DOI:
10.1039/D1NJ00290B
(Paper)
New J. Chem., 2021,
45, 8936-8941
Exploring different methods of cellulose extraction for 14C dating
Received
18th January 2021
, Accepted 23rd March 2021
First published on 29th April 2021
Abstract
In this study we aim to identify the optimal cellulose extraction protocol for 14C dating of wood, with a focus on glacial trees. To achieve this, we compare three cellulose extraction methods on the basis of cellulose yield and 14C age. The study is conducted on 12 wood samples of different species, in varying states of preservation with ages covering the full 14C age range. Cellulose is extracted from each sample following three different protocols selected from the literature: ABA-B, BABAB and 2Chlorox. The extracted cellulose was graphitised and dated with the MICADAS (Mini Carbon Dating System) at the ETH AMS laboratory. Although all three methods are considered efficient, the BABAB protocol, despite being a more aggressive procedure, allows the extraction of a sufficient amount of cellulose to be 14C dated and leads to the most reliable results, particularly for very old and background samples (samples with 14C content of zero).
Introduction
Cellulose (C6H10O5) is an abundant biopolymer. The use of this compound is spreading in many fields, including the biomedical, packaging and environmental sectors, thanks not only to its chemical and physical properties, but also as a renewable, sustainable and biodegradable material.4–6 In this study we chemically isolate cellulose from wood to exploit another of its unique qualities: its record of atmospheric carbon content.7,8 Through the photosynthesis process, atmospheric CO2 is stored as carbon in plants. During growth, the ratio of carbon isotopes in the plant tissues is in equilibrium with the atmosphere. After active growth has ceased, the radioactive carbon isotope (14C) concentration starts to decrease. The concentration of 14C can be measured with annual resolution from the cellulose extracted from tree rings.8 Radiocarbon is the most precise and universal tool for dating events of the last 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years,9 the limit of the radiocarbon method. Here we study sub-fossil tree remnants (wood fragments in which the organic component is not fully decomposed and is only partially fossilized) grown in the glacial period. We intend to improve the resolution of the radiocarbon calibration curve (used to convert 14C ages into calendar ages) beyond the current extent of the unbroken dendrochronological curve (back to 14
000 years,9 the limit of the radiocarbon method. Here we study sub-fossil tree remnants (wood fragments in which the organic component is not fully decomposed and is only partially fossilized) grown in the glacial period. We intend to improve the resolution of the radiocarbon calibration curve (used to convert 14C ages into calendar ages) beyond the current extent of the unbroken dendrochronological curve (back to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 years cal BP†) to allow more precise age determinations.9 Cellulose is the fraction of choice for dating trees.2,7,8,10 To obtain reliable 14C ages any contamination by extraneous carbon in the sub-fossil wood must be removed. Contamination can occur by young carbon, e.g. humic acid introduced from groundwater supply, or from 14C-free carbonates. For radiocarbon dating of Upper Pleistocene samples, contamination by young carbon is more problematic than contamination by fossil carbon: 1% of modern carbon contamination added to a sample of 37 000 14C BP will reduce its age by 5500 years, whereas 1% 14C-free carbon will increase the age by only 80 years. Therefore, when assessing the effectiveness of pretreatment procedures, older ages are generally considered more reliable. Several studies report cellulose extraction protocols for young Holocene trees as well as wood near/beyond the limit of 14C dating (>55
226 years cal BP†) to allow more precise age determinations.9 Cellulose is the fraction of choice for dating trees.2,7,8,10 To obtain reliable 14C ages any contamination by extraneous carbon in the sub-fossil wood must be removed. Contamination can occur by young carbon, e.g. humic acid introduced from groundwater supply, or from 14C-free carbonates. For radiocarbon dating of Upper Pleistocene samples, contamination by young carbon is more problematic than contamination by fossil carbon: 1% of modern carbon contamination added to a sample of 37 000 14C BP will reduce its age by 5500 years, whereas 1% 14C-free carbon will increase the age by only 80 years. Therefore, when assessing the effectiveness of pretreatment procedures, older ages are generally considered more reliable. Several studies report cellulose extraction protocols for young Holocene trees as well as wood near/beyond the limit of 14C dating (>55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years BP).1,3,11 We want to identify the most effective pretreatment procedure to extract cellulose and efficiently remove exogenous contamination from sub-fossil trees spanning the glacial period. We compare three different protocols, including a novel technique which has been recently published. We selected subfossil trees of different species from different time periods to identify the limits and capabilities of each extraction method. For most of the samples the age is already known, either from dendrochronology or from radiocarbon dating. The results are evaluated on the basis of the cellulose yield and the 14C age.
000 years BP).1,3,11 We want to identify the most effective pretreatment procedure to extract cellulose and efficiently remove exogenous contamination from sub-fossil trees spanning the glacial period. We compare three different protocols, including a novel technique which has been recently published. We selected subfossil trees of different species from different time periods to identify the limits and capabilities of each extraction method. For most of the samples the age is already known, either from dendrochronology or from radiocarbon dating. The results are evaluated on the basis of the cellulose yield and the 14C age.
