Recent quinone diazide based transformations via metal–carbene formation
Abstract
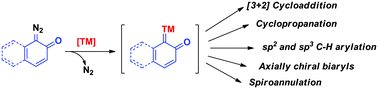
The diazo quinone or quinone diazide class of compounds has been extensively utilised to introduce phenol/naphthol moieties into hydrocarbons or nitrogen-containing heterocycles under transition metal catalysis. The reactions proceed via C–H insertion or migratory insertion of metal carbenes. In many cases, the site-selectivities were obtained via the guidance of directing groups. Various rearrangement reactions were explored with the metal carbenes generated from these diazo compounds. Further, interesting asymmetric reactions were studied to obtain phenol/naphthol containing compounds having stereogenic centers or axial chirality with high enantioselectivity. In this review, the recent progress on synthetic studies of metal carbenes generated from quinone diazide moieties has been summarized.
- This article is part of the themed collections: 2021 Focus and Perspective articles and NJC Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...