 Open Access Article
Open Access ArticleSynthesis, polytypism, and dehydration behaviour of nitrate-intercalated layered double hydroxides of Ca and Al†
Anand N.
Narayanappa
a,
Supreeth
Nagendran
 *b and
P. Vishnu
Kamath
*b and
P. Vishnu
Kamath
 a
a
aDepartment of Chemistry, Central College, Bangalore University, Bangalore 560 001, India. E-mail: supreethn4@gmail.com
bDepartment of Chemistry, University of Cambridge, Cambridge CB2 1EW, UK
First published on 16th March 2021
Abstract
Rapid precipitation of the hydroxide phase from a mixed metal nitrate solution comprising Ca2+ and Al3+ ions leads to the formation of a one-layered hexagonal polytype of [Ca–Al] layered double hydroxide. In contrast, slow precipitation results in a three-layered polytype of rhombohedral symmetry. Both polytypes comprise a stacking of positively charged metal hydroxide layers having the composition [Ca2Al(OH)6(H2O)2]+. The Ca2+ ions are seven coordinated with water molecules providing the seventh coordination. In the absence of any prior knowledge of the structure of the 1H polytype, translationengleiche and klassengleiche graphs were used to arrive at the space group and atom positions of the 1H polytype. Rietveld refinements of the structures of the two polytypes show that nitrate ions are intercalated in the interlayer gallery with its molecular plane inclined to the metal hydroxide layer. The angle of inclination in the 1H polytype (∼61°) is greater than that in the 3R polytype (∼30°). When the 1H polytype is completely dehydrated, the metal hydroxide layers undergo a rigid translation relative to one another resulting in (i) a 1H → 3R interpolytype transition, and (ii) the grafting of the nitrate ion to the metal hydroxide layer to satisfy the seventh coordination of the Ca2+ ion. The as-prepared 3R polytype has a mixed anion interlayer comprising nitrate and hydroxyl ions. On heating, this phase progressively loses crystallinity before decomposition.
1. Introduction
Hydrotalcite-like layered double hydroxides (LDHs) comprise a stacking of positively charged ionocovalently bonded metal hydroxide layers of the composition [Mg1−xM(III)x(OH)2]x+(M = Al3+, Cr3+, Fe3+; 0.2 ≤ x ≤ 0.33) (layer group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1) with anions in the interlayer region. Given the anisotropy in bonding, the metal hydroxide layers are stacked in different ways leading to a diversity of polytypes. Bookin and Drits1 were the first to comprehensively describe the complete universe of polytypes among LDH systems by assuming a cation-disordered layer. In this layer, the Mg2+ and M3+ ions are statistically distributed in all the six-coordinate cation sites in a close-packed AC-layer of hydroxyl ions. Using this pseudo-single cation layer, a one-layered (1H), three two-layered (2H1, 2H2, and 2H3), and two three-layered polytypes (3R1, 3R2) of hexagonal (H) and rhombohedral (R) symmetries were theoretically predicted. These polytypes differ from one another in the manner in which successive metal hydroxide layers are translated relative to each other. Known structures of mineral, as well as laboratory-synthesized LDHs, were found to belong to 2H1 (space group P63/mcm),2 3R1,3 and 3R24 (space group R
m1) with anions in the interlayer region. Given the anisotropy in bonding, the metal hydroxide layers are stacked in different ways leading to a diversity of polytypes. Bookin and Drits1 were the first to comprehensively describe the complete universe of polytypes among LDH systems by assuming a cation-disordered layer. In this layer, the Mg2+ and M3+ ions are statistically distributed in all the six-coordinate cation sites in a close-packed AC-layer of hydroxyl ions. Using this pseudo-single cation layer, a one-layered (1H), three two-layered (2H1, 2H2, and 2H3), and two three-layered polytypes (3R1, 3R2) of hexagonal (H) and rhombohedral (R) symmetries were theoretically predicted. These polytypes differ from one another in the manner in which successive metal hydroxide layers are translated relative to each other. Known structures of mineral, as well as laboratory-synthesized LDHs, were found to belong to 2H1 (space group P63/mcm),2 3R1,3 and 3R24 (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) polytypes.
m) polytypes.