Experimental
Materials and methods
Twelve wood samples within the age range of 14C dating were selected from our archive (Table 1). Samples (Pinus sylvestris L.) from Furadouro were found in an ancient forest bed under the sandy shore of the northern Atlantic coast of Portugal, near the city of Ovar, accessible only during rare events of minimum low tide.12 In this study, three sub-samples of the same tree section were dated. The sample from Revine (Larix decidua) was found in a larch forest preserved in colluvial and lacustrine deposits near the lake of Revine (Treviso) in the Italian Pre-Alps.13 Cairo Montenotte (Pinus sylvestris L.) is part of a sub-fossil pine tree stump sampled from glacial sediments in Savona (Italy) in 2018. The conifer wood sample from Reichwalde is a background sample,14 which was found in Miocene layers in the lignite mining area in Saxony, Germany.15 Wood samples from Győrújfalu (Fraxinus spec.) and Győrzámoly (Ulmus spec.) were found in Hungary, near the city of Győr, in Quaternary gravel sediments in a tributary of the Danube River. The Győrzámoly sample was previously dated at the Heidelberg radiocarbon laboratory to beyond the 14C dating limit (>55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years BP). Recently, samples from Győrújfalu were unsuccessfully pretreated (cellulose was not obtained) at Mannheim radiocarbon laboratory. To verify their preservation state, they were tested again. The sample from Illegio is a larch stump (Larix decidua) found in sediments dating to the LGM (Last Glacial Maximum) in the basin of the Tagliamento River in the Carnic alps of the Friuli-Venezia-Giulia region (Italy), as described in Monegato et al.16 The sample analysed here was found in situ together with another larch trunk, dated by Hajdas et al.17 The Late Glacial pine (Pinus sylvestris L.) from Breitenthal was found in a gravel pit in southern Germany near the Günz River, together with other pines. They were dendrochronologically analysed by Friedrich et al.18 Samples from Ebensfeld and Freising are Holocene oak (Quercus spec.) and pine (Pinus sylvestris L.) found in Quaternary deposits of the rivers Isar and Main in southern Germany. They were remnants of old riparian forests buried by river activity and found in gravel pits. They are parts of the Holocene oak and pine chronologies of central Europe.18,19
000 years BP). Recently, samples from Győrújfalu were unsuccessfully pretreated (cellulose was not obtained) at Mannheim radiocarbon laboratory. To verify their preservation state, they were tested again. The sample from Illegio is a larch stump (Larix decidua) found in sediments dating to the LGM (Last Glacial Maximum) in the basin of the Tagliamento River in the Carnic alps of the Friuli-Venezia-Giulia region (Italy), as described in Monegato et al.16 The sample analysed here was found in situ together with another larch trunk, dated by Hajdas et al.17 The Late Glacial pine (Pinus sylvestris L.) from Breitenthal was found in a gravel pit in southern Germany near the Günz River, together with other pines. They were dendrochronologically analysed by Friedrich et al.18 Samples from Ebensfeld and Freising are Holocene oak (Quercus spec.) and pine (Pinus sylvestris L.) found in Quaternary deposits of the rivers Isar and Main in southern Germany. They were remnants of old riparian forests buried by river activity and found in gravel pits. They are parts of the Holocene oak and pine chronologies of central Europe.18,19
Table 1 Sample information. Samples are ordered by age (from youngest to oldest)
| Bologna lab code BRA- |
Site |
Species |
Ref. |
Dendro-date or 14C age (if floating) |
| 3275 |
Ebensfeld, Germany |
Oak (Quercus spec.) |
Friedrich et al.;19 Friedrich et al.18 |
Ring 60–85 (72): 5569 BP (3620 BC) |
| 3276 |
Freising, Germany |
Pine (Pinus sylvestris L.) |
Friedrich et al.;19 Friedrich et al.18 |
Ring 20–40 (30): 10.872 BP (8923 BC) |
| 3272 |
Breitenthal, Germany |
Pine (Pinus sylvestris L.) |
Friedrich et al.19 |
Ring 50–80 (65): 11.840 BP (9891 BC) |
| 3274 |
Revine, Italy |
Larch (Larix decidua) |
Kromer et al.;20 Friedrich et al.;19 Kaiser et al.21 |
∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP 000 14C BP |
| 3271 |
Furadouro, Portugal |
Pine (Pinus sylvestris L.) |
This study |
∼27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP (untreated) 000 14C BP (untreated) |
| 3282 |
Furadouro, Portugal |
Pine (Pinus sylvestris L.) |
This study |
∼27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP (untreated) 000 14C BP (untreated) |
| 3283 |
Furadouro, Portugal |
Pine (Pinus sylvestris L.) |
This study |
∼27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP (untreated) 000 14C BP (untreated) |
| 3277 |
Illegio, Italy |
Larch (Larix decidua) |
This study |
Not dated |
| 3278 |
Cairo Montenotte, Italy |
Pine (Pinus sylvestris L.) |
This study |
Not dated |
| 3279 |
Győrújfalu, Hungary |
Ash (Fraxinus spec.) |
This study |
Background (probably Eemian) |
| 3281 |
Győrzámoly, Hungary |
Elm (Ulmus spec.) |
This study |
Background (probably Eemian) |
| 3273 |
Reichwalde, Germany |
Conifer (c.f. Chamaecyparis) |
Sookdeo et al.;14 Scott et al.22 |
Background (Miocene) |
Pretreatment methods
Three sub-samples were taken from each of the 12 trees, one for each protocol. Nine sub-samples weighed ∼200 mg, and 3 samples ∼40 mg to ensure that all methods could be applied in cases of limited material. All samples were chopped into small pieces and were put into 10 mL glass tubes and processed according to the three different protocols: ABA-B,1 BABAB2 and 2ChlorOx.3 The classic ABA method1,8,23 was tested with the addition of a final bleaching step (ABA-B).1 The BABAB method adds an initial overnight bath in alkaline solution to the ABA method followed by a final oxidative bleaching step.2 The recently published 2ChlorOx method involves an alkaline hypochlorite and an acidic chlorite oxidation bleaching step, repeated two times.3
ABA-B1.
