the substitutions 4-chloro (II), 4-bromo (III), and 4-nitro (IV) using HPLC-MS. We show that these are rapid reactions and that the products are stable for up to 24 h. Here, we suggest two alternative mechanisms (MAP3K5 inhibition and thiol reactivity) for the biological potential of a series of terpenoid-like chalcone derivatives. We anticipate that these findings can be explored to design additional derivatives with even more robust apoptotic activity.


 Please wait while we load your content...
Please wait while we load your content...