Evidence from NMR spectroscopy5,6 and powder diffraction7,8 showed that the trivalent cation (M) is ordered with respect to Mg2+ ions in the metal hydroxide layers. Cation ordering reduces the symmetry of the metal hydroxide layer (layer group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 1m). Using the cation-ordered metal hydroxide layer as the building block, the three-layered polytypes of rhombohedral symmetry (3R1 and 3R2), were shown to be one-layered polytypes of monoclinic symmetry (1M1/1M2) (space group C2/m) in which the stacking of the metal hydroxide layers is non-orthogonal (β ∼ 96°–106°), and the layers are translated by (±a/3, 0, z) relative to one another.9,10
1m). Using the cation-ordered metal hydroxide layer as the building block, the three-layered polytypes of rhombohedral symmetry (3R1 and 3R2), were shown to be one-layered polytypes of monoclinic symmetry (1M1/1M2) (space group C2/m) in which the stacking of the metal hydroxide layers is non-orthogonal (β ∼ 96°–106°), and the layers are translated by (±a/3, 0, z) relative to one another.9,10
The Ca2+-containing hydrocalumite-like LDHs are different from the better known hydrotalcite-like LDHs, as the Ca2+ ion is seven-coordinated. The seventh coordination is with the intercalated water molecule in the interlayer. To facilitate bonding with the intercalated water molecule, the Ca2+ ion is displaced to an acentric position away from the plane of the Al3+ ions. The resulting metal hydroxide layer [Ca2M(OH)6(H2O)2]+ is puckered. Although the layer group is the same as that in the cation-ordered hydrotalcite-like layer, not all stacking sequences are valid for the [Ca–Al] layer, as the puckered structure offers steric hindrance. The restriction on translation severely limits the diversity of polytypes in the [Ca–Al] LDHs. The best known hydrocalumite-like LDH with intercalated Cl− ions, also known as Friedel's salt, crystallizes as a three-layered rhombohedral polytype (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), in which the metal hydroxide layers are translated by (1/3, 2/3, z) relative to one another.11 The composition of Friedel's salt is [Ca2Al(OH)6(H2O)2]Cl. Hydrocalumite-like LDHs with other anions such as Br−, I−, ClO4−, and SO42−-hereinafter abbreviated to the symbol [Ca–Al–A] (A = anion), have also been assigned to the structure of the 3R polytype (space group R
), in which the metal hydroxide layers are translated by (1/3, 2/3, z) relative to one another.11 The composition of Friedel's salt is [Ca2Al(OH)6(H2O)2]Cl. Hydrocalumite-like LDHs with other anions such as Br−, I−, ClO4−, and SO42−-hereinafter abbreviated to the symbol [Ca–Al–A] (A = anion), have also been assigned to the structure of the 3R polytype (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ).12–14
).12–14
Among all the anions, the NO3− ion is unique in that its orientation depends on the layer charge as well as the degree of hydration. In the hydrotalcite-like LDH system, the NO3− ion is intercalated with its molecular plane parallel to the metal hydroxide layer when the layer charge is low (x ≤ 0.25). At high layer charge (x = 0.33), the nitrate ion orients itself with its molecular plane nearly perpendicular to the metal hydroxide layer.15 A similar change in the orientation of the intercalated nitrate ion is also brought about with a variation in the degree of hydration of the interlayer.16 Within the hydrocalumite system, there is relatively less work reported on the nitrate-intercalated LDHs, possibly due to the poor affinity of the metal hydroxide layer for the NO3− ion. In an earlier study, the [Ca–Al–NO3] LDH has been reported to be a two-layered polytype (space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1).17 In this work, the synthesis and structure refinement of the [Ca–Al–NO3] LDH is undertaken to study the polytypism if any, inherent to this system, and study the structural changes induced as a function of temperature and humidity.
c1).17 In this work, the synthesis and structure refinement of the [Ca–Al–NO3] LDH is undertaken to study the polytypism if any, inherent to this system, and study the structural changes induced as a function of temperature and humidity.
2. Experimental section
2.1 Synthesis
The 1H polytype of [Ca–Al–NO3] LDH was synthesized by rapid precipitation by the addition of NaOH (50 mL, 0.75 mol L−1) solution to a mixed metal nitrate solution containing Ca2+, and Al3+([Ca]/[Al] = 2) ions taken in an autoclave (volume 65 mL; 50% filling). Five times the stoichiometric requirement of nitrate ions taken in the form of NaNO3 was pre-dissolved in the mixed metal nitrate solution. The resulting slurry was aged at 90 °C for 48 h before being centrifuged and washed until the pH of the wash was 11.9.The 3R polytype of [Ca–Al–NO3] LDH was synthesized by slow addition (0.1 mL min−1) of the mixed metal nitrate solution ([Ca]/[Al] = 2, total concentration = 0.39 M) to a reservoir of NaNO3 solution (80 mL) containing ten times the stoichiometric excess of NO3− ions. A solution of NaOH (2 M) was dispensed using a Metrohm Model 836 Titrando, operating in the pH STAT mode. The pH of the reaction mixture was maintained at 11.5 at 60 °C. N2 gas was bubbled during precipitation and ageing to minimize carbonate contamination. At the end of the precipitation, the slurry was aged in the mother liquor under N2 blanket for 18 h under stirring. The precipitates were centrifuged, washed with boiled Type II water (specific resistance 15 MΩ cm, Millipore Academic water purification system), and dried in a hot air oven at 60 °C.