Samples were treated with 4% HCl to remove contamination from carbonates, then washed with MilliQ water, followed by a 4% NaOH bath, and a second 4% HCl step to remove any absorbed atmospheric CO2. Each step was carried out for ∼1 hour. A bleaching solution (5% NaClO2 and some drops of 4% HCl) to remove lignin and other contaminants was applied for ∼1 hour,24,25 then renewed to increase efficiency, as suggested by Capano et al.,1 and left for another hour. All procedures are carried out in a heater-block at 75 °C. The final extract was dried in an oven at 80 °C.
BABAB2.
Samples were left in a 4% NaOH bath at 75 °C overnight to dissolve the lignin (making the cellulose more accessible to reagents)2 and the humic acid (soluble at high pH) from the soil.26,27 The following day, the acid–base–acid steps (as described above for the ABA-B protocol) were performed. The final bleaching step to remove lignin, hemicellulose and other extractives24,25 was carried out for 2 hours at 75 °C, followed by 15 minutes in an ultrasonic bath at room temperature in the same bleach solution. Samples were then washed several times with MilliQ water to reach pH 5 and dried in an oven at 80 °C.
2ChlorOx3.
Two oxidative steps were applied. The first was performed in an alkaline environment at room temperature with NaClO and NaOH for 2 hours, followed by an acidic wash with HCl, and a second oxidative reaction with NaClO2 and HCl started at 70 °C for another 2 hours. These steps were then repeated. Following this procedure, samples were washed to neutrality and dried in an oven at 80 °C. This is in contrast to Gillespie's procedure,3 where the final product was freeze-dried.
After each step, samples were rinsed thoroughly with MilliQ water until the required pH was reached: ≤pH 10 before acid steps and ≥pH 4 before alkaline steps. To help solid–liquid separation samples were centrifuged and decanted. The weight of the initial dried sample and the weight of the dried final product were considered to calculate the cellulose yield % of each pretreated sample.
Graphitisation and 14C dating
At the Ionplus laboratory, 2.5–3.0 mg dried cellulose was weighed into aluminium cups, compressed and then combusted in an elemental analyser coupled to an AGE 3. The resulting CO2 was converted into graphite in the reactors using H2 and 3.5 mg iron powder as a catalyst. Graphite was pressed into a target (sample holders) ready for AMS analyses at the ETH laboratory in Zurich. Samples of phthalic acid anhydride (chemical blank) and oxalic acid II (radiocarbon standard), kindly provided by the Ionplus laboratory, were graphitised and measured together with our samples in the ETH MICADAS (Mini Radiocarbon Dating System).28
Results and discussion
The final cellulose yield of all pretreated samples is shown in Fig. 1. For two thirds of the triple tests, ABA-B and 2ChloOx resulted in a similar yield (within 5%). In contrast, for most samples, BABAB reduced the sample mass to lower than 35% of the initial weight. In general, all the pretreatment methods used in this study resulted in a substantial loss of the initial sample mass as only cellulose was targeted, which constitutes 40–44% of the dry weight of both soft- and hardwood trees25 and it is tightly connected to the state of wood preservation. 2ChlorOx was the most conservative among the considered protocols, together with ABA-B. Nevertheless, 50% of samples had a final yield between 20 and 40%, and the other half between 0 and 20%. In the BABAB procedure, in addition to the final bleaching stage, samples were first soaked in an alkaline bath of sodium hydroxide to dissolve humic acid and the polymeric molecules constituting lignin. The dissolution of lignin leads to a significant mass loss, as highlighted in Table 2: after the alkaline bath, 75% of our samples lost more than 30% of their initial weight. It is interesting to note the different behaviours of the background samples (BRA-3273, BRA-3279, BRA-3281) that show a significant loss of mass of 73% for BRA-3281, 50% for BRA-3279 and 46% for BRA-3273. At the end of the BABAB procedure, only BRA-3273 yielded sufficient cellulose to be radiocarbon dated. It is obvious that the pretreatment protocol adopted plays an important role in the isolation of cellulose and the quantity of datable material obtained. However, it is also true that the state of preservation, which is an intrinsic property of the sample, has to be taken into consideration when choosing the pretreatment method since it defines the available amount of cellulose. Samples BRA-3276, BRA-3279 and BRA-3281 are examples of this: for the last two samples (both background samples) the state of preservation was very low, and we could only extract 0–3% cellulose, which was not a sufficient amount to be 14C dated. Similarly, from the relatively young Holocene sample BRA-3276 only a small cellulose yield of <20% was obtained after the 2ChlorOx procedure, and <10% after the ABA-B and BABAB procedures. In contrast to the background samples, BRA-3276 is a relatively young (Holocene) pine wood which is poorly preserved. In general, we observed very different cellulose yields in both young (Holocene) and old (Upper Pleistocene) samples. There was no clear trend observable with burial time. Preservation depends on a combination of site-specific bedding conditions, burial time, and the durability of wood of different tree-species. In this respect, species such as oak, elm, pine and larch form heartwood in the inner part of the stem which contains phenolic compounds such as tannins, or other substances (i.e. resins), which make