Rapid gram-scale synthesis of Au/chitosan nanoparticles catalysts using solid mortar grinding†
K. Paul
Reddy
 a,
R. S.
Meerakrishna
b,
P.
Shanmugam
a,
R. S.
Meerakrishna
b,
P.
Shanmugam
 b,
Biswarup
Satpati
b,
Biswarup
Satpati
 c and
A.
Murugadoss
c and
A.
Murugadoss
 *a
*a
aDepartment of Inorganic Chemistry, University of Madras, Guindy Campus, Chennai-600025, India. E-mail: murugadoss@unom.ac.in
bOrganic and Bioorganic Chemistry Division, Council of Scientific and Industrial Research (CSIR), Central Leather Research Institute (CLRI), Adyar, Chennai-600020, India
cSurface Physics and Material Science Division, Saha Institute of Nuclear Physics, Kolkata-700064, India
First published on 27th November 2020
Abstract
Owing to the abundant functional groups present in the chitosan polymer, high density catalytic tiny gold particles with greater dispersion can be anchored on the chitosan powder using simple mortar and pestle. Chitosan-supported gold nanoparticles (NPs) with excellent control of size and shape were rapidly synthesized in gram-scale by solid-grinding without the need of any toxic solvents. The structure of catalysts and products was established by advanced instrumental and spectroscopic methods. The supported gold NPs functions as a heterogeneous catalyst for the homocoupling of phenylboronic acid and the aerobic oxidation of benzyl alcohol in water. The catalytic behaviour and activity of supported gold NPs was tuned/modulated by varying the ratio of chitosan polymer and gold precursor. Comparative studies showed that the solid chitosan supported gold catalyst exhibits superior catalytic activity and selectivity than the well known hydrophilic polymer-stabilized gold NPs catalysts prepared by the conventional solution-based methods.
Introduction
The gram-scale synthesis of highly stable gold nanoparticles (NPs) catalysts, with excellent size and shape controllability, has become one of the attractive research areas in various fields of science and technology, specifically in catalysis.1–4 The unique catalytic performance of polymer protected nanogold catalysts has been demonstrated in a number of chemical transformations such as alcohol oxidations,5–7 carbon–carbon bond formation reactions,8–10 and hydrogenations.11 In the last two decades, various hydrophilic functional polymers, such as polyvinyl alcohols, thiol-linked polyethylene glycol, polyvinyl 2-pyrrolidone, starch, and cellulose have been used as stabilizers as well as capping agents for the synthesis of well-defined size and shape tuneable colloidal nanogold catalysts.12–17 In contrast to solid metal oxide support, these hydrophilic polymers act as catalytically inert stabilizers due to multiple coordination sites, which are weakly bound to gold NPs surface without affecting the intrinsic chemical properties of gold. Multifunctional groups present in the hydrophilic polymers have the potential to control both the catalytic activity by donating the electronic charge and selectivity by activating the substrates during the catalytic reactions.18,19 These unique features make the colloidal nanogold as promising model catalysts for the fundamental studies of heterogeneous catalysis.Although significant progress has been made in the synthesis of such polymer-stabilized colloidal nanogold catalysts, these methodologies are mostly solution-based synthetic procedures, which are complex and tedious, as well as consume large amounts of solvents.20 In addition, these solution-based methods always lead to slow progress of nanogold catalysts-based technologies and are difficult to scale-up using laboratory/industrial synthetic procedures.21–23 Another important constraint in the solution-based approaches using functional polymers as a protective matrix is that it severely restricts both the scaling-up process and catalysis.24–27 Additionally, due to their inherent viscous nature, the solution-based approaches not only require multiple purifications/washing processes with excessive solvents but also need sophisticated equipment for purifications and drying to obtain the polymer-protected colloidal nanogold catalysts.24,25,28 In addition, it is always crucial to achieving high loading of catalytically active gold NPs with good dispersion and excellent catalytic performance of the functional polymer-stabilized nanogold catalysts using solution-based methods. Therefore, it is highly desirable to develop an alternative strategy that can overcome these drawbacks for the gram-scale preparation of the hydrophilic functional polymer-supported gold NPs.
Chitosan is β-1,4-linked poly(D-glucosamine) derived from the partial deacetylation of chitin.29,30 In contrast to other hydrophilic functional polymers, chitosan is an inexpensive, highly stable solid polymer with rich multifunctional groups and possesses great potential as excellent support for metal cluster catalysts.31–34 For example, Ag NPs can be supported on solid chitosan that was subsequently used for the synthesis of bimetallic Au–Ag alloy NPs, where the chitosan powder was used as both reducing and stabilizing agent for Ag and Au–Ag alloy NPs. Contrary to the solution-based processes, the gram-scale quantity of solid chitosan supported Au–Ag alloy NPs catalysts can be synthesized by mortar grinding.35 However, due to the weak reducing nature of chitosan, the synthesis of other noble metal NPs, such as palladium and gold NPs, by solid grinding was not feasible. Nevertheless, the gram-scale synthesis of PVP-stabilized Au NPs was achieved by using vibrating ball milling. However, this method produces an inhomogeneous size distribution of Au NPs, which limits its practical applications.36 Since solid grinding requires only a small amount of solvent or no solvent, it would have been highly promising if one could synthesize the solid chitosan supported gold NPs in gram-scale through the solid-grinding method, and this achievement would surely advance their practical applications.
Thus, herein, we report an efficient solid-grinding approach for the synthesis of the gram-scale quantity of solid chitosan supported gold NPs using NaBH4 as a reducing agent. Hence, a large amount of gold NPs (0.47–12.5 wt%) can be loaded onto the solid chitosan support, with gold NPs sizes ranging from 2.8 to 9.5 nm. This solid chitosan supported gold NPs were used as heterogeneous catalysts for the oxidative homocoupling of arylboronic acids into biphenyls and the aerobic oxidation of benzyl alcohols into corresponding aldehyde and acids in water under the open air. In contrast to conventional solution-based synthesis, solid chitosan supported gold NPs catalysts showed higher activity and selectivity for homocoupling of arylboronic acid reactions. More importantly, the catalytic activity of chitosan-supported nanogold can be easily modulated by changing the mole ratio of the chitosan polymer and gold precursor using the simple solid-grinding method. To our knowledge, this is the first report in which the tuning/modulation of the catalytic activity of hydrophilic polymer-supported metal NPs was achieved by varying the mole ratio of the polymer and metal precursor using simple mortar and pestle without the use of solvents.