2.2 Characterization
The Ca content was determined by Atomic Absorption Spectroscopy (Shimadzu Model AA-6650 atomic absorption spectrometer). The Al content was estimated by gravimetry. The nitrate content was estimated by ion chromatography (Metrohm Model 861 Advanced Compact Ion Chromatograph with Metrosep SUP5 150 column). The presence of NO3− anions was qualitatively verified by the presence of its characteristic absorption bands in the infrared spectra (Bruker Alpha-P FTIR spectrometer, diamond ATR, 400–4000 cm−1, resolution 4 cm−1). The water content was estimated by thermogravimetric analysis (Mettler Toledo TGA/SDTA 851e system driven by STARe 7.01 software, 25–900 °C, heating rate 5 °C min−1, flowing N2). All the LDHs were characterized by Powder X-ray Diffraction (Bruker Model D8 Advance Powder Diffractometer, Cu Kα source, λ = 1.5418 Å, Ni filter) operating in reflection geometry. The surface morphologies of the LDHs were observed using the Scanning Electron Microscope (SEM) images recorded on the VEGA3 TESCAN. Code APPPLEMAN, a part of the PROZKI suite of programs was used to index the PXRD patterns.As the first step in the structure refinement procedure, the layer to layer relationship was established by the simulation of the major Bragg reflections using code DIFFaX. These simulations facilitated the construction of a partial structure model comprising the most probable stacking sequence of the metal hydroxide layers. In the next step, the position and orientation of the intercalated nitrate anions were determined using Code FOX (Free Objects for Crystallography). Within the formalism employed in Code FOX, the nitrate anion is introduced as a free molecule into interlayer space and is permitted to randomly translate and rotate. After each step, the PXRD pattern is calculated and compared with the observed pattern. A Monte Carlo process is used to minimize the error. The various R parameters are used as error functions. A sufficient number of steps are carried out to fully explore the entire volume of the interlayer space. The position and orientation of the nitrate ion are periodically visualized to monitor the nonbonding contact distances between the nitrate ion and the metal hydroxide layer. In this process, the structure is optimized in direct space and a structure model is generated. This structure model is exported into code GSAS (General Structure Analysis System) to complete the refinement conventionally in reciprocal space. Where required difference Fourier maps are evaluated to account for the missing electron density and locate water molecules. CSD-1991917 (1H polytype), -1991904 (dehydrated 3R polytype), and -1991916 (as-prepared 3R polytype) contain the supplementary crystallographic data for this manuscript. These data can be obtained free of charge from “The Cambridge Crystallographic Data Center” through http://www.ccdc.cam.ac.uk/structures.
3. Results and discussion
The CaO–Al2O3–H2O phase diagram includes several ternary hydrated oxide/hydroxide phases which form competitively with hydrocalumite-like phases. The latter crystallizes in a layered structure, characterized by the appearance of basal reflections (00l) in the low angle region (5–25° 2θ) and two sharp hk0, hkl reflections in the high angle region (55–60°) in their powder X-ray diffraction (PXRD) patterns. Several coprecipitation reactions were carried out by empirically varying the precipitation conditions such as the concentration of the base, the total volume of the reaction mixture, pH, and the ageing time. Two distinctly different layered phases were obtained following the synthesis conditions described in Fig. 1. The PXRD pattern of one of the phases was indexed to a one-layered cell of hexagonal symmetry, while the PXRD pattern of the other was indexed to a three-layered cell of rhombohedral symmetry (Fig. S1 and Table S1, ESI†). The former phase is hitherto not reported. The basal spacing of the hexagonal phase, co = c = 8.62 Å, (c = nco, where n is the number of layers in a unit cell and co is the distance between two consecutive metal hydroxide layers) is comparable to that of the rhombohedral phase, co = c/3 = 8.48 Å, showing that the disposition of the intercalated species in the two phases is very similar. The two phases also have comparable a-parameters (∼5.75 Å) showing that the ionocovalently bonded metal hydroxide layer is invariant, and they differ only in the manner in which the layers are stacked. This is a feature characteristic of polytypism and we label the two phases as 1H (H: hexagonal), and 3R (R: rhombohedral) respectively. | ||
| Fig. 1 A schematic representation of the synthesis, structure, and temperature/humidity induced polytypism in (a) 1H, and (b) 3R polytypes of [Ca–Al–NO3] LDH. | ||
Chemical analysis of the two phases carried out by a combination of different independent techniques showed that the [Ca2+]/[Al3+] ∼ 2, the nominal value in both the phases. The 1H polytype showed a [Al3+]/[NO3−] ∼ 1, close to the nominal value, whereas this ratio was ∼0.5 in the 3R polytype. In the absence of any other anion, as evidenced by ion chromatography and infrared spectroscopy (see Fig. S2 for IR spectra, ESI†), the nitrate deficiency was compensated by the inclusion of hydroxyl ions in the interlayer. Taken together with intercalated water content estimated by TGA (Fig. S3, ESI†), the approximate formulae of the two LDHs were found to be [Ca2Al(OH)6]NO3·2H2O and [Ca2Al(OH)6](NO3)0.5(OH)0.5·2H2O for 1H and 3R polytypes respectively (Table 1). The morphology of both the 1H and 3R polytypes comprises hexagonal platelets with a small degree of edge deformation (Fig. S4, ESI†).