it much more resistant to decay than other types of wood, i.e. ash.29,30 The absence of residual cellulose from the background sample BRA-3279 may be due to a species-specific low resistance of ash, as a non-heartwood. Regarding the influence of burial conditions on the preservation of the trunk, we observe a similar situation between our sites, which enables good preservation of wood, even though the preservation status of individual trees from the different sites depends on the combination of all preservation factors. The subfossil trees from Hungary were found in Interglacial fluvial gravels which were part of the aquifer of the Danube River valley.15 As the trees were submerged in groundwater for most of the glacial period, they were predominantly exposed to anoxic bacterial decay. Trees from Furadouro were remnants of a pine forest in a lagoon close to the sea between 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–20
100–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 years BP, when the Atlantic ocean was below the present level.12 The site was covered by fluvial sandy sediments during the Pleniglacial phase of the last ice age. Due to sea-level rise in the post-glacial period, the trees were submerged after that time, which explains the low level of bacterial destruction (i.e. good cellulose preservation). The larch trees from Illegio17 (BRA-3277) and Revine13 (BRA-3274) and the pine from Cairo Montenotte (BRA-3278) were found in glacio-fluvial and colluvial sediments which kept the wood permanently under anoxic conditions and lead to remarkably good cellulose preservation, particularly at Revine and Cairo Montenotte. In comparison, the lower level of preservation at Illegio (BRA-3277) may be the result of local bedding conditions at that site. For the Holocene trees (BRA-3275; BRA-3276; BRA-3272) found in fluvial sediments,18,19 the permanently water-saturated conditions below the groundwater table was the crucial factor for high levels of preservation. The variation in preservation may be due to the local situation. The good preservation of the oak (BRA-3275) is due to the natural durability of this species due to the high content of tannins in oak heartwood.30
700 years BP, when the Atlantic ocean was below the present level.12 The site was covered by fluvial sandy sediments during the Pleniglacial phase of the last ice age. Due to sea-level rise in the post-glacial period, the trees were submerged after that time, which explains the low level of bacterial destruction (i.e. good cellulose preservation). The larch trees from Illegio17 (BRA-3277) and Revine13 (BRA-3274) and the pine from Cairo Montenotte (BRA-3278) were found in glacio-fluvial and colluvial sediments which kept the wood permanently under anoxic conditions and lead to remarkably good cellulose preservation, particularly at Revine and Cairo Montenotte. In comparison, the lower level of preservation at Illegio (BRA-3277) may be the result of local bedding conditions at that site. For the Holocene trees (BRA-3275; BRA-3276; BRA-3272) found in fluvial sediments,18,19 the permanently water-saturated conditions below the groundwater table was the crucial factor for high levels of preservation. The variation in preservation may be due to the local situation. The good preservation of the oak (BRA-3275) is due to the natural durability of this species due to the high content of tannins in oak heartwood.30
 |
| | Fig. 1 Comparison of sample cellulose yields (%) following pretreatment with the different methods (2ChlorOx, ABA-B, BABAB). Samples are ordered by age (from youngest to oldest). | |
Table 2 Mass loss (%) for each sample after a 4% NaOH overnight bath at 75 °C in the BABAB procedure
| Bologna lab code BRA- |
Mass loss (%) |
| BRA-3275 |
39 |
| BRA-3276 |
32 |
| BRA-3272 |
30 |
| BRA-3274 |
22 |
| BRA-3271 |
51 |
| BRA-3282 |
58 |
| BRA-3283 |
27 |
| BRA-3277 |
41 |
| BRA-3278 |
39 |
| BRA-3279 |
50 |
| BRA-3281 |
73 |
| BRA-3273 |
46 |
The efficiency of contamination removal was evaluated through the 14C dating shown in Table 3 and Fig. 2. For the three dendro-dated samples (top row of Fig. 2) the calibrated ranges of the 14C ages cover the known calendar date. The measured ages of the sample pairs/triplets do not show significant variations. The results of the different pretreatment methods fall within the 2σ error range for 7 out of the 9 samples dated (background sample BRA-3273 excluded). The most significant difference is observed for sample BRA-3278: the ABA-B and BABAB extracts date to the background age level, whereas the 2ChlorOx extract is significantly younger. For BRA-3282, the BABAB extract is 2.7σ younger than the mean of the ABA-B and 2ChlorOx extracts. For the background sample BRA-3273, the F 14C (Fraction Modern, defined as the ratio of 14C specific activity of the sample and of the radiocarbon standard OxII, normalised to δ13C, as described by Stenström et al.31) values of the ABA-B and BABAB extracts (shown in Table 4) are essentially identical (0.00265), whereas the 2ChlorOx extract is higher (0.00290) by 2σ compared to the other two methods. It is also interesting to compare the age results obtained for sample BRA-3271 which was previously speed-dated (i.e. AMS dating of CO2 gas of untreated sample)32 and dated again in this study after the application of different pretreatment procedures. The result obtained from the untreated sample is younger by 2200 14C years than the ages obtained from cellulose after pretreatment in this study. Contamination by 0.9% modern carbon in the untreated sample would account for this discrepancy.