Results and discussion
Grinding of white-solid chitosan, with few microlitres of highly concentrated HAuCl4, results in the formation of the yellow solid, which subsequently changed into a dark greyish powder by the addition of solid NaBH4, thereby indicating the formation of gold NPs in the solid mixtures. As shown in Table 1, the molar ratio of gold and chitosan polymer (in glucosamine monomer unit) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42. This solid mixture was then purified with water using filter paper. It should be mentioned that neither unreduced gold ions nor gold NPs leached out from the solid chitosan during the washing and filtration (Fig. S1, ESI†) process. This observation indicates that the reduction of gold ions quantitatively proceeded, and the gold NPs were effectively anchored on the chitosan polymer. In this synthetic strategy, the process of supporting the gold NPs on the solid chitosan gets completed within 5–10 min, which is much shorter than 3 to 12 h or even a day that is often required in the solution-based process. More specifically, no solvents were required; hence, the solubility of hydrophilic polymers, molecular weight, and their viscosity problem can, in principle, be ignored.37 To verify this, the conventional solution approach was used to prepare the chitosan-stabilized gold NPs by maintaining the 1
42. This solid mixture was then purified with water using filter paper. It should be mentioned that neither unreduced gold ions nor gold NPs leached out from the solid chitosan during the washing and filtration (Fig. S1, ESI†) process. This observation indicates that the reduction of gold ions quantitatively proceeded, and the gold NPs were effectively anchored on the chitosan polymer. In this synthetic strategy, the process of supporting the gold NPs on the solid chitosan gets completed within 5–10 min, which is much shorter than 3 to 12 h or even a day that is often required in the solution-based process. More specifically, no solvents were required; hence, the solubility of hydrophilic polymers, molecular weight, and their viscosity problem can, in principle, be ignored.37 To verify this, the conventional solution approach was used to prepare the chitosan-stabilized gold NPs by maintaining the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 molar ratio of gold and chitosan. For example, when the chitosan was dissolved in an aqueous acidic solution, followed by the addition of HAuCl4 and NaBH4, the formation of a gel was observed (see Fig. S2, ESI†).33 Further, it was observed that gold NPs were severally aggregated in the gel. This result clearly reveals that the solid-grinding strategy was facile and effective for the direct dispersion of gold NPs on the chitosan polymer support. Note that additional loading of catalytically active metals in the form of tiny NPs with greater dispersion onto the solid support materials is of paramount importance for the development of high-performance metal catalysts.38,39 Owing to the significant content of primary amines, hydroxyls, and esters functional groups in the chitosan polymer, the solid chitosan might function as an excellent platform for loading high-density gold NPs without significant aggregations. To investigate whether chitosan would be superior support material for the loading of large amounts of gold NPs, we prepared different solid chitosan supported gold NPs by changing the mole ratio of the chitosan and gold precursor using the solid-grinding method.
42 molar ratio of gold and chitosan. For example, when the chitosan was dissolved in an aqueous acidic solution, followed by the addition of HAuCl4 and NaBH4, the formation of a gel was observed (see Fig. S2, ESI†).33 Further, it was observed that gold NPs were severally aggregated in the gel. This result clearly reveals that the solid-grinding strategy was facile and effective for the direct dispersion of gold NPs on the chitosan polymer support. Note that additional loading of catalytically active metals in the form of tiny NPs with greater dispersion onto the solid support materials is of paramount importance for the development of high-performance metal catalysts.38,39 Owing to the significant content of primary amines, hydroxyls, and esters functional groups in the chitosan polymer, the solid chitosan might function as an excellent platform for loading high-density gold NPs without significant aggregations. To investigate whether chitosan would be superior support material for the loading of large amounts of gold NPs, we prepared different solid chitosan supported gold NPs by changing the mole ratio of the chitosan and gold precursor using the solid-grinding method.
| Sample name | Initial amount of gold (mmol) | Amount of chitosan (mmol) | Gold loading (wt%) | Mole ratio of gold and chitosan | Particle size (nm) | Effective surface area (m2 g−1) | Amount of Au from ICP-OES (mmol) |
|---|---|---|---|---|---|---|---|
| a Glucosamine monomer unit. b Determined from Langmuir-adsorption isotherm. | |||||||
| Chit–Au1 | 0.02 | 0.84 | 2.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
5.6 ± 1.5 | 5.5 × 103 | 0.0198 |
| Chit–Au2 | 0.1 | 0.84 | 12.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
9.5 ± 2.7 | 1.5 × 103 | 0.0981 |
| Chit–Au3 | 0.02 | 4.2 | 0.47 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
3.8 ± 1.7 | 4.9 × 103 | 0.0195 |
| Chit–Au4 | 0.02 × 5 | 0.84 × 5 | 2.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
2.8 ± 1.7 | 2.8 × 104 | 0.0197 |
As summarized in Table 1, hereafter, the different contents of gold NPs supported on chitosan are denoted as Chit–Au1, Chit–Au2, Chit–Au3, and Chit–Au4. The gold content in each solid chitosan was determined using ICP-OES, and the values obtained indicated that the gold ions quantitatively reduced and successfully anchored onto the solid chitosan support. Notably, the synthesis of gold NPs supported on solid chitosan using simple mortar and pestle was reproducible several times (see Fig. S3, ESI†). Fig. 1a shows the photographic images of chitosan-supported nanogold powder. Depending on the content of gold and chitosan or vice versa, the color of the solution changes from dark to pale red. The color in the aqueous acidic solutions was produced by the light scattering due to the surface plasmon resonance band (SPR) of gold NPs.40 The UV-visible spectra shown in Fig. 1c demonstrate that the wavelength maxima and intensity of the SPR band were highly sensitive to the content of gold and chitosan polymers in the samples. As compared to Chit–Au1, Chit–Au2 exhibited an intense SPR band at a longer wavelength (550 nm). Though both samples contain the same amount of chitosan support (Table 1), more amount of gold content in Chit–Au2 may lead to the aggregation