| Polytype | Ca2+ (mol%) | Al3+ (mol%) | NO3− (mol%) | Total mass loss (%) | Approximate formula |
|---|---|---|---|---|---|
| Values in the parentheses correspond to the nominal composition. | |||||
| 1H | 0.67 (0.67) | 0.33 (0.33) | 0.33 (0.33) | 48.0 (46.9) | [Ca2Al(OH)6]NO3·2H2O |
| 3R | 0.67 (0.67) | 0.33 (0.33) | 0.17 (0.33) | 47.0 (42.7) | [Ca2Al(OH)6](NO3)0.5(OH)0.5·2H2O |
3.1 Structure refinement of the 1H polytype
In the absence of any previous report of a 1H polytype in the hydrocalumite-like LDH system, structure refinement of this polytype is of much interest. The following questions arise in the choice of a suitable structure model for the 1H polytype:(i) What is the plausible space group?
(ii) Since the structure of the metal hydroxide layer is known, can a partial structure model be constructed?
(iii) How are the intercalated nitrate ions and water molecules packed in the interlayer?
Among the hydrotalcite-like LDHs, the cation ordered mineral pyroaurite [Mg–Fe–CO3] was found to crystallize in the P63/mcm space group.2 This structure is a 2H1 polytype in which successive metal hydroxide layers are related by a mirror plane. If a single metal hydroxide layer is represented by the symbol P and its mirror image by ![[P with combining macron]](https://www.rsc.org/images/entities/char_0050_0304.gif) , the stacking sequence in mineral pyroaurite is ⋯P
, the stacking sequence in mineral pyroaurite is ⋯P![[P with combining macron]](https://www.rsc.org/images/entities/char_0050_0304.gif) P
P![[P with combining macron]](https://www.rsc.org/images/entities/char_0050_0304.gif) ⋯. In an earlier study, the nitrate-intercalated hydrocalumite-like LDH was also assigned to a two-layered polytype of hexagonal symmetry within the space group P
⋯. In an earlier study, the nitrate-intercalated hydrocalumite-like LDH was also assigned to a two-layered polytype of hexagonal symmetry within the space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1.17 In the P
c1.17 In the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1 space group, the successive layers are related by inversion symmetry. The P
c1 space group, the successive layers are related by inversion symmetry. The P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1 space group appears as a subgroup in the translationengleiche graphs of two hexagonal summits, namely, P63/mcm, and P6/mcc.18
c1 space group appears as a subgroup in the translationengleiche graphs of two hexagonal summits, namely, P63/mcm, and P6/mcc.18
The PXRD pattern of the 1H polytype reported here could be simulated by stacking the [Ca–Al] metal hydroxide layer using the stacking vector (0, 0, 1) (see Fig. S5, ESI†). This shows the stacking sequence is ⋯PPP⋯ and successive layers are not related to one another either by reflection or by inversion. The space group of the 1H polytype should therefore be a subgroup of P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1. Elimination of the c-glide yields the P
c1. Elimination of the c-glide yields the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (Scheme 1) in both summits.
space group (Scheme 1) in both summits.
The hydrocalumite-like LDHs with intercalated Cl−, Br−, or ClO4− anions have been assigned to R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group. The P
space group. The P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) is related to R
is related to R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) by the klassengleiche relationship18 due to the common crystal class. The following transformations in the special positions are observed.
by the klassengleiche relationship18 due to the common crystal class. The following transformations in the special positions are observed.