Table 3 Carbon amount (%) following combustion and AMS 14C results of samples pretreated following the ABA-B, BABAB and 2ChlorOx protocols
| Bologna lab code BRA- |
Method |
Carbon content (%) |
F14C |
±1σ |
Age (14C BP) |
±1σ |
| 3275 |
BABAB |
44.0 |
0.54978 |
0.00145 |
4806 |
21 |
| 2ChlorOx |
34.9 |
0.55050 |
0.00141 |
4795 |
21 |
| 3276 |
BABAB |
42.4 |
0.30513 |
0.00097 |
9535 |
25 |
| 2ChlorOx |
40.3 |
0.30510 |
0.00097 |
9536 |
26 |
| 3272 |
BABAB |
44.7 |
0.28228 |
0.00094 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
27 |
| 2ChlorOx |
44.2 |
0.28292 |
0.00093 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 142 142 |
26 |
| 3274 |
BABAB |
42.2 |
0.15275 |
0.00068 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 093 |
36 |
| 2ChlorOx |
40.9 |
0.15584 |
0.00068 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 932 |
35 |
| 3271 |
ABA-B |
42.4 |
0.02589 |
0.00035 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 352 352 |
109 |
| BABAB |
41.6 |
0.02666 |
0.00035 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
107 |
| 2ChlorOx |
44.7 |
0.02636 |
0.00035 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 207 |
108 |
| 3282 |
ABA-B |
43.5 |
0.02592 |
0.00035 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
110 |
| BABAB |
40.4 |
0.02740 |
0.00036 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 897 |
106 |
| 2ChlorOx |
47.0 |
0.02606 |
0.00035 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 297 297 |
109 |
| 3283 |
BABAB |
45.3 |
0.02603 |
0.00036 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309 309 |
110 |
| 2ChlorOx |
51.4 |
0.02631 |
0.00036 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 223 |
105 |
| 3277 |
ABA-B |
39.9 |
0.01820 |
0.00033 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183 183 |
144 |
| BABAB |
40.8 |
0.01748 |
0.00032 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 505 |
149 |
| 2ChlorOx |
31.6 |
0.01802 |
0.00032 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 261 |
145 |
| 3278 |
ABA-B |
42.2 |
0.00169 |
0.00025 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
1 207 |
| BABAB |
41.3 |
0.00112 |
0.00025 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 613 613 |
1 807 |
| 2ChlorOx |
43.2 |
0.00316 |
0.00026 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254 254 |
661 |
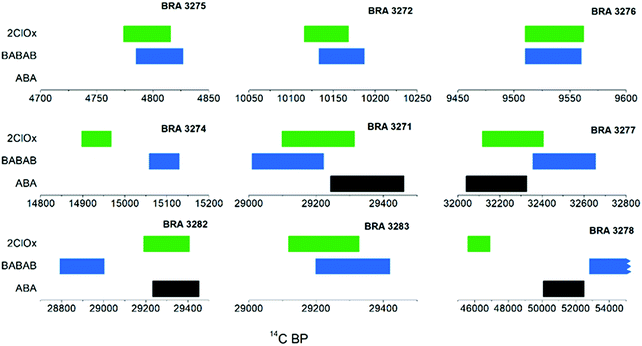 |
| | Fig. 2 Comparison of 14C age ranges (1σ) of the pretreatment methods. 7 out of 9 samples (background sample BRA-3273 excluded) are within 2σ error range. BRA-3278 and BRA-3282 show the most significant differences. | |
Table 4 Background F14C values measured in this study
| Bologna lab code BRA- |
Method |
F14C |
±1σ |
| 3273 |
ABA-B |
0.00264 |
0.00008 |
| BABAB |
0.00265 |
0.00009 |
| 2ChlorOx |
0.00290 |
0.00009 |
Conclusions
The extraction and dating of cellulose from glacial sub-fossil wood are a key tool for improving the resolution of the radiocarbon calibration curve beyond the Holocene period. Cellulose extraction is fundamental to ensure correct dating. We aimed to determine the most effective extraction procedure on the basis of cellulose yield and 14C age, particularly focussing on trees grown in the glacial period (older than 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 years cal BP). In light of the obtained results, the ABA-B method was sufficiently conservative in term of cellulose yield and efficiently removed contamination even from samples older than 30
226 years cal BP). In light of the obtained results, the ABA-B method was sufficiently conservative in term of cellulose yield and efficiently removed contamination even from samples older than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years cal BP. The 2ChlorOx method led to almost the same final yield of cellulose compared to ABA-B, but appeared slightly less capable of removing young contamination in very old and background samples. The BABAB method is the harshest pretreatment method to apply in the case of poorly preserved trees, and it led to the highest loss of material during pretreatment but the results were the most reliable. Therefore, the pretreatment procedure must be chosen according to the preservation state of the wood sample. Based on this study, we have adopted BABAB as our standard pretreatment protocol since it is the most adequate method and guarantees high 14C-dating quality. From our data and other studies referred to here, we can confirm that ABA-B pretreatment protocol represents a suitable alternative, especially for smaller sample quantities and wood samples with lower preservation status.
000 years cal BP. The 2ChlorOx method led to almost the same final yield of cellulose compared to ABA-B, but appeared slightly less capable of removing young contamination in very old and background samples. The BABAB method is the harshest pretreatment method to apply in the case of poorly preserved trees, and it led to the highest loss of material during pretreatment but the results were the most reliable. Therefore, the pretreatment procedure must be chosen according to the preservation state of the wood sample. Based on this study, we have adopted BABAB as our standard pretreatment protocol since it is the most adequate method and guarantees high 14C-dating quality. From our data and other studies referred to here, we can confirm that ABA-B pretreatment protocol represents a suitable alternative, especially for smaller sample quantities and wood samples with lower preservation status.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This project is funded by the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (grant agreement no. 803147 RESOLUTION, https://site.unibo.it/resolution-erc/en). We thank Prof. Nuno Bicho and the Ph.D. candidate Pedro Horta of the University of Algarve, Portugal, for the work done and the suggestions given to us during the RESOLUTION fieldwork in Portugal. We thank Dr. Giovanni Monegato and Prof. Alessandro Fontana for providing the Illegio sample and Prof. Alessandro Michetti for providing Cairo Montenotte sample. We are grateful to Joël Bourquin, Dr. Simon Fahrni and Dr. David Ramrath of the Ionplus laboratory of Zurich who made available to us the graphitisation equipment (AGE 3) and the pneumatic sample press (PSP), in addition to their personal availability and technical support. We acknowledge Dr. Lukas Wacker of the Laboratory of Ion Beam Physics of ETH for providing the AMS dates and for his personal availability. We thank Dr. Helmut Dalitz for technical and administrative support. We thank Prof. Luca Prodi, whose advice helped to improve this manuscript. We are very grateful to Dr. Helen Fewlass for her fundamental input and a British colleague who edited the English and considerably improved the manuscript. We acknowledge the Editor, Prof. P. Junk, and two anonymous reviewers for their constructive and helpful comments.
References
- M. Capano, C. Miramont, F. Guibal, B. Kromer, T. Tuna, Y. Fagault and E. Bard, Radiocarbon, 2018, 60, 51–74 CrossRef CAS.