of gold NPs on the support, which resulted in the shifting of the SPR band to a longer wavelength in Chit–Au2 as compared to Chit–Au1. However, as the amount of chitosan was increased by five times, the SPR peak significantly reduced in Chit–Au3. This result indicated that the more content of the chitosan support leads to the high dispersion of gold NPs. However, an increase in the content of both gold and chitosan in Chit–Au4 resulted in a narrow SPR band at 525 nm. To understand the shifting of the SPR band and to investigate the size and morphology of chitosan-supported nanogold, high-resolution TEM analysis was performed. As shown in Fig. 2a–d and Fig. S4 (ESI†), the gold NPs in all four samples were nearly spherical in shapes, with the distribution of gold NPs being homogeneous throughout the chitosan support. Further, in SAED patterns, these particles exhibited clear diffraction rings (the inset image in Fig. S4d, ESI†), corresponding to d-spacing of 2.27, 1.96, 1.39, and 1.19 Å for (111), (200), (220), and (311) planes of the fcc structure of metallic gold, respectively (JCPDS No. 04-0784).41–43 The size distribution histogram plots were constructed for all four samples by analyzing >250 particles from the HRTEM images of corresponding samples, and particles sizes were determined to be 5.6 ± 1.5, 9.5 ± 2.7, 3.8 ± 1.7, and 2.8 ± 1.7 nm for Chit–Au1, Chit–Au2, Chit–Au3, and Chit–Au4, respectively (Fig. 2e–h and Table 1). Both HRTEM images and histogram plots revealed that on increasing the amount of chitosan support in Chit–Au3 and Chit–Au4, not only the size of gold NPs was reduced, but also the particles were greatly dispersed homogeneously without bulk or lumps of aggregations on the solid chitosan support (see Fig. S4, ESI†). It is reported elsewhere that a higher amount of stabilizing polymer instigates a large number of nucleation sites for the growth of more number of tiny particles.44,45 Moreover, due to this high dispersion of smaller gold NPs on the chitosan support, the SPR band was significantly blue-shifted in both Chit–Au3 and Chit–Au4. In contrast to Chit–Au1, the bigger-sized gold NPs were obtained for Chit–Au2. Obviously, the loading of more amounts of gold NPs on less amount of solid support tends to form bigger-sized particles due to aggregation that causes the red shifting of the SPR band in Chit–Au2. Note that more loading of metals on solid support materials like metal–organic framework and other mesoporous materials leads to the formation of rods and wire-like structures by aggregation due to weak interaction between metals and support functional groups.46–48 In contrast, the multifunctional groups present in the chitosan not only strongly interacts with the gold atoms but also provides a large number of adsorption site for high dispersion of gold NPs. This observation was further supported by the FTIR study of chitosan-supported gold NPs (Fig. S5, ESI†). The absorption bands at 1659 cm−1 (amide I for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration), 1598 cm−1 (NH2 bending in primary amines), 1552 cm−1 (NH bending in amide II vibration), and 3422 cm−1 (OH and NH vibration) slightly shifted with reduced intensities. The shifting of absorption bands with peak intensity reduction compared to free chitosan can be attributed to the interaction between chitosan and gold NPs.49,50 In addition, the peak reduction was very significant in the case of Chit–Au2, which is due to the more loading of gold NPs on the chitosan support.
O vibration), 1598 cm−1 (NH2 bending in primary amines), 1552 cm−1 (NH bending in amide II vibration), and 3422 cm−1 (OH and NH vibration) slightly shifted with reduced intensities. The shifting of absorption bands with peak intensity reduction compared to free chitosan can be attributed to the interaction between chitosan and gold NPs.49,50 In addition, the peak reduction was very significant in the case of Chit–Au2, which is due to the more loading of gold NPs on the chitosan support.
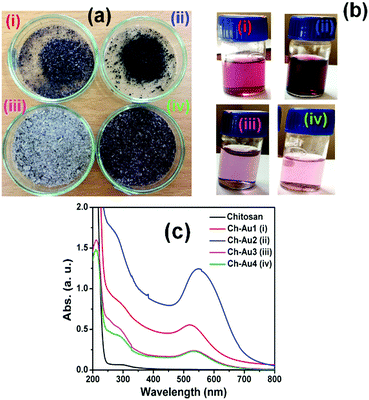 | ||
| Fig. 1 (a) Photographic images of solid chitosan supported gold NPs powder, (b) corresponding powders dissolved in 1% (v/v) aqueous acetic acid solution, and their (c) UV-visible absorption spectra. | ||
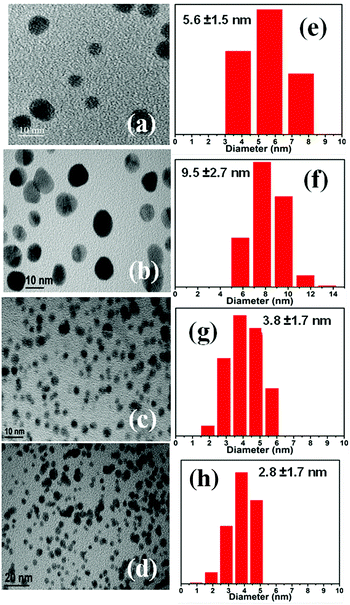 | ||
| Fig. 2 TEM images and corresponding particles size histogram plot (a and e) Chit–Au1, (b and f) Chit–Au2, (c and g) Chit–Au3, and (d and h) Chit–Au4. | ||
The electronic structure of chitosan-supported gold NPs was analyzed using XPS and XANES. The two peaks at 83.7 and 87.9 eV in the XPS spectra were observed due to the spin–orbit coupling of Au 4f (Fig. S6b; ESI†), thereby ensuring the complete reduction of Au3+ to Au(0) during solid grinding. The reduction of white line intensity at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 eV compared to Au foil in the XANES spectra at Au-L3 edge (Fig. S7, ESI†) implies the significant reduction of d-holes51–53 in the chitosan-supported gold NPs, which confirms the formation of gold NPs in Chit–Au1. These results clearly demonstrate that chitosan can be used as superior support materials for both the loading of the high density of gold NPs and preparation of different sized gold particles using the simple solid-grinding method.
923 eV compared to Au foil in the XANES spectra at Au-L3 edge (Fig. S7, ESI†) implies the significant reduction of d-holes51–53 in the chitosan-supported gold NPs, which confirms the formation of gold NPs in Chit–Au1. These results clearly demonstrate that chitosan can be used as superior support materials for both the loading of the high density of gold NPs and preparation of different sized gold particles using the simple solid-grinding method.
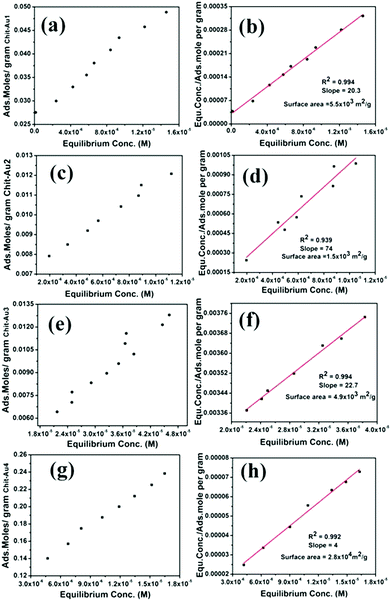
To determine the available surface area of gold NPs supported on solid chitosan, the Langmuir adsorption isotherm was used with P-nitro thiophenol (PNTP) as a probe ligand.54,55 Various concentrations of PNTP were mixed with few milligrams of solid chitosan-supported gold NPs in aqueous solutions. The number of moles of adsorbed PNTP on the gold NPs surface was calculated from a calibration curve using the extinction coefficient of PNTP (refer to ESI†).55 It should be mentioned that PNTP adsorbs selectively only on gold NPs surface and not on chitosan polymer. As shown in Fig. 3, the adsorption isotherm was plotted between the number of moles of adsorbed PNTP per gram of gold NPs and the equilibrium concentration of PNTP for four samples to determine the surface area of supported gold NPs. Interestingly, all four samples showed a straight line in the adsorption isotherm. Langmuir isotherm plots were then used to determine the available surface area of gold NPs on the chitosan support.