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
|
|---|---|---|
| Ca, H2O | 6c (0, 0, z) | 2c (0, 0, z), 2d (1/3, 2/3, z) |
| Al | 3a (0, 0, 0) | 1a (0, 0, 0) |
In the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, Al3+ occupies the 3a (0, 0, 0) position while Ca2+ occupies the 6c (2/3, 1/3, z) position (symmetry equivalent to (0, 0, z)). In the P
space group, Al3+ occupies the 3a (0, 0, 0) position while Ca2+ occupies the 6c (2/3, 1/3, z) position (symmetry equivalent to (0, 0, z)). In the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, the rhombohedral 3a reduces to a site of lower degeneracy of 1a (0, 0, 0), and the rhombohedral 6c (0, 0, z) splits into two sites of lower degeneracy of 2c (0, 0, z) and 2d (1/3, 2/3, z). The placement of Al3+ at 1a (0, 0, 0) and Ca at 2d (1/3, 2/3, z) in P
space group, the rhombohedral 3a reduces to a site of lower degeneracy of 1a (0, 0, 0), and the rhombohedral 6c (0, 0, z) splits into two sites of lower degeneracy of 2c (0, 0, z) and 2d (1/3, 2/3, z). The placement of Al3+ at 1a (0, 0, 0) and Ca at 2d (1/3, 2/3, z) in P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) provides for the correct composition. The O atom of the intercalated water molecule is also placed at 2d (1/3, 2/3, z) to satisfy its relationship with the position of the Ca2+ ion. The O atom of the OH− ion was placed in the general 6g (x, y, z) site and the coordinates were refined to generate the approximate bond lengths and bond angles of the metal hydroxide layer.
provides for the correct composition. The O atom of the intercalated water molecule is also placed at 2d (1/3, 2/3, z) to satisfy its relationship with the position of the Ca2+ ion. The O atom of the OH− ion was placed in the general 6g (x, y, z) site and the coordinates were refined to generate the approximate bond lengths and bond angles of the metal hydroxide layer.
In this manner, a partial structure model for the 1H polytype was constructed, and input into code FOX.19 The nitrate ion was introduced into the interlayer space as a free molecule (N–O bond length 1.26 Å; bond angle 120°) and allowed to translate and rotate. The N atom of the nitrate ion was confined midway in the interlayer space (z = 0.5) to eliminate crystal chemically unphysical positions. After each step, the powder diffraction pattern was computed and compared with the observed pattern and the error was minimized using a Monte Carlo procedure. The goodness of fit parameters, Rp and Rwp were used as error functions. This procedure amounts to structure optimization in direct space and provides an approximate structure model in situations where none exists. The structure optimization was terminated at Rp = 0.07, and Rwp = 0.15. The structure model obtained at this stage was exported to code GSAS20 for completing the refinement in the reciprocal space. An orientation parameter was introduced to correct for the residual intensity under the basal reflections (00l). The resulting Rietveld fit obtained after refining the position of Ca2+, hydroxyl O atom (Oh), and intercalated water (Ow) yields a featureless difference profile (Fig. 2 and Tables 2, 3). The refined structure (Fig. 3) shows that the nitrate ion is oriented with its molecular plane inclined at ∼61° to the metal hydroxide layer. The bond lengths and bond angles (Table S2, ESI†) show that two of the three oxygen atoms of the nitrate ion (ON2 and ON3) are strongly hydrogen-bonded to the metal hydroxide layer (ON2–Oh 2.59 Å; ON3–Oh 2.66 Å). ON3 is also strongly hydrogen-bonded to the intercalated water (ON3–Ow 2.36 Å).