- M. Němec, L. Wacker, I. Hajdas and H. Gäggeler, Radiocarbon, 2010, 52, 1358–1370 CrossRef.
- R. Gillespie, Radiocarbon, 2019, 61, 131–139 CrossRef CAS.
- O. Nechyporchuk, M. N. Belgacem and J. Bras, Ind. Crops Prod., 2016, 93, 2–25 CrossRef CAS.
- M. Prakash Menon, R. Selvakumar, P. Suresh kumar and S. Ramakrishna, RSC Adv., 2017, 7, 42750–42773 RSC.
- W. H. Danial, R. M. Taib, M. A. A. Samah, R. M. Salim and Z. A. Majid, RSC Adv., 2020, 42400–42407 RSC.
- K. J. Anchukaitis, M. N. Evans, T. Lange, D. R. Smith, S. W. Leavitt and D. P. Schrag, Anal. Chem., 2008, 80, 2035–2041 CrossRef CAS PubMed.
- J. B. Gaudinski, T. E. Dawson, S. Quideau, E. A. G. Schuur, J. S. Roden, S. E. Trumbore, D. R. Sandquist, S.-W. Oh and R. E. Wasylishen, Anal. Chem., 2005, 77, 7212–7224 CrossRef CAS PubMed.
- P. J. Reimer, W. E. N. Austin, E. Bard, A. Bayliss, P. G. Blackwell, C. Bronk Ramsey, M. Butzin, H. Cheng, R. L. Edwards, M. Friedrich, P. M. Grootes, T. P. Guilderson, I. Hajdas, T. J. Heaton, A. G. Hogg, K. A. Hughen, B. Kromer, S. W. Manning, R. Muscheler, J. G. Palmer, C. Pearson, J. van der Plicht, R. W. Reimer, D. A. Richards, E. M. Scott, J. R. Southon, C. S. M. Turney, L. Wacker, F. Adolphi, U. Büntgen, M. Capano, S. M. Fahrni, A. Fogtmann-Schulz, R. Friedrich, P. Köhler, S. Kudsk, F. Miyake, J. Olsen, F. Reinig, M. Sakamoto, A. Sookdeo and S. Talamo, Radiocarbon, 2020, 62, 725–757 CrossRef CAS.
- K. Schollaen, H. Baschek, I. Heinrich, F. Slotta, M. Pauly and G. Helle, Dendrochronologia, 2017, 44, 133–145 CrossRef.
- A. Hogg, J. Southon, C. Turney, J. Palmer, C. B. Ramsey, P. Fenwick, G. Boswijk, U. Büntgen, M. Friedrich, G. Helle, K. Hughen, R. Jones, B. Kromer, A. Noronha, F. Reinig, L. Reynard, R. Staff and L. Wacker, Radiocarbon, 2016, 58, 709–733 CrossRef CAS.
- H. M. Granja, T. A. M. Dte Groot and A. L. Costa, Sedimentology, 2008, 55, 1203–1226 CrossRef.
- G. Casadoro, E. Corona, F. Massari, M. G. Moretto, A. Paganelli, F. Terenziani and V. Toniello, Bollettino del Comitato Glaciologico Italiano, 1976, 22–63 Search PubMed.
- A. Sookdeo, B. Kromer, U. Büntgen, M. Friedrich, R. Friedrich, G. Helle, M. Pauly, D. Nievergelt, F. Reinig, K. Treydte, H.-A. Synal and L. Wacker, Radiocarbon, 2019, 62, 891–899 CrossRef.
- M. Friedrich, M. Knipping, P. Kroft, A. Renno, S. Schmidt, O. Ullrich and J. Vollbrecht, Arbeits- und Forschungsberichte zur sächsischen Bodendenkmalpflege, 2001, 43, 21–94 Search PubMed.
- G. Monegato, C. Ravazzi, M. Culiberg, R. Pini, B. Miloš, G. Calderoni, J. Jež and R. Perego, Palaeogeogr., Palaeoclimatol., Palaeoecol., 2015, 436, 23–40 CrossRef.
- I. Hajdas, L. Hendriks, A. Fontana and G. Monegato, Radiocarbon, 2017, 59, 727–737 CrossRef CAS.
- M. Friedrich, S. Remmele, B. Kromer, J. Hofmann, M. Spurk, K. Felix Kaiser, C. Orcel and M. Küppers, Radiocarbon, 2004, 46, 1111–1122 CrossRef CAS.
- M. Friedrich, B. Kromer, M. Spurk, J. Hofmann and K. Felix Kaiser, Quaternary International, 1999, 61, 27–39 CrossRef.
- B. Kromer, M. Spurk, S. Remmele, M. Barbetti and V. Joniello, Radiocarbon, 1997, 40, 351–358 CrossRef.
- K. F. Kaiser, M. Friedrich, C. Miramont, B. Kromer, M. Sgier, M. Schaub, I. Boeren, S. Remmele, S. Talamo, F. Guibal and O. Sivan, Quat. Sci. Rev., 2012, 36, 78–90 CrossRef.
- E. M. Scott, G. T. Cook and P. Naysmith, Radiocarbon, 2016, 52, 859–865 CrossRef.
- J. R. Southon and A. L. Magana, Radiocarbon, 2010, 52, 1371–1379 CrossRef CAS.
- J. Grumo, L. j. Jabber, J. Patricio, M. R. Magdadaro, A. Lubguban and A. Alguno, J. Appl. Fundam. Sci., 2017, 9, 124–133 CAS.
-
R. Shmulsky and P. D. Jones, Forest products and wood science: an introduction, Wiley-Blackwell, Chichester, West Sussex, UK, Ames, Iowa, 2011 Search PubMed.
- A. Baglieri, A. Ioppolo, M. Nègre and M. Gennari, Org. Geochem., 2007, 38, 140–150 CrossRef CAS.
- S. Khatami, Y. Deng, M. Tien and P. G. Hatcher, J. Environ. Qual., 2019, 48, 1565–1570 CrossRef CAS.
- L. Wacker, G. Bonani, M. Friedrich, I. Hajdas, B. Kromer, M. Němec, M. Ruff, M. Suter, H. A. Synal and C. Vockenhuber, Radiocarbon, 2010, 52, 252–262 CrossRef CAS.
- A. Taylor, B. Gartner and J. Morrell, Wood Fiber Sci., 2002, 34, 587–611 CAS.
- J. Baar, Z. Paschová, T. Hofmann, T. Kolář, G. Koch, B. Saake and P. Rademacher, Holzforschung, 2019, 74, 47–59 Search PubMed.
-
K. E. Stenström, G. Skog, E. Georgiadou, J. Genberg and A. Johansson, Nuclear Physics, Lund University, 2011, pp. 1–17 Search PubMed.
- A. Sookdeo, L. Wacker, S. Fahrni, C. P. McIntyre, M. Friedrich, F. Reinig, D. Nievergelt, W. Tegel, B. Kromer and U. Büntgen, Radiocarbon, 2017, 59, 933–939 CrossRef CAS.
Footnote |
| † Conventional term for radiocarbon calibrated dates corresponding to calendar years considering 1950 as present. Cal – (calibrated) BP – before present (1950). |
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Michael
Friedrich
a,
Michael
Friedrich
 b,
Bernd
Kromer
b,
Bernd
Kromer
 c,
Dragana
Paleček
c,
Dragana
Paleček
 a and
Sahra
Talamo
a and
Sahra
Talamo
 a
a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years,9 the limit of the radiocarbon method. Here we study sub-fossil tree remnants (wood fragments in which the organic component is not fully decomposed and is only partially fossilized) grown in the glacial period. We intend to improve the resolution of the radiocarbon calibration curve (used to convert 14C ages into calendar ages) beyond the current extent of the unbroken dendrochronological curve (back to 14
000 years,9 the limit of the radiocarbon method. Here we study sub-fossil tree remnants (wood fragments in which the organic component is not fully decomposed and is only partially fossilized) grown in the glacial period. We intend to improve the resolution of the radiocarbon calibration curve (used to convert 14C ages into calendar ages) beyond the current extent of the unbroken dendrochronological curve (back to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 years cal BP†) to allow more precise age determinations.9 Cellulose is the fraction of choice for dating trees.2,7,8,10 To obtain reliable 14C ages any contamination by extraneous carbon in the sub-fossil wood must be removed. Contamination can occur by young carbon, e.g. humic acid introduced from groundwater supply, or from 14C-free carbonates. For radiocarbon dating of Upper Pleistocene samples, contamination by young carbon is more problematic than contamination by fossil carbon: 1% of modern carbon contamination added to a sample of 37 000 14C BP will reduce its age by 5500 years, whereas 1% 14C-free carbon will increase the age by only 80 years. Therefore, when assessing the effectiveness of pretreatment procedures, older ages are generally considered more reliable. Several studies report cellulose extraction protocols for young Holocene trees as well as wood near/beyond the limit of 14C dating (>55
226 years cal BP†) to allow more precise age determinations.9 Cellulose is the fraction of choice for dating trees.2,7,8,10 To obtain reliable 14C ages any contamination by extraneous carbon in the sub-fossil wood must be removed. Contamination can occur by young carbon, e.g. humic acid introduced from groundwater supply, or from 14C-free carbonates. For radiocarbon dating of Upper Pleistocene samples, contamination by young carbon is more problematic than contamination by fossil carbon: 1% of modern carbon contamination added to a sample of 37 000 14C BP will reduce its age by 5500 years, whereas 1% 14C-free carbon will increase the age by only 80 years. Therefore, when assessing the effectiveness of pretreatment procedures, older ages are generally considered more reliable. Several studies report cellulose extraction protocols for young Holocene trees as well as wood near/beyond the limit of 14C dating (>55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years BP).1,3,11 We want to identify the most effective pretreatment procedure to extract cellulose and efficiently remove exogenous contamination from sub-fossil trees spanning the glacial period. We compare three different protocols, including a novel technique which has been recently published. We selected subfossil trees of different species from different time periods to identify the limits and capabilities of each extraction method. For most of the samples the age is already known, either from dendrochronology or from radiocarbon dating. The results are evaluated on the basis of the cellulose yield and the 14C age.
000 years BP).1,3,11 We want to identify the most effective pretreatment procedure to extract cellulose and efficiently remove exogenous contamination from sub-fossil trees spanning the glacial period. We compare three different protocols, including a novel technique which has been recently published. We selected subfossil trees of different species from different time periods to identify the limits and capabilities of each extraction method. For most of the samples the age is already known, either from dendrochronology or from radiocarbon dating. The results are evaluated on the basis of the cellulose yield and the 14C age.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years BP). Recently, samples from Győrújfalu were unsuccessfully pretreated (cellulose was not obtained) at Mannheim radiocarbon laboratory. To verify their preservation state, they were tested again. The sample from Illegio is a larch stump (Larix decidua) found in sediments dating to the LGM (Last Glacial Maximum) in the basin of the Tagliamento River in the Carnic alps of the Friuli-Venezia-Giulia region (Italy), as described in Monegato et al.16 The sample analysed here was found in situ together with another larch trunk, dated by Hajdas et al.17 The Late Glacial pine (Pinus sylvestris L.) from Breitenthal was found in a gravel pit in southern Germany near the Günz River, together with other pines. They were dendrochronologically analysed by Friedrich et al.18 Samples from Ebensfeld and Freising are Holocene oak (Quercus spec.) and pine (Pinus sylvestris L.) found in Quaternary deposits of the rivers Isar and Main in southern Germany. They were remnants of old riparian forests buried by river activity and found in gravel pits. They are parts of the Holocene oak and pine chronologies of central Europe.18,19
000 years BP). Recently, samples from Győrújfalu were unsuccessfully pretreated (cellulose was not obtained) at Mannheim radiocarbon laboratory. To verify their preservation state, they were tested again. The sample from Illegio is a larch stump (Larix decidua) found in sediments dating to the LGM (Last Glacial Maximum) in the basin of the Tagliamento River in the Carnic alps of the Friuli-Venezia-Giulia region (Italy), as described in Monegato et al.16 The sample analysed here was found in situ together with another larch trunk, dated by Hajdas et al.17 The Late Glacial pine (Pinus sylvestris L.) from Breitenthal was found in a gravel pit in southern Germany near the Günz River, together with other pines. They were dendrochronologically analysed by Friedrich et al.18 Samples from Ebensfeld and Freising are Holocene oak (Quercus spec.) and pine (Pinus sylvestris L.) found in Quaternary deposits of the rivers Isar and Main in southern Germany. They were remnants of old riparian forests buried by river activity and found in gravel pits. They are parts of the Holocene oak and pine chronologies of central Europe.18,19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP
000 14C BP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP (untreated)
000 14C BP (untreated)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP (untreated)
000 14C BP (untreated)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 14C BP (untreated)
000 14C BP (untreated)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–20
100–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 years BP, when the Atlantic ocean was below the present level.12 The site was covered by fluvial sandy sediments during the Pleniglacial phase of the last ice age. Due to sea-level rise in the post-glacial period, the trees were submerged after that time, which explains the low level of bacterial destruction (i.e. good cellulose preservation). The larch trees from Illegio17 (BRA-3277) and Revine13 (BRA-3274) and the pine from Cairo Montenotte (BRA-3278) were found in glacio-fluvial and colluvial sediments which kept the wood permanently under anoxic conditions and lead to remarkably good cellulose preservation, particularly at Revine and Cairo Montenotte. In comparison, the lower level of preservation at Illegio (BRA-3277) may be the result of local bedding conditions at that site. For the Holocene trees (BRA-3275; BRA-3276; BRA-3272) found in fluvial sediments,18,19 the permanently water-saturated conditions below the groundwater table was the crucial factor for high levels of preservation. The variation in preservation may be due to the local situation. The good preservation of the oak (BRA-3275) is due to the natural durability of this species due to the high content of tannins in oak heartwood.30
700 years BP, when the Atlantic ocean was below the present level.12 The site was covered by fluvial sandy sediments during the Pleniglacial phase of the last ice age. Due to sea-level rise in the post-glacial period, the trees were submerged after that time, which explains the low level of bacterial destruction (i.e. good cellulose preservation). The larch trees from Illegio17 (BRA-3277) and Revine13 (BRA-3274) and the pine from Cairo Montenotte (BRA-3278) were found in glacio-fluvial and colluvial sediments which kept the wood permanently under anoxic conditions and lead to remarkably good cellulose preservation, particularly at Revine and Cairo Montenotte. In comparison, the lower level of preservation at Illegio (BRA-3277) may be the result of local bedding conditions at that site. For the Holocene trees (BRA-3275; BRA-3276; BRA-3272) found in fluvial sediments,18,19 the permanently water-saturated conditions below the groundwater table was the crucial factor for high levels of preservation. The variation in preservation may be due to the local situation. The good preservation of the oak (BRA-3275) is due to the natural durability of this species due to the high content of tannins in oak heartwood.30

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160
160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 142
142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093
093![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932
932![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 352
352![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115
115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207
207![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343
343![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897
897![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 297
297![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309
309![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223
223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183
183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505
505![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261
261![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285
285![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 613
613![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254
254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 years cal BP). In light of the obtained results, the ABA-B method was sufficiently conservative in term of cellulose yield and efficiently removed contamination even from samples older than 30
226 years cal BP). In light of the obtained results, the ABA-B method was sufficiently conservative in term of cellulose yield and efficiently removed contamination even from samples older than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years cal BP. The 2ChlorOx method led to almost the same final yield of cellulose compared to ABA-B, but appeared slightly less capable of removing young contamination in very old and background samples. The BABAB method is the harshest pretreatment method to apply in the case of poorly preserved trees, and it led to the highest loss of material during pretreatment but the results were the most reliable. Therefore, the pretreatment procedure must be chosen according to the preservation state of the wood sample. Based on this study, we have adopted BABAB as our standard pretreatment protocol since it is the most adequate method and guarantees high 14C-dating quality. From our data and other studies referred to here, we can confirm that ABA-B pretreatment protocol represents a suitable alternative, especially for smaller sample quantities and wood samples with lower preservation status.
000 years cal BP. The 2ChlorOx method led to almost the same final yield of cellulose compared to ABA-B, but appeared slightly less capable of removing young contamination in very old and background samples. The BABAB method is the harshest pretreatment method to apply in the case of poorly preserved trees, and it led to the highest loss of material during pretreatment but the results were the most reliable. Therefore, the pretreatment procedure must be chosen according to the preservation state of the wood sample. Based on this study, we have adopted BABAB as our standard pretreatment protocol since it is the most adequate method and guarantees high 14C-dating quality. From our data and other studies referred to here, we can confirm that ABA-B pretreatment protocol represents a suitable alternative, especially for smaller sample quantities and wood samples with lower preservation status.