Though all four samples exhibited a higher surface area, the highest surface area ca. 2.8 × 104 m2 g−1 was observed only for Chit–Au4. This may be due to the high dispersion of a large number of small-sized gold NPs on the chitosan support. Compared to Chit–Au3, Chit–Au1 showed higher surface area, ca. 5.5 × 103 m2 g−1, even though Chit–Au3 had smaller-sized gold NPs. Since Chit–Au3 contained a large amount of chitosan polymer than Chit–Au1, so either some of the gold NPs may be buried inside the chitosan polymer, or the exposed surface atoms of gold NPs may be covered by polymers. Both concomitantly affected the thiol binding at gold NPs surfaces, which in turn reduced the available surface area of gold NPs in Chit–Au3. In contrast, the Langmuir isotherm plots of Chit–Au2 show a higher slope of 74 g mol−1, and the corresponding surface area was found to be much lower than the other three samples.
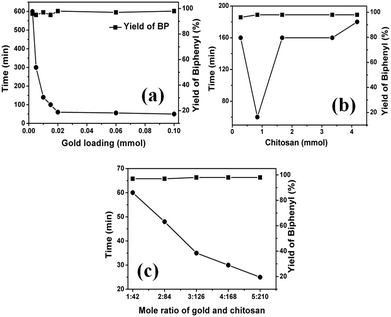
This result clearly implies that the available surface area of gold NPs supported on solid chitosan depends not only on the size of gold NPs but also on the amount of chitosan polymer. Therefore, the higher available surface area of supported gold NPs could be easily synthesized in gram scale by simple solid-grinding. Solid-grinding with mortar and pestle using chitosan as unique support allowed the rapid preparation of supported catalysts with an improved loading efficiency and greater uniformity of gold NPs, which were then employed in the homocoupling of phenylboronic acid in water at room temperature under air (see ESI† for reaction details). Note that these solid chitosan supported gold NPs functioned as heterogeneous catalysts during the reactions. As shown in Fig. 4a, when the amount of gold loading was increased from 0.0025 to 0.1 mmol on the fixed amount of chitosan support (0.84 mmol), the time required for the complete conversion of phenylboronic acid into biphenyl sharply reduced, indicating that the gold NPs supported on solid chitosan actively catalyzed the homocoupling reaction. After 0.02 mmol of gold, the reaction time did not reduce sharply and remained almost constant even at higher gold content. This result indicates that when gold loading exceeds more than 0.02 mmol on 0.84 mmol chitosan, then the aggregation of gold NPs occurs, which in turn reduces the total available surface areas and thus decreases the catalytic activity of gold NPs (Chit–Au2 details in Table 1). To study whether more content of chitosan support exerts any impact on the catalytic activity of gold NPs, the amount of chitosan support was increased from 0.28 to 4.2 mmol, and the amount of gold was kept constant (0.02 mmol Au) during the solid-grinding. As depicted in Fig. 4b, when chitosan polymer was increased or decreased from 0.84 mmol, the catalytic activity of gold NPs decreased. This could be either due to the aggregation of gold NPs that might occur at a lesser amount of chitosan or due to partial blockage of some of the active sites of gold NPs at a higher amount of chitosan polymer. These both concurrently affected the catalytic activity of gold NPs. Based on these results, 0.02 mmol of Au on 0.84 mmol of chitosan, i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 mole ratio of gold and chitosan was chosen as an optimum catalysts for homocoupling reactions. To demonstrate that the solid grinding is a facile and promising technique for the gram-scale synthesis of chitosan-supported nanogold catalysts, both gold and chitosan amount was increased up to five times while maintaining the 1
42 mole ratio of gold and chitosan was chosen as an optimum catalysts for homocoupling reactions. To demonstrate that the solid grinding is a facile and promising technique for the gram-scale synthesis of chitosan-supported nanogold catalysts, both gold and chitosan amount was increased up to five times while maintaining the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 mole ratio of gold and chitosan. Note that due to the highly viscous nature of chitosan polymer in aqueous solutions, it is impossible to synthesize the catalytically active chitosan-stabilized gold NPs in gram scale using solution-based methods (see details in ESI,† Table S1). As the amount of both gold and chitosan were gradually increased while maintaining the 1
42 mole ratio of gold and chitosan. Note that due to the highly viscous nature of chitosan polymer in aqueous solutions, it is impossible to synthesize the catalytically active chitosan-stabilized gold NPs in gram scale using solution-based methods (see details in ESI,† Table S1). As the amount of both gold and chitosan were gradually increased while maintaining the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 mole ratio, the catalytic activity of gold NPs was greatly increased (Fig. 4c). These results strongly demonstrate that the chitosan can be used as excellent support for the high loading of gold NPs. Thus, highly active and selective Au NPs catalysts supported on chitosan could be achieved in gram-scale rapidly using simple mortar-solid grinding, which is not possible in the solution-based method.
42 mole ratio, the catalytic activity of gold NPs was greatly increased (Fig. 4c). These results strongly demonstrate that the chitosan can be used as excellent support for the high loading of gold NPs. Thus, highly active and selective Au NPs catalysts supported on chitosan could be achieved in gram-scale rapidly using simple mortar-solid grinding, which is not possible in the solution-based method.
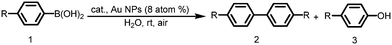
It is interesting to note that solid chitosan supported gold catalysts possess excellent selectivity towards the formation of biphenyl regardless of the amount of gold and chitosan. As chitosan possesses an excellent matrix to activate the conversion of phenylboronic acids into tetra-coordinated phenylboronate ester at the gold surfaces, it completely eliminated the use of inorganic bases and increased the selectivity for biphenyl formation.19,56 Since all the reactions were carried out in the water in the pH range 6.5–7.0, only a trace amount of phenol (>2–3%) formation was observed, which is inevitable due to the formation of superoxide species around gold NPs surfaces.19,57,58
To examine the versatility of these chitosan-supported nanogold catalysts under optimized conditions (0.02 mmol Au on 0.84 mmol chitosan), the homocoupling reactions involving other p-substituted arylboronic acids were tested. Phenylboronic acid possessing electron-donating groups, such as 4-methyl and 4-methoxy arylboronic acids, afforded excellent yields and selectivity for corresponding biphenyl in 2 h (entries 2 and 3, Table 2), even though they took longer reaction times than phenylboronic acids. Phenylboronic acids with electron-withdrawing groups, such as 4-fluoro, 4-chloro, and 4-cyano substituents (entries 4, 5, and 6, Table 2), gave the corresponding biaryls in 97%, 97%, and 94% yields and the corresponding phenols in 0%, 2%, and 6% yields after 2, 3, and 5 h, respectively. This result demonstrates that this catalyst works very efficiently in terms of achieving higher activity and selectivity for both electron-donating and withdrawing substituents present on the phenylboronic acid.