| As-prepared | Dehydrated | |
|---|---|---|
| a March–Dollase orientation parameters along 001a, 200b, 003c and 2-10d planes respectively. | ||
| Molecular formula | [Ca2Al(OH)6][NO3]·2H2O | [Ca6Al3(OH)6][NO3]3 |
| Crystal symmetry | Trigonal | Rhombohedral |
| Space group |
P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
| Cell parameters | a = 5.74 Å; c = 8.62 Å | a = 5.75 Å; c = 23.98 Å |
| Volume of the cell (Å3) | 246.95 | 685.96 |
| Data points | 3750 | 3652 |
| Parameters refined | 35 | 42 |
| Orientation parametera | 0.41a, 0.48b | 0.68c, 1.94d |
| R wp | 0.13 | 0.12 |
| R p | 0.10 | 0.10 |
| R(F2) | 0.16 | 0.14 |
| χ 2 | 2.1 | 3.1 |
| Atom type | Wyckoff position | x | y | z | Occupancy |
|---|---|---|---|---|---|
| Ca | 2d | 1/3 | 2/3 | 0.06542 | 1.0 |
| Al | 1a | 0 | 0 | 0 | 1.0 |
| Oh | 6g | −0.05528 | 0.24071 | 0.12113 | 1.0 |
| Ow | 2d | 1/3 | 2/3 | 0.36265 | 1.0 |
| N | 6g | 0.53146 | 0.36294 | 0.5 | 0.167 |
| ON1 | 6g | 0.72582 | 0.34849 | 0.46385 | 0.167 |
| ON2 | 6g | 0.53637 | 0.48380 | 0.63231 | 0.167 |
| ON3 | 6g | 0.32777 | 0.25787 | 0.40300 | 0.167 |
3.2 Dehydration behaviour of 1H polytype
As seen in the TGA profile (Fig. S3, ESI†), the 1H polytype is completely dehydrated at 140 °C. The in situ PXRD pattern shows compression of the basal spacing by ∼0.63 Å and was indexed to a three-layered cell of rhombohedral symmetry with a = 5.74 Å, and c = 23.98 Å (Table S3, ESI†). This shows a temperature-induced 1H → 3R interpolytype transition (Fig. 1a), which is reversible and achieved by cooling and rehydration at relative humidity 50% (see Fig. S6, ESI†). A DIFFaX21,22 simulation of the PXRD pattern (see Fig. S7, ESI†) using the stacking vector (1/3, 2/3, z) generates all the Bragg reflections of the PXRD pattern obtained at 140 °C. This enabled the construction of a partial structure model, based on the reported structure of Friedel's salt.11 Using the same methodology employed earlier, for the 1H polytype, the structure was refined and the Rietveld fit (Fig. 4 and Table 2) yielded acceptable R-parameters. The refined structure (Fig. 5 and Table 4) shows that in the absence of intercalated water, the nitrate ion has moved to a new position and reoriented itself in such a way as to provide the seventh coordination for the Ca2+ ion. A comparison of the 1H and 3R structures in the polyhedral representation (Fig. 1) exemplifies this change. The Ca–ON3 bond length at 2.34 Å is close to the Ca–Oh bond length at 2.46 Å (Table S2, ESI†), showing that the ON3 atom of the nitrate ion is well within the first coordination sphere of the Ca2+ ion, thereby stabilizing the structure. The angle between the plane of the nitrate ion and the metal hydroxide layer at ∼57° is similar to that observed in the 1H polytype.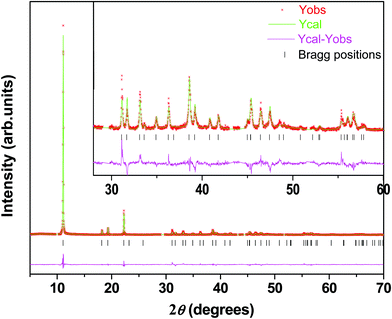 | ||
| Fig. 4 A Rietveld fit of the PXRD pattern of the 3R polytype obtained by the dehydration of the 1H polytype of [Ca–Al–NO3] LDH. | ||
| Atom type | Wyckoff position | x | y | z | Occupancy |
|---|---|---|---|---|---|
| Ca | 6c | 1/3 | 2/3 | 0.02305 | 1.0 |
| Al | 3a | 0 | 0 | 0 | 1.0 |
| Oh | 18f | 0.30494 | 0.06787 | 0.04376 | 1.0 |
| N | 18f | 0.16021 | 0.04897 | 0.51814 | 0.167 |
| ON1 | 18f | 0.42726 | 0.15943 | 0.51963 | 0.167 |
| ON2 | 18f | 0.03469 | −0.07875 | 0.47833 | 0.167 |
| ON3 | 18f | 0.04655 | 0.12311 | 0.54944 | 0.167 |
3.3 Structure refinement of the as-prepared 3R polytype
The as-prepared 3R polytype obtained by slow precipitation was found to be nitrate-deficient (Table 1). The deficiency was compensated by the inclusion of OH− ions to balance the charge on the metal hydroxide layer. Such an assumption can be justified on several grounds.(i) The slow precipitation was carried out at high pH (11.5) thus exposing the LDH slurry to a preponderance of OH− ions in the mother liquor.
(ii) The intercalated nitrate ion is known to be a good leaving group in anion exchange reactions.
(iii) The intercalated OH− ions form strong hydrogen bonds with the metal hydroxide layer and have the capacity to exchange protons with intercalated water.
All these factors operate synergistically to promote nitrate exchange for hydroxyl ions from the solution. The total mass loss observed in the TGA profile (observed 47%, expected 42.7%) supports the NO3−–OH− mixed anion composition of the interlayer. Structure refinement was carried out by the methodology already described using a combination of codes FOX and GSAS, by fixing the anion site occupancy to the experimentally determined anion composition. The resulting Rietveld fit (Fig. 6 and Table 5) is satisfactory with acceptable R values. The molecular plane of the nitrate ion is inclined to the plane of the metal hydroxide layer at ∼30° (Fig. 7 and Table 6). One of the oxygen atoms of the intercalated nitrate ion (ON2) is within H-bonding distances of the metal hydroxide layer (Table S4, ESI†).
| Molecular formula | [Ca2Al(OH)6][NO3]0.5(OH)0.5·2H2O |
|---|---|
| a March–Dollase orientation parameters along 003a, and 202b planes respectively. | |
| Crystal symmetry | Rhombohedral |
| Space group | R 3 |
| Cell parameters | a = 5.75 Å; c = 25.46 Å |
| Volume of the cell (Å3) | 729.80 |
| Data points | 3546 |
| Parameters refined | 35 |
| Orientation parametera | 0.38a, 0.5b |
| R wp | 0.14 |
| R p | 0.11 |
| R(F2) | 0.10 |
| χ 2 | 5.3 |
| Atom type | Wyckoff position | x | y | z | Occupancy |
|---|---|---|---|---|---|
| Ca | 6c | 2/3 | 1/3 | 0.0203 | 1.0 |
| Al | 3a | 0 | 0 | 0 | 1.0 |
| Oh | 18f | 0.24503 | −0.03009 | 0.03361 | 1.0 |
| Ow | 6c | 2/3 | 1/3 | 0.11865 | 1.0 |
| N | 18f | 0.63117 | 0.05927 | 0.17018 | 0.083 |
| O1 | 18f | 0.57481 | 0.19306 | 0.14388 | 0.111 |
| O2 | 18f | 0.45803 | −0.19091 | 0.17143 | 0.111 |
| O3 | 18f | 0.85657 | 0.12686 | 0.18301 | 0.111 |
On heating the LDH, the PXRD pattern shows a steady compression of the non-00l reflections, showing turbostratic disorder, before the complete breakdown of the structure (Fig. S8 (ESI†) and Fig. 1b).
The 1H and 3R polytypes differ from one another in the stacking sequence of the metal hydroxide layers. While in the 1H polytype, the metal hydroxide layers stacked one above another [stacking vector (0, 0, 1)], in the 3R polytype, successive layers are rigidly translated relative to one another by (1/3, 2/3, z)/(2/3, 1/3, z). The choice of the stacking sequence, from among the complete universe of possibilities is determined by the symmetry of the anions and the strength of their interaction with the metal hydroxide layers. Anions generally mediate the stacking, by choosing a stacking sequence which provides interlayer sites of local symmetry which match their molecular symmetry.23
Within the hydrocalumite-like structure, the local symmetry of the interlayer sites is determined by the six closely spaced hydroxyl ions, three each chosen from adjacent metal hydroxyl layers lining the interlayer gallery is the same in both the 1H and 3R polytypes (C3 and D3d). Thereby, the strength of bonding between the metal hydroxide layers and the interlayer atoms is comparable in both polytypes, so that the nucleation of one in preference to the other is determined by kinetic rather than thermodynamic factors. This also generates the possibility of interpolytype transitions driven by small changes in temperature and/or humidity. This work demonstrates the conversion of 1H → 3R polytype, upon temperature-induced dehydration involving a rigid translation of the metal hydroxide layers relative to one another. Rigid translation of the layers requires relatively less energy. Consequently, the 1H polytype on temperature-induced dehydration gives the 3R polytype. This transformation is reversible and occurs on rehydration in the ambient condition. The as-prepared 3R polytype on heating loses crystallinity and decomposes without undergoing any interpolytype transition. This behaviour is attributed to the presence of intercalated OH− ions in a mixed anion interlayer. The strong hydrogen bonding of the intercalated hydroxyl ions anchors the metal hydroxide layer, thereby preventing rigid translation of the layers.
4. Conclusions
Two different polytypes of [Ca–Al–NO3] LDHs, 1H and 3R, were synthesized by varying the synthesis conditions. The structures of the as-prepared phases of 1H and 3R polytypes were refined in space groups P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and R
and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) respectively. The nitrate ions are tilted with respect to the metal hydroxide layer in both the 1H and 3R polytypes but differ in the angle of tilt. In the 1H polytype, the nitrate ion makes an angle of 61° whereas in the 3R polytype it is 30° to the metal hydroxide layer. The two polytypes exhibit different dehydration behaviours. The 1H polytype undergoes a phase transformation to 3R polytype on temperature-induced dehydration. Structure refinement of the dehydrated phase shows that the ion migrates to a new position to satisfy the seventh coordination to Ca through one of its oxygen atoms. The as-prepared 3R polytype on dehydration results in a faulted structure characterized by broad reflections in the PXRD pattern suggesting a highly disordered and liquid-like interlayer.
respectively. The nitrate ions are tilted with respect to the metal hydroxide layer in both the 1H and 3R polytypes but differ in the angle of tilt. In the 1H polytype, the nitrate ion makes an angle of 61° whereas in the 3R polytype it is 30° to the metal hydroxide layer. The two polytypes exhibit different dehydration behaviours. The 1H polytype undergoes a phase transformation to 3R polytype on temperature-induced dehydration. Structure refinement of the dehydrated phase shows that the ion migrates to a new position to satisfy the seventh coordination to Ca through one of its oxygen atoms. The as-prepared 3R polytype on dehydration results in a faulted structure characterized by broad reflections in the PXRD pattern suggesting a highly disordered and liquid-like interlayer.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
ANN and PVK thank the Department of Science and Technology, Government of India, for financial support. SN thanks the Royal Society (United Kingdom) and Science and Engineering Research Board (Government of India) for the award of Newton-Bhabha International Fellowship (NIF/R1/180075).References
- A. S. Bookin and V. A. Drits, Clays Clay Miner., 1993, 41, 551–557 CrossRef CAS.
- H. F. W. Taylor, Min. Mag., 1969, 37, 338–342 CrossRef CAS.
- M. Bellotto, B. Rebours, O. Clause, J. Lynch, D. Bazin and E. Elkaim, J. Phys. Chem., 1996, 100, 8527–8534 CrossRef CAS.
- S. Radha, S. V. Prasanna and P. V. Kamath, Cryst. Growth Des., 2011, 11, 2287–2293 CrossRef CAS.
- P. J. Sideris, U. G. Nielsen, Z. Gan and C. P. Grey, Science, 2008, 321, 113–117 CrossRef CAS PubMed.
- P. J. Sideris, F. Blanc, Z. Gan and C. P. Grey, Chem. Mater., 2012, 24, 2449–2461 CrossRef CAS.
- H. Roussel, V. Briois, E. Elkaim, A. de Roy and J. P. Besse, J. Phys. Chem. B, 2000, 104, 5915–5923 CrossRef CAS.
- L. Bigey, C. Depege, A. de Roy and J. P. Besse, J. Phys. IV, 1997, 7, 949–950 CrossRef CAS.
- K. Jayanthi, S. Nagendran and P. V. Kamath, Inorg. Chem., 2015, 54, 8388–8395 CrossRef CAS PubMed.
- S. Marappa and P. V. Kamath, Ind. Eng. Chem. Res., 2015, 54, 11075–11079 CrossRef CAS.
- L. Vieille, I. Rousselot, F. Leroux, J.-P. Besse and C. Taviot-Guého, Chem. Mater., 2003, 15, 4361–4368 CrossRef CAS.
- A. N. Narayanappa and P. V. Kamath, Eur. J. Inorg. Chem., 2019, 4647–4652 CrossRef CAS.
- A. Mesbah, M. Francois, C. Cau-Dit-Coumes, F. Frizon, Y. Filinchuk, F. Leroux, J. Ravaux and G. Renaudin, Cem. Concr. Res., 2011, 41, 504–509 CrossRef CAS.
- G. Renaudin and M. François, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 835–838 CrossRef.
- S.-L. Wang and P.-C. Wang, Colloids Surf., 2007, 292, 131–138 CrossRef CAS.
- M. Jobaggy and N. Iyi, J. Phys. Chem. C, 2010, 114, 18153–18158 CrossRef.
- I. Rousselot, C. Taviot-Guého, F. Leroux, P. Léone, P. Palvadeau and J. P. Besse, J. Solid State Chem., 2002, 167, 137–144 CrossRef CAS.
- H. Wondratschek and U. Müller, International Tables for Crystallography, Wiley, West Sussex, UK, 2010, vol. A1 Search PubMed.
- V. Favre-Nicolin and R. Černý, J. Appl. Crystallogr., 2002, 35, 734–743 CrossRef CAS.
- A. C. Larson and R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR, 2004, pp. 86–748 Search PubMed.
- M. M. J. Treacy, J. M. Newsam and M. W. Deem, Proc. R. Soc. London, Ser. A, 1991, 433, 499–520 CrossRef.
- M. M. J. Treacy and M. W. Deem and J. M. Newsam, DIFFaX version 1.812, http://www.public.asu.edu/~mtreacy/DIFFaX.html, 2005.
- H. W. F. Taylor, Min. Mag., 1973, 39, 377–389 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj00148e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |






