Toward an efficient and eco-friendly route for the synthesis of dimeric 2,4-diacetyl phloroglucinol and its potential as a SARS-CoV-2 main protease antagonist: insight from in silico studies†
Abstract
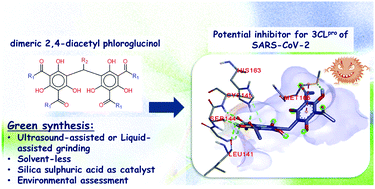
As a consequence of the unavailability of an anti-viral drug for SARS-CoV-2, the prospect of developing an antiviral drug is of great importance in this current emergency of the COVID-19 pandemic era. To support the enduring research on the improvement of COVID-19 therapeutics, herein, we demonstrated the development of highly effective and eco-friendly synthetic routes for the synthesis of dimeric 2,4-diacetyl phloroglucinol 4a, a potent antibiotic and malarial drug candidate via phenol–aldehyde condensation over silica sulphuric acid (SSA) catalyst and the arylation of dimethylammonium salts under greener protocols, i.e., ultrasound-assisted (US) and liquid assisted grinding (LAG) methods. Through an environmental assessment, the cleanness and environmental efficiency of the developed protocols were evidenced to be improved compared with a recently published alternative synthetic pathway, which was indicated by the presence of higher efficiency and lower environmental impact. In addition, we accomplished molecular docking studies to permit the rapid screening of possible therapeutic ligands of 4a and its derivatives along with N3, Chloroquine, and Remdesivir as references against the crystal structure for the recently released 3-chymotrypsin-like cysteine protease (3CLpro) of SARS-CoV-2. Exclusively, our finding showed that compound 4d with an excellent binding affinity of −8.1 kcal occupies the active site in varying ways at the N3 binding site of 3CLpro, confirming its fitness as a good candidate for the therapeutic action against COVID-19. Furthermore, molecular target prediction, pharmacokinetic studies, prediction of the activity spectra for substances (PASS), density-functional theory (DFT), and the structure–activity relationship (SAR) analysis were also established to highlight the prominence of this chemical scaffold.


 Please wait while we load your content...
Please wait while we load your content...