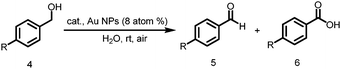
Encouraged by these results, we were further interested to investigate the potential of solid chitosan supported nanogold catalysts in the aerobic oxidation of benzyl alcohols into corresponding aldehydes and acids. Thus, the catalyst was tested for the oxidation of various p-substituted benzyl alcohols in aqueous solutions in the presence of 300 mol% K2CO3 using molecular oxygen as an oxidant. Surprisingly, solid chitosan supported nanogold, used as heterogeneous catalysts, works very efficiently for the oxidation of various p-substituted benzyl alcohols into corresponding aldehyde and acids (Table 3). For example, the oxidation of benzyl alcohol in the presence of the supported gold catalyst gave corresponding acids in 99% yield after 90 min. Hydroxyl and methoxy substituted benzyl alcohols were oxidized quantitatively into corresponding aldehydes in 25 and 5 min (entries 2 and 3, Table 3), respectively. In contrast, the electron-withdrawing substituent of p-nitro benzyl alcohol was almost converted into the corresponding acids in 150 min under the same reaction conditions (entry 4, Table 3). This experimental result showed that the strong electron-donating groups present on benzyl alcohols not only increased the rate of the oxidation reactions but also exclusively afforded the desired aldehyde products. Further, 1-indanol was selectively oxidized into 1-indanone with 99% yield after 80 min (entry 5, Table 3).
In typical solid materials supported metal nanoparticle catalysts, the recovery and reusability of the catalyst is an important property from an economic and industrial point of view. Therefore, the reusability of solid chitosan supported nanogold catalysts was established in both the homocoupling of 4-methyl phenylboronic acid and aerobic oxidation of p-hydroxyl benzyl alcohols under the optimized reaction conditions, as listed in entry 2 of Tables 2 and 3. After the completion of each homocoupling and alcohol oxidation reaction, the solid catalysts were centrifuged, washed with water, dried, and successfully reused for five cycles without notable loss of activity and selectivity. As shown in Fig. S8 (in ESI†), p-methyl phenylboronic acids were exclusively converted into the corresponding biphenyl with excellent yield over the five cycles. However, the oxidation of p-hydroxyl benzyl alcohol gave more selectively the corresponding aldehydes, and the yields over the five cycles were 99%, 95%, 98%, 96%, and 94%, respectively. Fig. S9 (in ESI†) shows the TEM micrograph of recovered catalysts obtained from the homocoupling reaction of p-methyl phenylboronic acids after the fifth cycle. As can be seen from these images, the gold NPs were well distributed over the chitosan support without any noticeable aggregations. Therefore, it can be realized that gold NPs on the chitosan support were preserved even after the fifth cycle. The ICP-OES analysis of the corresponding filtrate showed that no gold was leached from the catalyst, indicating that the gold NPs were effectively anchored on the chitosan support. Next, we investigated that whether the solid chitosan supported gold NPs catalysts prepared by physical mixing of gold chloride and NaBH4 with the chitosan in pestle and mortar exhibits comparable or better catalytic activity as compared to other hydrophilic polymer-stabilized gold NPs catalysts. Hence, other well-known polymers such as PVA and PVP-stabilized gold NPs catalysts were prepared using mortar and pestle (refer to ESI†), and their catalytic activity was tested for the homocoupling of phenylboronic acid (entry 1, Table 2). It should be mentioned that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 ratio of gold and PVA/PVP in monomer was maintained during solid grinding. As indicated in entries 2 and 3 in Table 4, PVA and PVP-stabilized gold NPs exhibited inferior catalytic activity toward the homocoupling of phenylboronic acid, indicating both PVA and PVP were incapable of stabilizing the Au NPs effectively in solid grinding using a mortar and pestle (Fig. S10 and Table S2, ESI†).
42 ratio of gold and PVA/PVP in monomer was maintained during solid grinding. As indicated in entries 2 and 3 in Table 4, PVA and PVP-stabilized gold NPs exhibited inferior catalytic activity toward the homocoupling of phenylboronic acid, indicating both PVA and PVP were incapable of stabilizing the Au NPs effectively in solid grinding using a mortar and pestle (Fig. S10 and Table S2, ESI†).
| Entry | Method | Catalysts | Amount of gold (mmol) | Time (h) | Yield (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| 2 | 3 | ||||||
| SG and SB are solid-grinding and solution-based, respectively.a 0.25 mmol of phenylboronic acid.b 0.17 mmol of phenylboronic acid.c 0.24 mmol of phenylboronic acid.d 0.5 mmol of phenylboronic acid.e −105 °C used for the coupling reaction. | |||||||
| 1 | SG | Chit/Au | 0.005a | 5 | 98 | Trace | This work |
| 2 | SG | PVA/Au | 0.005a | 5 | ND | ND | See ESI |
| 3 | SG | PVP/Au | 0.005a | — | ND | ND | See ESI |
| 3 | SB | Chit/Au | 0.005a | 9 | 93 | 6 | 19 |
| 4 | SB | Starch/Au | 0.005a | 7 | 96 | 4 | 19 |
| 5 | SB | PNIPAM/Au | 12–36b | 2–12 | <95 | Trace | 59 and 60 |
| 6 | SB | PS-co-PMAA/Au | 6.8c | 8e | 99 | — | 61 |
| 7 | SB | PS-PAMAM/Au | 0.007d | 24 | 99 | — | 62 |
| 8 | SB | PVP/Au | 0.005a | 15 | 31 | 69 | 19 and 63 |
Indeed, the comparative studies between other hydrophilic polymer-stabilized gold NPs using the conventional solution-based methods reported in the literature and chitosan-supported gold catalysts obtained from solid-grinding clearly demonstrated that the chitosan-supported gold NPs works as efficient catalysts in the homocoupling of phenylboronic acid. For example, though starch can effectively stabilize the gold NPs; however, the catalytic activity was lost at the end of the coupling reaction due to aggregation. When the same reaction was carried out using PVP-stabilized gold NPs under quasi-homogeneous conditions in an aqueous acidic solution, only 17% of biphenyl product was obtained after 9 h. Despite the composite of the PNIPAM/Au system possess interesting thermosensitive properties, the present catalytic system shows quite a high catalytic activity. This comparative study clearly indicates that chitosan possesses an excellent matrix for the synthesis of highly efficient and versatile supported gold catalysts in gram-scale using simple mortar and pestle by solid-grinding. Further, the deposited gold on solid metal oxides such as TiO2 and MAO possesses interesting catalytic activities;64,65 however, their preparation was more complex and laborious compared to the present catalysts system. This result further demonstrates that solid-grinding with simple mortar and pestle could be used as a promising tool to achieve highly efficient heterogeneous metal catalysts.
Conclusions
In conclusion, a facile and sustainable approach was developed for the gram-scale synthesis of chitosan-supported gold NPs by solid grinding. Abundant hydroxyl and amine functional groups present in the chitosan polymer allowed rapid preparation of supported catalysts with an improved loading efficiency and greater uniformity of gold NPs using mortar and pestle. The supported gold NPs works as efficient heterogeneous catalysts and exhibits outstanding activity and selectivity in oxidative homocoupling of phenylboronic acids and aerobic oxidation of alcohols in water (the greener solvent). It was also observed that the catalytic activity of solid chitosan supported gold NPs can be easily modulated by changing the mole ratio of chitosan polymer and gold precursors. Further, the catalytic activity comparison studies between the other well-known hydrophilic polymer-stabilized colloidal nanogold catalysts, prepared from the conventional solution-based methods, and the present supported gold catalysts indicates that solid chitosan supported gold NPs possess superior catalytic activity and selectivity. Therefore, rapid gram-scale preparation of the biopolymer chitosan supported gold NPs by simple solid-grinding can inspire more studies on the design and application of the solid polymer-supported metal catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. M. acknowledges DST-SERB, New Delhi for the award of financial support vide grant no: YSS/2015/001124. We also thank UGC-DAE Consortium for Scientific Research (CSR-IC-ISUM-06/CRS-289/2019-20/1341). Characterization studies acquired from GNR Instrumental facility, University of Madras, Guindy Campus is greatly acknowledged. Thanks to Dr S. N. Jha, for XANES analysis at Indus-2, RRCAT, India.Notes and references
- T. Ishida, N. Kinoshita, H. Okatsu, T. Akita, T. Takei and M. Haruta, Influence of the Support and the Size of Gold Clusters on Catalytic Activity for Glucose Oxidation, Angew. Chem., Int. Ed., 2008, 47(25), 9265–9268 CrossRef CAS PubMed.
- L. X. Dien, T. Ishida, A. Taketoshi, Q.-D. Truong, H. D. Chinh, T. Honma, T. Murayama and M. Haruta, Supported gold cluster catalysts prepared by solid grinding using a non-volatile organogold complex for low-temperature CO oxidation and the effect of potassium on old particle size, Appl. Catal., B, 2019, 241, 539–547 CrossRef CAS.
- T. Ishida, M. Nagaoka, T. Akita and M. Haruta, Chem. – Eur. J., 2008, 14, 8456–8460 CrossRef CAS PubMed.
- L. X. Dien, Q. D. Truong, T. Murayama, H. D. Chinh, A. Taketoshi, I. Honma, M. Haruta and T. Ishida, Gold Nanoparticles Supported on Nb2O5 for Low-Temperature CO Oxidation and as Cathode Materials for Li-ion Batteries, Appl. Catal., A, 2020, 603, 117747 CrossRef CAS.
- M. Stratakis and H. Garcia, Catalysis by supported gold nanoparticles: beyond aerobic oxidative processes, Chem. Rev., 2012, 112, 4469–4506 CrossRef CAS PubMed.
- T. Tsukuda, H. Tsunoyama and H. Sakurai, Aerobic Oxidations Catalyzed by Colloidal Nanogold, Chem. – Asian J., 2011, 6, 736–748 CrossRef CAS PubMed.
- Y. Zhang, X. Cui, F. Shi and Y. Deng, Nano-gold catalysis in fine chemical synthesis, Chem. Rev., 2012, 112, 2467–2505 CrossRef CAS.
- J. Garcia-Calvo, V. Garcia-Calvo, S. Vallejos, F. C. Garcia, M. Avella, J. M. Garcia and T. Torroba, Surface coating by gold nanoparticles on functional polymers: on-demand portable catalysts for Suzuki reactions, ACS Appl. Mater. Interfaces, 2016, 8, 24999–25004 CrossRef CAS PubMed.
- H. Tsunoyama, H. Sakurai, N. Ichikuni, Y. Negishi and T. Tsukuda, Colloidal Gold Nanoparticles as Catalyst for Carbon-Carbon Bond Formation: Application to Aerobic Homocoupling of Phenylboronic Acid in Water, Langmuir, 2004, 20, 11293–11296 CrossRef CAS PubMed.
- W. Jang, H. Byun and J. H. Kim, Encapsulated gold nanoparticles as a reactive quasi-homogeneous catalyst in base-free aerobic homocoupling reactions, ChemCatChem, 2020, 12(3), 705–709 CrossRef CAS.
- M. Haruta, Gold rush, Nature, 2005, 437(7062), 1098–1099 CrossRef CAS PubMed.
- E. Oh, K. Susumu, A. J. Makinen, J. R. Deschamps, A. L. Huston and I. L. Medintz, Colloidal stability of gold nanoparticles coated with multithiol-poly(ethylene glycol) ligands: importance of structural constraints of the sulfur anchoring groups, J. Phys. Chem. C, 2013, 117(37), 18947–18956 CrossRef CAS.
- K. M. Koczkur, S. Mourdikoudis, L. Polavarapu and S. E. Skrabalak, Polyvinylpyrrolidone (PVP) in nanoparticle synthesis, Dalton Trans., 2015, 44, 17883–17905 RSC.
- A. Villa, D. Wang, D. S. Su and L. Prati, Gold sols as catalysts for glycerol oxidation: The role of stabilizer, ChemCatChem, 2009, 1(4), 510–514 CrossRef CAS.
- K. Wongmanee, S. Khuanamkam and S. Chairam, Gold nanoparticles stabilized by starch polymer and their use as catalyst in homocoupling of phenylboronic acid, J. King Saud Univ., Sci., 2017, 29(5), 547–552 CrossRef.
- L. M. Rossi, J. L. Fiorio, M. A. S. Garcia and C. P. Ferraz, The Role and Fate of Capping Ligands in Colloidally Prepared Metal Nanoparticle Catalysts, Dalton Trans., 2018, 47, 5889–5915 RSC.
- J. Van Rie and W. Thielemans, Cellulose–gold nanoparticle hybrid materials, Nanoscale, 2017, 9, 8525–8554 RSC.
- H. Tsunoyama, N. Ichikuni, H. Sakurai and T. Tsukuda, Effect of Electronic Structures of Au Clusters Stabilized by Poly(N-Vinyl-2-Pyrrolidone) on Aerobic Oxidation Catalysis, J. Am. Chem. Soc., 2009, 131, 7086–7093 CrossRef CAS PubMed.
- R. N. Dhital, A. Murugadoss and H. Sakurai, Dual Roles of Polyhydroxy Matrices in the Homocoupling of Arylboronic Acids Catalyzed by Gold Nanoclusters under Acidic Conditions, Chem. – Asian J., 2012, 7, 55–59 CrossRef CAS PubMed.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Toward greener nanosynthesis, Chem. Rev., 2007, 107(6), 2228–2269 CrossRef CAS PubMed.
- T. Tsuzki, Commercial scale production of inorganic nanoparticles, Int. J. Nanotechnol., 2009, 6(5–6), 567–578 CrossRef.
- P. F. M. de Oliveira, R. M. Torresi, F. Emmerling and P. H. C. Camargo, Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles, J. Mater. Chem. A, 2020, 8, 16114–16141 RSC.
- B. Donoeva and P. E. de Jongh, Colloidal Au catalyst preparation: selective removal of polyvinylpyrrolidone from active Au sites, ChemCatChem, 2018, 10, 989–997 CrossRef CAS PubMed.
- H. Li, J. V. John, S. J. Byeon, M. S. Heo, J. H. Sung, K. H. Kim and I. Kim, Controlled accommodation of metal nanostructures within the matrices of polymer architectures through solution-based synthetic strategies, Prog. Polym. Sci., 2014, 39, 1878–1907 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, Effect of Catalysis on the Stability of Metallic Nanoparticles:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Suzuki Reaction Catalyzed by PVP-Palladium Nanoparticles, J. Am. Chem. Soc., 2003, 125(27), 8340–8347 CrossRef CAS PubMed.
Suzuki Reaction Catalyzed by PVP-Palladium Nanoparticles, J. Am. Chem. Soc., 2003, 125(27), 8340–8347 CrossRef CAS PubMed. - S. Haesuwannakij, Y. Yakiyama and H. Sakurai, Partially Fluoride-Substituted Hydroxyapatite as a Suitable Support for the Gold-Catalyzed Homocoupling of Phenylboronic Acid: An Example of Interface Modification, ACS Catal., 2017, 7(4), 2998–3003 CrossRef CAS.
- Y. Mikami, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Catalytic Activity of Unsupported Gold Nanoparticles, Catal. Sci. Technol., 2013, 3, 58–69 RSC.
- L. M. Dias Ribeiro de Sousa Martins, S. A. C. Carabineiro, J. Wang, B. G. M. Rocha, F. J. Maldonado-Hodar and A. J. Latourrette de Oliveira Pombeiro, Supported Gold Nanoparticles as Reusable Catalysts for Oxidation Reactions of Industrial Significance, ChemCatChem, 2017, 9, 1211–1221 CrossRef.
- L. Q. Wu, A. P. Gadre, H. Yi, M. J. Kastantin, G. W. Rubloff, W. E. Bentley, G. F. Payne and R. Ghodssi, Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface, Langmuir, 2002, 18, 8620–8625 CrossRef CAS.
- E. I. Rabea, M. E. T. Badawy, C. V. Stevens, G. Smagghe and W. Steurbaut, Chitosan as antimicrobial agent: applications and mode of action, Biomacromolecules, 2003, 4(6), 1457–1465 CrossRef CAS PubMed.
- A. Murugadoss and A. Chattopadhyay, A ‘green’ chitosan–silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst, Nanotechnology, 2018, 19(1), 015603 CrossRef PubMed.
- M. Lee, B. Y. Chen and W. Den, Chitosan as a Natural Polymer for Heterogeneous Catalysts Support: A Short Review on Its Applications, Appl. Sci., 2015, 5(4), 1272–1283 CrossRef CAS.
- A. Murugadoss and H. Sakurai, Chitosan-Stabilized Gold, Gold-Palladium, and Gold-Platinum Nanoclusters as Efficient Catalysts for Aerobic Oxidation of Alcohols, J. Mol. Catal. A: Chem., 2011, 341, 1–6 CrossRef CAS.
- W. Wang and H. Cui, Chitosan-luminol reduced gold nanoflowers: from one-pot synthesis to morphology-dependent SPR and chemiluminescence sensing, J. Phys. Chem. C, 2008, 112, 10759–10766 CrossRef CAS.
- A. Murugadoss, N. Kai and H. Sakurai, Synthesis of Bimetallic Gold-Silver Alloy Nanoclusters by Simple Mortar Grinding, Nanoscale, 2012, 4, 1280–1282 RSC.
- D. Debnath, S. H. Kim and K. E. Geckeler, The first solid-phase route to fabricate and size-tune gold nanoparticles at room temperature, J. Mater. Chem., 2009, 19, 8810–8816 RSC.
- P. F. M. de Oliveira, J. Quiroz, D. C. de Oliveira and P. H. C. Camargo, A mechano-colloidal approach for the controlled synthesis of metal nanoparticles, Chem. Commun., 2019, 55, 14267–14270 RSC.
- H. Schreyer, R. Eckert, S. Immohr, J. de Bellis, M. Felderhoff and F. Schuth, A general process for the direct dry synthesis of supported metal catalysts, Angew. Chem., Int. Ed., 2019, 58, 11262–11265 CrossRef CAS PubMed.
- B. Gole, U. Sanyal and P. Sarathi Mukherjee, A smart approach to achieve an exceptionally high loading of metal nanoparticles supported by functionalized extended frameworks for efficient catalysis, Chem. Commun., 2015, 51, 4872–4875 RSC.
- L. M. Liz-Marzan, M. Giersig and P. Mulvaney, Synthesis of Nanosized Gold–Silica Core–Shell Particles, Langmuir, 1996, 12(18), 4329–4335 CrossRef CAS.
- X. B. Qian, W. Peng, Y. B. Shao and J. H. Huang, Synthesis and visible light-driven photocatalytic hydrogen production, Int. J. Hydrogen Energy, 2018, 43(4), 2160–2170 CrossRef CAS.
- D. Su, S. Dou and G. Wang, Gold nanocrystals with variable index facets as highly effective cathode catalysts for lithium–oxygen batteries, NPG Asia Mater., 2015, 7(1), e155 CrossRef CAS.
- R. Torres-Mendieta, D. Ventura-Espinosa, S. Sabater, J. Lancis, G. Minguez-Vega and J. A. Mata, situ decoration of graphene sheets with gold nanoparticles synthetized by pulsed laser ablation in liquids, Sci. Rep., 2016, 6, 30478 CrossRef CAS PubMed.
- A. Q. Zhang, L. J. Cai, L. Sui, D. J. Qian and M. Chen, Reducing properties of polymers in the synthesis of noble metal nanoparticles, Polym. Rev., 2013, 53(2), 240–276 CrossRef CAS.
- X. Xia, Z. Qiang, G. Bass, M. L. Becker and B. D. Vogt, Morphological control of hydrothermally synthesized cobalt oxide particles using poly(vinyl pyrrolidone), Colloid Polym. Sci., 2019, 297, 59–67 CrossRef CAS.
- J. Han, J. Cho, J. C. Kim and R. Ryoo, Confinement of supported metal catalysts at high loading in the mesopore network of hierarchical zeolites, with access via the microporous windows, ACS Catal., 2018, 8(2), 876–879 CrossRef CAS.
- D. Kunwar, S. Zhou, A. DeLaRiva, E. J. Peterson, H. Xiong, X. I. Pereira-Hernandez, S. C. Purdy, R. ter Veen, H. H. Brongersma, J. T. Miller and H. Hashiguchi, Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support, ACS Catal., 2019, 9(5), 3978–3990 CrossRef CAS.
- X. Y. Hao, Y. Q. Zhang, J. W. Wang, W. Zhou, C. Zhang and S. Liu, A novel approach to prepare MCM-41 supported CuO catalyst with high metal loading and dispersion, Microporous Mesoporous Mater., 2006, 88(1–3), 38–47 CrossRef CAS.
- A. Regiel-Futyra, M. Kus-Liskiewicz, V. Sebastian, S. Irusta, M. Arruebo, G. Stochel and A. Kyziol, Development of noncytotoxic chitosan–gold nanocomposites as efficient antibacterial materials, ACS Appl. Mater. Interfaces, 2015, 7(2), 1087–1099 CrossRef CAS PubMed.
- Q. Li, F. Lu, G. Zhou, K. Yu, B. Lu, Y. Xiao, F. Dai, D. Wu and G. Lan, Silver inlaid with gold nanoparticle/chitosan wound dressing enhances antibacterial activity and porosity, and promotes wound healing, Biomacromolecules, 2017, 18, 3766–3775 CrossRef CAS PubMed.
- A. Murugadoss, K. Okumura and H. Sakurai, Bimetallic AuPd Nanocluster Catalysts with Controlled Atomic Gold Distribution for Oxidative Dehydrogenation of Tetralin, J. Phys. Chem. C, 2012, 116, 26776–26783 CrossRef CAS.
- E. K. Gibson, A. M. Beale, C. R. A. Catlow, A. Chutia, D. Gianolio, A. Gould, A. Kroner, K. M. Mohammed, M. Perdjon, S. M. Rogers and P. P. Wells, Restructuring of AuPd nanoparticles studied by a combined XAFS/DRIFTS approach, Chem. Mater., 2015, 27, 3714–3720 CrossRef CAS.
- P. Dash, T. Bond, C. Fowler, W. Hou, N. Coombs and R. W. Scott, Rational design of supported PdAu nanoparticle catalysts from structured nanoparticle precursors, J. Phys. Chem. C, 2009, 113, 12719–12730 CrossRef CAS.
- M. A. Mahmoud, B. Garlyyev and M. A. El-Sayed, Determining the mechanism of solution metallic nanocatalysis with solid and hollow nanoparticles: homogeneous or heterogeneous, J. Phys. Chem. C, 2013, 117, 21886–21893 CrossRef CAS.
- K. Paul Reddy, K. Jaiswal, B. Satpati, C. Selvaraju and A. Murugadoss, High yield synthesis of branched gold nanoparticles as excellent catalysts for the reduction of nitroarenes, New J. Chem., 2017, 41, 11250–11257 RSC.
- R. N. Dhital and H. Sakurai, Oxidative Coupling of Organoboron Compounds, Asian J. Org. Chem., 2014, 3(6), 668 CrossRef CAS.
- S. Karanjit, M. Ehara and H. Sakurai, Mechanism of the aerobic homocoupling of phenylboronic acid on Au20−: A DFT study, Chem. – Asian J., 2015, 10, 2397–2403 CrossRef CAS PubMed.
- S. Carrettin, J. Guzman and A. Corma, Supported gold catalyzes the homocoupling of phenylboronic acid with high conversion and selectivity, Angew. Chem., Int. Ed., 2005, 44, 2242–2245 CrossRef CAS PubMed.
- P. N. Eyimegwu, J. A. Lartey and J. H. Kim, Gold-Nanoparticle-Embedded Poly(N-isopropylacrylamide) Microparticles for Selective Quasi-Homogeneous Catalytic Homocoupling Reactions, ACS Appl. Nano Mater., 2019, 2(9), 6057–6066 CrossRef CAS.
- W. Jang, H. Byun and J. H. Kim, Encapsulated gold nanoparticles as a reactive quasi-homogeneous catalyst in base-free aerobic homocoupling reactions, ChemCatChem, 2020, 12(3), 705–709 CrossRef CAS.
- X. Zhang, H. Zhao and J. Wang, Recyclable Au(I) Catalyst for Selective Homocoupling of Arylboronic Acids: Significant Enhancement of Nano-Surface Binding for Stability and Catalytic Activity, J. Nanosci. Nanotechnol., 2010, 10, 5153–5160 CrossRef CAS PubMed.
- J. Zheng, S. Lin, X. Zhu, B. Jiang, Z. Yang and Z. Pan, Reductant-directed formation of PS-PAMAM-supported gold nanoparticles for use as highly active and recyclable catalysts for the aerobic oxidation of alcohols and the homocoupling of phenylboronic acids, Chem. Commun., 2012, 48, 6235–6237 RSC.
- Vinsen, Y. Uetake and H. Sakurai, Selective Oxidative Hydroxylation of Arylboronic Acids by Colloidal Nanogold Catalyzed in Situ Generation of H2O2 from Alcohols Under Aerobic Conditions, Bull. Chem. Soc. Jpn., 2020, 93, 299–301 CrossRef CAS.
- L. Wang, W. Zhang, D. S. Su, X. Meng and F.-S. Xiao, Supported Au nanoparticles as efficient catalysts for aerobic homocoupling of phenylboronic acid, Chem. Commun., 2012, 48, 5476–5478 RSC.
- W. Jang, J. Yun, L. Ludwig, S. G. Jang, J. Y. Bae, H. Byun and J.-H. Kim, Comparative Catalytic Properties of Supported and Encapsulated Gold Nanoparticles in Homocoupling Reactions, Front. Chem., 2020, 8, 834–842 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: More TEM images, XPS, XANES and Langmuir-isotherm plots for supported gold catalysts. Experimental procedure and reproduced spectra of products. See DOI: 10.1039/d0nj04255b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |