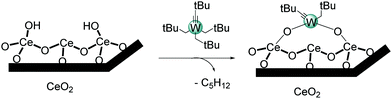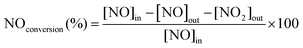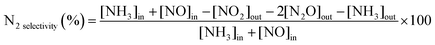Open Access Article
Open Access ArticleWell-defined surface tungstenocarbyne complex through the reaction of [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) CtBu)(CH2tBu)3] with CeO2: a highly stable precatalyst for NOx reduction with NH3†
CtBu)(CH2tBu)3] with CeO2: a highly stable precatalyst for NOx reduction with NH3†
Cherif
Larabi
a,
Cuirong
Chen
a,
Nicolas
Merle
ab,
Marc
Charlin
a,
Kai C.
Szeto
a,
Aimery
De Mallmann
a,
Anass
Benayad
c,
Karima
B. Meziane
 b,
Akim
Kaddouri
d,
Hai P.
Nguyen
*e and
Mostafa
Taoufik
b,
Akim
Kaddouri
d,
Hai P.
Nguyen
*e and
Mostafa
Taoufik
 *a
*a
aUniversité Lyon 1, Institut de Chimie Lyon, CPE Lyon CNRS, UMR 5128 CP2M, PCM, 43 Bd du 11 Novembre 1918, 69616 Villeurbanne Cedex, France. E-mail: mostafa.TAOUFIK@univ-lyon1.fr
bUniversité de Lille, CNRS, UMR 8516 - LASIRE - Laboratoire de Spectroscopie pour les Interactions, la Réactivité et l'Environnement, F-59000 Lille, France
cUniversité Grenoble Alpes, CEA-LITEN, 17 rue des Martyrs, 38054 Grenoble Cedex 9, France
dUniversité Lyon 1 - CNRS, UMR 5256, IRCELYON, 2 Avenue Albert Einstein, F-69626 Villeurbanne, France
eToyota Motor Europe, 1930 Zaventem, Belgium. E-mail: Hai.P.Nguyen@toyota-europe.com
First published on 7th June 2021
Abstract
A novel well-defined precatalyst for ammonia-selective catalytic reduction of NOx (NH3-SCR), namely, [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3]/CeO2, was prepared by surface organometallic chemistry and then characterized. Due to the high dispersion of the active phase, this catalyst showed excellent activity after calcination at 500 °C, described by up to 99% conversion of NOx, high N2 selectivity, broad operation temperature window (225–500 °C), and extremely high durability for the selective catalytic reduction of NOx with NH3.
CtBu)(CH2tBu)3]/CeO2, was prepared by surface organometallic chemistry and then characterized. Due to the high dispersion of the active phase, this catalyst showed excellent activity after calcination at 500 °C, described by up to 99% conversion of NOx, high N2 selectivity, broad operation temperature window (225–500 °C), and extremely high durability for the selective catalytic reduction of NOx with NH3.
Introduction
Significant worldwide increase in stationary thermal power plants and mobile combustion engines has resulted in the production of large quantities of carbon dioxide and nitrogen oxides (NOx). NOx emissions contribute to ecosystem damage, serious health issues, and worsen climate change via the greenhouse effect, acid rains, and ozone destruction.1 Therefore, the emission-level legislation of this pollutant has been drastically tightened.2,3NOx emissions have reduced through improvements such as exhaust gas recirculation, homogeneous charge compression ignition technologies, and optimized injection systems, as well as improved air control.4 These reducing measures can lower NOx emissions, albeit with a net increase in particulate matter (PM) amount and unburned hydrocarbons. As PM and NOx emissions are totally interdependent, a decline in the former triggers a rise in the latter, and vice versa. Thus, to improve the combustion engine efficiency and minimize fuel consumption, it is desirable to operate at a lean fuel mixture, which is typically the case for diesel engines.5 Under these conditions, in addition to a complete combustion product (CO2 and H2O), a significant amount of NOx is produced. Therefore, exhaust gas after-treatment systems are necessary in order to meet stringent harmful-emission limits. Catalysis has seen impressive developments in the field of NOx reduction since the beginning of the eighties by the implementation of three-way catalyst systems. However, the composition of diesel exhaust brings new challenges that mobilized industries, researchers, and authorities need to conform to by creating many improvements and innovative measures. The most effective and useful method for NOx removal without compromising engine performances is the selective catalytic reduction (SCR) method assisted with reducing agents (NH3 or hydrocarbon).6,7 The most efficient approach is SCR using ammonia (NH3-SCR). This process leads to high NOx conversions at fairly low temperatures and in large temperature ranges.8 Initially, this technology is set up in power plants and industrial installations since many years; however, currently, they are extensively adopted in heavy-duty and light-duty trucks as well as locomotives and ships.
A wide range of SCR catalysts have been developed. The most commercially viable and utilized catalysts are TiO2-supported V2O5 with WO3 or MoO3 as promotors.9,10 Although the catalytic activity of this class of materials is acceptable, they suffer from several weaknesses such as low selectivity, narrow operating temperature range, and poor deNOx activity at low temperatures; however, the most critical is another central environmental issue—the release of deleterious and toxic VOx species.11 Hence, alternative catalysts based on transition (W, Nb, Mo, Zr, Ta)12 and rare-earth (Ce, Y) metals have been further developed.13 In particular, relevant studies on new catalytic systems such as WO3–CeO2, Nb2O5–CeO2, MnOx–WO3–CeO2, Cu–zeolite, and Fe–zeolite have brought important improvements in the field of NOx reduction.13–15 Due to the oxygen buffering capacity and redox properties of ceria, it is widely used in these processes.16 The major drawbacks of pure ceria are related to low thermal resistance and weak acidity. Hence, in order to improve these properties, new ceria-based catalysts have been developed by introducing other rare-earth or transition metal oxides such as ZrO2, TiO2, and their modification with WO3, resulting in improvements in redox properties, thermal stability, and surface acidity.13,16
Recently, through several mechanistic studies, it has been found that the catalytic activity depends not only on the acidic and redox properties of the material,10,17 but also on the metal–support interactions.18 In particular, the nature of the supported tungsten species is a determining factor. The reported conventional catalysts are normally prepared by uncontrolled impregnation, resulting in different species, including isolated surface tungsten sites, WxOy clusters, amorphous WO3, and Ce2(WO4)3.19 Indeed, all these phases are observed by any characterization technique and this can therefore complicate surface species identification and obtaining structure–activity relationships.
It has been proposed that single-site heterogeneous catalysts with a controlled coordination sphere of metals are highly effective for the selective reduction of NOx.20 The key step is the formation of isolated, supported metal species that improve metal dispersion and increase metal–support interactions. Therefore, a powerful approach known as surface organometallic chemistry (SOMC) can be applied in order to prepare such single-site species. This methodology involves the controlled grafting of a suitable organometallic precursor onto a support, creating a firmly bonded surface-based organometallic fragment. Previous studies have shown that catalytic materials prepared through this approach can lead to enhanced activity and facilitate further mechanistic studies.21 Moreover, great efforts have been devoted toward achieving high-performance catalysts via a chemical design. It is believed that the missing link for the use of catalysts in automotive applications is related to increasing the amount of nanosized and atomic-scale catalysts without impinging on their size, structure, shape, and interactions. This can be carefully monitored by SOMC.22
However, commercial SCR catalysts are loaded with high amounts of WO323,24 for ensuring higher stability and sufficient acidity, thereby increasing the NH3 adsorption strength that improves the activity and also the selectivity by inhibiting the oxidation of NH3.17 This phenomenon is also found in the case of industrial metathesis catalysts, where a high amount of metal such as tungsten (∼10 wt%) is required to reach high catalytic activities, despite the fact that only a low fraction of metal sites is active.25 Based on studies that unraveled the structure of the active species,26–28 SOMC has led to the development of well-defined highly active catalysts that contain only a low amount of metal.29–33 On the other hand, the use of SOMC for oxidizing support and for deNOx applications is rare. We hereby report the first example of a highly active deNOx catalyst with low metal loading prepared by the grafting of a Schrock-type tungsten complex on ceria along with its characterization.
Results and discussion
Grafting of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CtBu)(CH2tBu)3 on ceria (CeO2–200) surface by SOMC
CtBu)(CH2tBu)3 on ceria (CeO2–200) surface by SOMC
SOMC methodology has never been investigated on a ceria support for the grafting of a tungsten complex. Prior to catalyst preparation, the ceria support (CeO2) is characterized. The IR spectrum of ceria dehydroxylated at 200 °C shown in Fig. 1 reveal four ν(O–H) vibration bands attributable to different structures of cerium hydroxyl on the surface (terminal and bridging O–H), as reported in the literature (Fig. S1, ESI†).34,35 The intensity of the band at 3714 cm−1 associated with isolated OH groups is weak and the spectrum is rather dominated by the broad signal centered at 3630 cm−1, which can be attributed to bridged hydroxyl groups. In addition, a large band centered at 3527 cm−1 corresponds to a residual cerium oxy-hydroxide phase located within the pores.36 To achieve the grafting and functionalization of surface hydroxides under optimum conditions, it is desirable to know their amount. Among the different quantification methods, a chemical titration using Al(iBu)3, a surface reaction with a highly reactive organometallic complex, has shown to be reliable.37 This complex quantitatively reacts in pentane with surface hydroxyl groups by releasing one equivalent of isobutane per OH (Fig. S2, ESI†). The quantification of isobutane by GC shows that CeO2–200 contains 0.7 mmol OH g−1.
 | ||
Fig. 1 DRIFT spectrum of (a) ceria dehydroxylated at 200 °C and (b) after the grafting of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3. CtBu)(CH2tBu)3. | ||
The grafting reaction of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3 on CeO2–200 is carried out in pentane at room temperature for 4 h. Elemental analysis of this material reveals the presence of 3.3 wt% W, which corresponds to ∼0.18 mmol W g−1. The amount of carbon is found to be 2.16 wt% (1.79 mmol C g−1), which gives a C/W ratio of 9.95. Furthermore, a quantitative GC analysis of the gas released during the grafting process shows the formation of 0.38 mmol of neopentane, which is ∼1.9 tBuCH3 per W. This corresponds to a partial consumption of surface hydroxyls (∼55% of the initial surface O–H groups). Overall, the data are consistent with the formation of bipodal surface species bearing two ligands by a protonolysis with surface hydroxyls, leading to a concomitant release of around two neopentane per grafted tungsten atom, as highlighted in Scheme 1.
CtBu)(CH2tBu)3 on CeO2–200 is carried out in pentane at room temperature for 4 h. Elemental analysis of this material reveals the presence of 3.3 wt% W, which corresponds to ∼0.18 mmol W g−1. The amount of carbon is found to be 2.16 wt% (1.79 mmol C g−1), which gives a C/W ratio of 9.95. Furthermore, a quantitative GC analysis of the gas released during the grafting process shows the formation of 0.38 mmol of neopentane, which is ∼1.9 tBuCH3 per W. This corresponds to a partial consumption of surface hydroxyls (∼55% of the initial surface O–H groups). Overall, the data are consistent with the formation of bipodal surface species bearing two ligands by a protonolysis with surface hydroxyls, leading to a concomitant release of around two neopentane per grafted tungsten atom, as highlighted in Scheme 1.
The textural properties of CeO2–200 as well as W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200 were examined by nitrogen adsorption–desorption isotherm measurements; the physisorption isotherms and pore size distribution are depicted in Fig. S3 (ESI†). As shown, the shape of these material isotherms corresponds to type V according to IUPAC classification and shows an H2-type hysteresis loop (characteristic of capillary condensation between the aggregates constituting the solid). The initial increase in the adsorption capacity at low relative pressures is due to monolayer adsorption. The upward deviation in the range of P/P0 = 0.5–0.7 for the support and catalyst is associated with the progressive filling of space between the aggregates of particles. The neat ceria has a specific surface area of ∼205 ± 10 m2 g−1. The surface area slightly decreases upon functionalization with the transition metal complex by reducing the value to 190 ± 10 m2 g−1. The pore volume also decreases from 0.24 ± 0.01 to 0.21 ± 0.01 cm3 g−1.
CtBu)(CH2tBu)3/CeO2–200 were examined by nitrogen adsorption–desorption isotherm measurements; the physisorption isotherms and pore size distribution are depicted in Fig. S3 (ESI†). As shown, the shape of these material isotherms corresponds to type V according to IUPAC classification and shows an H2-type hysteresis loop (characteristic of capillary condensation between the aggregates constituting the solid). The initial increase in the adsorption capacity at low relative pressures is due to monolayer adsorption. The upward deviation in the range of P/P0 = 0.5–0.7 for the support and catalyst is associated with the progressive filling of space between the aggregates of particles. The neat ceria has a specific surface area of ∼205 ± 10 m2 g−1. The surface area slightly decreases upon functionalization with the transition metal complex by reducing the value to 190 ± 10 m2 g−1. The pore volume also decreases from 0.24 ± 0.01 to 0.21 ± 0.01 cm3 g−1.
The grafting reaction was monitored by DRIFT spectroscopy (Fig. 1). After surface functionalization of ceria by W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3, the isolated ν(CeO–H) band at 3712 cm−1 disappeared. The new bands appearing in the ranges of 3100–2850 cm−1 and 1620–1400 cm−1 are characteristic of aliphatic ν(C–H) and δ(C–H) vibrations, respectively, of the perhydrocarbyl ligands coordinated to surface tungsten. Noteworthily, a band at 2120 cm−1, which is absent in the neat ceria sample (Fig. 1a), is observed (Fig. 1b). This signal has already been found and ascribed to the forbidden 2F5/2 → 2F7/2 electronic transition of the subsurface Ce3+ (due to the partial reduction of ceria).38 Moreover, the DRIFT spectrum of the resulting material (Fig. 1b) shows a partial consumption of the other OH vibration bands, located between 3700 and 3600 cm−1, while a new broad band appears, resulting from the interaction of some OH groups with tungsten alkyl ligands. The evolution of signals in this spectral region with grafting has already been described for the reaction of W(
CtBu)(CH2tBu)3, the isolated ν(CeO–H) band at 3712 cm−1 disappeared. The new bands appearing in the ranges of 3100–2850 cm−1 and 1620–1400 cm−1 are characteristic of aliphatic ν(C–H) and δ(C–H) vibrations, respectively, of the perhydrocarbyl ligands coordinated to surface tungsten. Noteworthily, a band at 2120 cm−1, which is absent in the neat ceria sample (Fig. 1a), is observed (Fig. 1b). This signal has already been found and ascribed to the forbidden 2F5/2 → 2F7/2 electronic transition of the subsurface Ce3+ (due to the partial reduction of ceria).38 Moreover, the DRIFT spectrum of the resulting material (Fig. 1b) shows a partial consumption of the other OH vibration bands, located between 3700 and 3600 cm−1, while a new broad band appears, resulting from the interaction of some OH groups with tungsten alkyl ligands. The evolution of signals in this spectral region with grafting has already been described for the reaction of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3 with alumina dehydroxylated at 500 °C, where only the terminal tetrahedral Al sites react completely.39
CtBu)(CH2tBu)3 with alumina dehydroxylated at 500 °C, where only the terminal tetrahedral Al sites react completely.39
The X-ray diffraction analyses, as shown in Fig. S4 (ESI†), reveal that the samples exhibit the same diffraction peaks at 28.5°, 33.1°, 47.5°, 56.4°, 59.1°, 69.7°, and 79.1°, characteristic of the cubic fluorite structure of CeO2.40 This observation suggests that the functionalization did not affect the crystalline structure of ceria. From the diffraction pattern, the mean size of microcrystals can be evaluated from the Scherrer's equation. The average crystal sizes consequently estimated, as summarized in Table S1 (ESI†), indicated that the ceria particles have the tendency to agglomerate during the grafting of the tungsten complex, which corroborates the marginal reduction in the surface area.
1H MAS solid-state NMR spectrum of the resulting material, as depicted in Fig. S5a (ESI†), shows large signals at −3 and 0.1 ppm, tentatively attributed to the methyl of neopentyl and neopentylidyne ligands, respectively. The 1H NMR data are less informative due to the broadening and shifting of the signal positions owing to the presence of paramagnetic species (Ce3+).41,42 Moreover, from a 30% 13C-labelled molecular complex, the 13C CP MAS NMR data highlighted in Fig. S5b (ESI†) show an intense and broad signal centered at 26 ppm assigned to the methyl of t-Bu fragments and a broad—albeit weak—signal at 80 ppm, which can be assigned to the methylene carbons of the neopentyl fragment (CH2tBu). A weak signal at 287 ppm can be ambitiously attributed to the quaternary carbons of carbyne ligands. The broadening of the resonances and their shift to a higher field is presumably due to paramagnetic Ce3+ ions in trigonal and cubic sites present in the ceria support as already identified.41,42
X-ray photoelectron spectroscopy (XPS) analyses are carried out in order to provide more information on the oxidation state of the constituent elements of the sample (W, Ce, O). Fig. S6 (ESI†) shows the representative XPS spectra for Ce 3d, O 1s, and W 4f. Generally, ten features are found in the Ce 3d region due to the pairs of spin–orbit doublet, as shown in Fig. S6a and b (ESI†). Six peaks are labelled as v, v′′, v′′′ (3d5/2) and u, u′′, u′′′ (3d3/2), associated to Ce4+ and u0, u′, v0, v′ attributable to Ce3+ (3d104f1), as described in the literature.43,44 The total fraction of Ce3+ is estimated by taking the fitted Ce3+ peak areas to the total deconvoluted spectra (%Ce3+ = (Ce3+/(Ce4+ + Ce3+) and %Ce4+ = (100–%Ce3+))).45 The concentration of Ce3+ in the thermally treated CeO2 is evaluated to be ∼32%, with respect to the total amount of Ce; therefore, the main oxidation state of ceria is Ce4+ (68%). Noteworthily, after the grafting of the organometallic complex, the amount of Ce3+ marginally increases to 34% (Table S2, ESI†), suggesting the presence of more surface oxygen vacancies.46 It is widely reported that the concomitant presence of Ce3+ and Ce4+ offers an oxygen-buffering capacity and redox properties that can promote NOx dissociation.47,48 The O 1s spectra of CeO2 and W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2 are compared in Fig. S6c and d (ESI†). The peaks are quite large, leading to two binding energy contributions for O2−: 531 eV and a shoulder at 532.5 eV, assigned to lattice oxygen (O2−) of CeO2 (denoted Oβ)47 and surface oxygen (denoted as Oα) such as (O−) in the defect oxide or OH,46 respectively. The relative amounts of calculated Oα (Oα/(Oα + Oβ)) are 45% for neat CeO2 and 37% for W(
CtBu)(CH2tBu)3/CeO2 are compared in Fig. S6c and d (ESI†). The peaks are quite large, leading to two binding energy contributions for O2−: 531 eV and a shoulder at 532.5 eV, assigned to lattice oxygen (O2−) of CeO2 (denoted Oβ)47 and surface oxygen (denoted as Oα) such as (O−) in the defect oxide or OH,46 respectively. The relative amounts of calculated Oα (Oα/(Oα + Oβ)) are 45% for neat CeO2 and 37% for W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200. The partial suppression of Oα can be attributed to their interaction with the grafted W.19 The chemisorbed oxygen may promote the oxidation of NO to NO2, inducing “fast SCR,” resulting in a higher overall SCR catalytic activity.49 Simultaneously, it can also promote the high-temperature oxidation of NH3 and affect the selectivity.18 Thus, an optimal amount of Oα is desirable in order to increase the reaction rate by not too much since it might affect the selectivity.50 The XPS analysis was also used to investigate the oxidation state of tungsten loaded on the support. The W 4f signal is depicted in Fig. S6e (ESI†): it shows the presence of two signals attributable to W 4f5/2 and W 4f7/2 at 37.5 and 35.3
CtBu)(CH2tBu)3/CeO2–200. The partial suppression of Oα can be attributed to their interaction with the grafted W.19 The chemisorbed oxygen may promote the oxidation of NO to NO2, inducing “fast SCR,” resulting in a higher overall SCR catalytic activity.49 Simultaneously, it can also promote the high-temperature oxidation of NH3 and affect the selectivity.18 Thus, an optimal amount of Oα is desirable in order to increase the reaction rate by not too much since it might affect the selectivity.50 The XPS analysis was also used to investigate the oxidation state of tungsten loaded on the support. The W 4f signal is depicted in Fig. S6e (ESI†): it shows the presence of two signals attributable to W 4f5/2 and W 4f7/2 at 37.5 and 35.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV, respectively, after deconvolution. These values are characteristic of W (+VI) for the grafted complex. The W 4f peaks overlap with the Ce 5s peaks, as shown in Fig. S6f (ESI†), inducing discrepancies for curve deconvolution. Nevertheless, these results are consistent with the presence of only W (+VI), but maybe with some marginal structural heterogeneities. Electron paramagnetic resonance (EPR) spectroscopy of the W(
eV, respectively, after deconvolution. These values are characteristic of W (+VI) for the grafted complex. The W 4f peaks overlap with the Ce 5s peaks, as shown in Fig. S6f (ESI†), inducing discrepancies for curve deconvolution. Nevertheless, these results are consistent with the presence of only W (+VI), but maybe with some marginal structural heterogeneities. Electron paramagnetic resonance (EPR) spectroscopy of the W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200 material (Fig. S7, ESI†) did not show any signal of isolated W(V) ions with an expected g factor between 1.39 and 1.85.51 This suggests that all tungsten atoms are present in the oxidation state of W(VI). The sample with 3.3 wt% W was studied by X-ray absorption spectroscopy (Table 1 and Fig. 2) in order to shed light on the structure of the supported species.
CtBu)(CH2tBu)3/CeO2–200 material (Fig. S7, ESI†) did not show any signal of isolated W(V) ions with an expected g factor between 1.39 and 1.85.51 This suggests that all tungsten atoms are present in the oxidation state of W(VI). The sample with 3.3 wt% W was studied by X-ray absorption spectroscopy (Table 1 and Fig. 2) in order to shed light on the structure of the supported species.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2. aThe errors generated by the EXAFS fitting program “RoundMidnight” are indicated in parentheses
CtBu)(CH2tBu)3/CeO2. aThe errors generated by the EXAFS fitting program “RoundMidnight” are indicated in parentheses
| Type of neighbor | Number of neighbors | Distance (Å) | σ 2 (Å2) |
|---|---|---|---|
| a Δk: [1.8–14.5 Å−1] − ΔR: [0.5–4.0 Å]; S02 = 0.94; ΔE0 = 4.7 ± 1.2 eV (the same for all shells); fit residue: ρ = 5.6%; quality factor: (Δχ)2/ν = 2.36 (ν = 15/30). b Shell constrained to a parameter above. | |||
W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) CMe3 CMe3 |
1.1(2) | 1.78(1) | 0.0011(6) |
W-![[O with combining low line]](https://www.rsc.org/images/entities/b_i_char_004f_0332.gif) Ce Ce |
1.9(3) | 1.78b | 0.0011b |
W-![[C with combining low line]](https://www.rsc.org/images/entities/b_i_char_0043_0332.gif) H2CMe3 H2CMe3 |
1.0(2) | 2.26(3) | 0.0011b |
W-![[O with combining low line]](https://www.rsc.org/images/entities/b_i_char_004f_0332.gif) (Ce) (Ce) |
1.9(5) | 2.69(2) | 0.0015(10) |
W-![[O with combining low line]](https://www.rsc.org/images/entities/b_i_char_004f_0332.gif) (Ce) (Ce) |
3.0(8) | 2.94(3) | 0.0015b |
W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) C C![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) Me3 Me3 |
1.1b | 3.25(6) | 0.0015b |

|
1.0(4) | 3.58(3) | 0.0015b |
 | ||
Fig. 2 W LIII-edge k2-weighted EXAFS (left) and its Fourier transform (right) for W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200 material. Solid lines: experimental; dashed lines: spherical wave theory. CtBu)(CH2tBu)3/CeO2–200 material. Solid lines: experimental; dashed lines: spherical wave theory. | ||
For the first peak of the Fourier transform (right in Fig. 2), two levels of coordinated light atoms back-scatterers could be evidenced at ∼1.78 Å and 2.25 Å. Considering the level at 1.78 Å, two types of atoms, namely, O and C, were considered: oxygen coming from the ceria surface and carbon, from a carbyne ligand. The parameters thus extracted from the fit of the EXAFS signal are in agreement with a bipodal structure, (–O)2W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu), with around two oxygen atoms at 1.78(1) Å, around one carbon atom at 1.78(1) Å, and another carbon atom at 2.25(3) Å, attributed most probably to the carbon atoms of neopentylidyne and neopentyl ligands, respectively. The W-O distance seems marginally short but tungsten has been found to be surrounded by around six oxygen atoms at 1.787(1) Å in a W0.2Ce0.8O2 mixed metal oxide.52 Moreover, the two carbon back-scatterers are located at distances in the range of W
CtBu)(CH2tBu), with around two oxygen atoms at 1.78(1) Å, around one carbon atom at 1.78(1) Å, and another carbon atom at 2.25(3) Å, attributed most probably to the carbon atoms of neopentylidyne and neopentyl ligands, respectively. The W-O distance seems marginally short but tungsten has been found to be surrounded by around six oxygen atoms at 1.787(1) Å in a W0.2Ce0.8O2 mixed metal oxide.52 Moreover, the two carbon back-scatterers are located at distances in the range of W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and W–C triple and single bonds, respectively, as observed for [W(
C and W–C triple and single bonds, respectively, as observed for [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCMe3)(
CCMe3)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCMe3)(CH2CMe3)(dmpe)] molecular complex (1.785(8) Å for W
CHCMe3)(CH2CMe3)(dmpe)] molecular complex (1.785(8) Å for W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu and 2.258(9) Å for W-CH2tBu).53 Similar parameters were obtained when fitting the k3χ(k) spectrum. The fit could also be improved by adding further layers of back-scatters, particularly two types of oxygen atoms at 2.69(2) and 2.94(2) Å and only around one cerium atom at 3.57(3) Å. The inclusion of tungsten as a second neighbor was not statistically validated. Therefore, this EXAFS study suggests a single-site structure ((–O)2W(
CtBu and 2.258(9) Å for W-CH2tBu).53 Similar parameters were obtained when fitting the k3χ(k) spectrum. The fit could also be improved by adding further layers of back-scatters, particularly two types of oxygen atoms at 2.69(2) and 2.94(2) Å and only around one cerium atom at 3.57(3) Å. The inclusion of tungsten as a second neighbor was not statistically validated. Therefore, this EXAFS study suggests a single-site structure ((–O)2W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)), as shown in Fig. 3, where the tungsten atom in a pseudo-octahedral environment can be tentatively proposed (in the cerium oxide crystal,54 the Ce–O bond distance is around 2.34 Å, and the shortest Ce–Ce distance is around 3.83 Å).
CtBu)(CH2tBu)), as shown in Fig. 3, where the tungsten atom in a pseudo-octahedral environment can be tentatively proposed (in the cerium oxide crystal,54 the Ce–O bond distance is around 2.34 Å, and the shortest Ce–Ce distance is around 3.83 Å).
 | ||
Fig. 3 Proposed structure for the species resulting from the grafting of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3 onto CeO2–200. CtBu)(CH2tBu)3 onto CeO2–200. | ||
Finally, W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200 has been submitted for HRTEM study. The distribution of atomic tungsten on the surface of ceria was confirmed in an indirect manner by HRTEM and STEM analyses (Fig. S8, ESI†). Indeed, the combination of HRTEM and STEM with EDX analyses showed the homogeneity of the sample. No observable cluster or nanoparticles were observed even under extensive magnification (∼1 nm), but at the same time, the EDX examination confirmed the presence of W.
CtBu)(CH2tBu)3/CeO2–200 has been submitted for HRTEM study. The distribution of atomic tungsten on the surface of ceria was confirmed in an indirect manner by HRTEM and STEM analyses (Fig. S8, ESI†). Indeed, the combination of HRTEM and STEM with EDX analyses showed the homogeneity of the sample. No observable cluster or nanoparticles were observed even under extensive magnification (∼1 nm), but at the same time, the EDX examination confirmed the presence of W.
The reactivity of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3 with CeO2 dehydroxylated at 200 °C has been investigated. The grafting occurs on Ce–OH groups by protonolysis, affording bipodal surface species, as suggested by the quantification of released neopentane and elemental analysis. Further characterizations by DRIFT and solid-state NMR confirm the presence of neopentyl fragments on the material. Tungsten—mainly in the oxidation state VI—has been revealed by XPS and EXAFS. XPS further provides quantitative information about the Ce3+/Ce4+ ratio on the surface upon grafting. A more representative environment around tungsten on the surface is proposed by the EXAFS data. Surprisingly, there is only one cerium atom close to the tungsten center. The proposed model (Fig. 3) fits well with the data and comprises isolated bipodal tungsten surface species, as indicated by the elemental analysis and HRTEM.
CtBu)(CH2tBu)3 with CeO2 dehydroxylated at 200 °C has been investigated. The grafting occurs on Ce–OH groups by protonolysis, affording bipodal surface species, as suggested by the quantification of released neopentane and elemental analysis. Further characterizations by DRIFT and solid-state NMR confirm the presence of neopentyl fragments on the material. Tungsten—mainly in the oxidation state VI—has been revealed by XPS and EXAFS. XPS further provides quantitative information about the Ce3+/Ce4+ ratio on the surface upon grafting. A more representative environment around tungsten on the surface is proposed by the EXAFS data. Surprisingly, there is only one cerium atom close to the tungsten center. The proposed model (Fig. 3) fits well with the data and comprises isolated bipodal tungsten surface species, as indicated by the elemental analysis and HRTEM.
Catalytic activity tests
The precatalyst W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200 was calcined under dry air at 500 °C for 16 h, allowing the removal of organic ligands (Fig. S9, ESI†) and affording catalyst 1 before evaluating the selective reduction of NOx in a continuous flow reactor. The temperature dependencies of NOx reduction are shown in Fig. 4. 1 shows more than 95% conversion of NOx between 280 and 400 °C (Fig. 4, 1st cycle). Indeed, a high activity at low temperature (200–250 °C) with a sharp increase in conversion with temperature is observed. The selectivity for N2 is outstandingly high until 400 °C (Fig. 4). The fresh catalyst starts to oxidize NH3 from ∼400 °C, resulting in a fast drop in NO conversion and N2 selectivity. However, upon repeating the SCR cycles, it can be observed that the same catalyst becomes more stable, selective, and temperature-resistant. Full NO conversion is maintained until 550 °C. Such evolution indicates that the surface sites responsible for NH3 oxidation have gradually been inhibited. After five catalytic recycle tests, the catalyst seems to be stable and converts more than 99% NOx between 250 and 500 °C. This suggests that the surface species occur under the catalytic conditions (O2, NH3, NO, and high temperature) to achieve stable and more active species.
CtBu)(CH2tBu)3/CeO2–200 was calcined under dry air at 500 °C for 16 h, allowing the removal of organic ligands (Fig. S9, ESI†) and affording catalyst 1 before evaluating the selective reduction of NOx in a continuous flow reactor. The temperature dependencies of NOx reduction are shown in Fig. 4. 1 shows more than 95% conversion of NOx between 280 and 400 °C (Fig. 4, 1st cycle). Indeed, a high activity at low temperature (200–250 °C) with a sharp increase in conversion with temperature is observed. The selectivity for N2 is outstandingly high until 400 °C (Fig. 4). The fresh catalyst starts to oxidize NH3 from ∼400 °C, resulting in a fast drop in NO conversion and N2 selectivity. However, upon repeating the SCR cycles, it can be observed that the same catalyst becomes more stable, selective, and temperature-resistant. Full NO conversion is maintained until 550 °C. Such evolution indicates that the surface sites responsible for NH3 oxidation have gradually been inhibited. After five catalytic recycle tests, the catalyst seems to be stable and converts more than 99% NOx between 250 and 500 °C. This suggests that the surface species occur under the catalytic conditions (O2, NH3, NO, and high temperature) to achieve stable and more active species.
 | ||
| Fig. 4 NOx conversion and N2 selectivity over 1 as a function of temperature during 7 cycles. Feed composition: 300 ppm NO, 350 ppm NH3, and 10 vol% O2 in He. | ||
Moreover, the long-term stability of the catalyst at 300 °C was also studied. The results shown in Fig. S10 (ESI†) indicate that the activity of the catalysts remains stable for more than 50 h, with a conversion of about 99%. To extend the behavior of these catalysts for deNOx-related reactions, two experiments with separate (NO + O2) and (NH3 + O2) feeds were performed. The results of NO and NH3 oxidation versus temperature, as shown in Fig. S11 (ESI†), show that the oxidation of NO into NO2 occurs at low temperatures, ∼150 °C, and it linearly increases with the temperature to reach a pseudo-plateau between 280 and 380 °C before increasing gradually again. The first part of the curve can be explained by the role of the surface oxygen available that can promote the oxidation of NO into NO2 to reach a plateau when oxygen is consumed. Then, thermal oxidation can follow. In contrast, the oxidation of NH3 did not take place before the reaction temperature reaches 300 °C, where a sharp increase was observed for temperatures higher than 300 °C. This can explain the high activity observed for this SOMC catalyst (1), where the oxidation of NO into NO2 is favored over NH3 oxidation, where NO2 can promote fast SCR.
Additional catalysis cycles have been investigated in the presence of 5% water (Fig. 5), reflecting more realistic conditions in combustion engines. Although the activity in the first cycle is far lower, which can be explained by the strong adsorption of water on the surface, the same tendency upon multiple recycling tests remains the same. After each catalytic run, the activity improves.
 | ||
| Fig. 5 NOx conversion and N2 selectivity over 1 as a function of temperature during 5 cycles. Feed composition: 300 ppm NO, 350 ppm NH3, 5 vol% H2O, and 10 vol% O2 in He. | ||
This better activity is due to better W dispersion on ceria, where only isolated sites are obtained in the case of the catalyst prepared via the SOMC approach, contrary to catalysts prepared by classical methods (2) composed of a statistical distribution of surface species (Fig. S12, ESI†). Therefore, the catalytic behavior is highly dependent on the preparation method. The well-controlled distribution of W species on the surface of ceria prepared through advanced surface organometallic functionalization could result in a higher concentration and strength of Brønsted acids sites19,46 or in the formation of WδCe1−δO2−δ clusters on the surface, which are highly active and more accessible to the reactants.
Conclusions
The present work describes the first example of a deNOx catalyst prepared by SOMC. The grafting of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3 on CeO2 dehydroxylated at 200 °C affords isolated bipodal species, as revealed by DRIFT, solid-state NMR, elemental analysis, TEM, XPS, and EXAFS. The catalyst transforms NO and NH3 into N2 in a typical exhaust gas composition after calcination at 500 °C, with almost full conversion between 280 and 400 °C. Importantly, when multiple SCR cycles were studied, the catalytic performance increased, leading to the full conversion of NO in the 220–500 °C temperature range, including that in the presence of 5 vol% water. Such an evolution is surprising, and it is presumably due to the inhibition of NH3 oxidation by flawed sites of ceria. The good dispersion of tungsten may also increase the acidity and oxygen-buffering capacity of the material and therefore results in a more active SCR catalyst. Ongoing experiments using in operando techniques (EXAFS and DRIFT) during the recycling process should provide information on the evolution of the catalyst when increasing the number of SCR cycles and explain the higher activity by the identification of different sites on the surface.
CtBu)(CH2tBu)3 on CeO2 dehydroxylated at 200 °C affords isolated bipodal species, as revealed by DRIFT, solid-state NMR, elemental analysis, TEM, XPS, and EXAFS. The catalyst transforms NO and NH3 into N2 in a typical exhaust gas composition after calcination at 500 °C, with almost full conversion between 280 and 400 °C. Importantly, when multiple SCR cycles were studied, the catalytic performance increased, leading to the full conversion of NO in the 220–500 °C temperature range, including that in the presence of 5 vol% water. Such an evolution is surprising, and it is presumably due to the inhibition of NH3 oxidation by flawed sites of ceria. The good dispersion of tungsten may also increase the acidity and oxygen-buffering capacity of the material and therefore results in a more active SCR catalyst. Ongoing experiments using in operando techniques (EXAFS and DRIFT) during the recycling process should provide information on the evolution of the catalyst when increasing the number of SCR cycles and explain the higher activity by the identification of different sites on the surface.
Experimental
All syntheses were performed under pure and dry argon, using standard Schlenk techniques and a glovebox. Solvents were purified and dried according to standard procedures. C6D6 (Aldrich, 99.8%) was distilled over Na/benzophenone and stored on NaK.Characterization methods
Elemental analyses were performed at Mikroanalytisches Labor Pascher (Remagen, Germany). Gas-phase analyses were performed on a Hewlett Packard 5890 series II gas chromatograph equipped with a flame ionization detector and KCl/Al2O3 column (30 m × 0.32 mm). Diffuse reflectance infrared spectra were collected in a Nicolet 6700 FT-IR spectrophotometer at a resolution of 4 cm−1. An airtight IR cell with CaF2 windows was employed and the final spectra comprise 64 scans. Solution NMR spectra were recorded on an Avance 300 Bruker spectrometer. All chemical shifts were measured relative to residual 1H or 13C resonances in a deuterated solvent: C6D6, δ 7.15 ppm for 1H, 128 ppm for 13C. 1H and 13C solid-state NMR spectra were recorded on a Bruker Avance 500 spectrometer with a conventional double-resonance 4 mm CP-MAS probe. The samples were introduced under argon in a zirconia rotor (4 mm), which was then tightly closed. In all the experiments, the rotation frequency was set to 10 kHz. Chemical shifts were given with respect to tetramethylsilane (TMS) as an external reference for 1H and 13C NMR. X-ray diffraction was performed on a Siemens D8 diffractometer at Bragg–Brentano geometry (ISA, Villeurbanne). The instrument is equipped with a Cu tube (λ = 1.5406 Å). The electron paramagnetic resonance (EPR) continuous-wave (CW) measurements were performed through a sealed quartz tube on an X-band Bruker ELEXSYS E500 spectrometer operating at 9.8 GHz. The spectra were recorded with the same modulation frequency (100 kHz), the same modulation of amplitude, and the same power level for each sample. In order to avoid the saturation effect, the measurements were made at different microwave power levels (0.1–4 mW). The modulation of amplitude (1–5 G) was adjusted for the same reason. The low-temperature measurements were made with cryogenic systems. TEM measurement was executed on an environmental tomographic transmission electron microscope by FEI TITAN (IRCE Lyon, Villeurbanne). TGA was performed on a Mettler Toledo TGA2 Stare system.The extended X-ray absorption fine-structure (EXAFS) spectra were acquired at ELETTRA, using the XAFS beamline (experiment code: 20145422)55 at room temperature at the tungsten LIII-edge. A pair of Si(111) crystals were used as the monochromator and the harmonics were rejected by the detuning of the second crystal. The spectra were recorded in the transmission mode between 9.9 and 11.43 keV. Three scans were collected for each sample. Each dataset was simultaneously collected with a W metal foil reference (11206.7 eV), and it was later aligned according to that reference (maximum in the first derivative of the first peak of the W foil). The W-supported sample was packaged within a nitrogen-filled glovebox in a double airtight sample holder equipped with Kapton windows. The data analyses were carried out using the “Athena” program and the EXAFS fitting “RoundMidnight” program, from the “MAX” package, using spherical waves. The FEFF8 program was used to calculate the theoretical files for phases and amplitudes based on model clusters of atoms. The scale factor, S02 = 0.94, was evaluated from [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)Np3] molecular complex diluted in BN and conditioned as a pellet (one carbon at 1.76(1) Å, with three carbon atoms at 2.10(1) Å in the first coordination sphere, and one carbon atom at 3.25(3) Å and three carbon atoms at 3.34(3) Å). The refinements were carried out by fitting the structural parameters Ni, Ri, σi, and energy shift, ΔE0 (the same for all shells).
CtBu)Np3] molecular complex diluted in BN and conditioned as a pellet (one carbon at 1.76(1) Å, with three carbon atoms at 2.10(1) Å in the first coordination sphere, and one carbon atom at 3.25(3) Å and three carbon atoms at 3.34(3) Å). The refinements were carried out by fitting the structural parameters Ni, Ri, σi, and energy shift, ΔE0 (the same for all shells).
Materials
Ceria (CeO2) HAS-5 ACTALYS 922 from Solvay (“Rare Earth La Rochelle”) was calcined for 16 h at 500 °C under a flow of dry air and then evacuated under a vacuum at a high temperature. After rehydration in an inert atmosphere, ceria was partially dehydroxylated at 200 °C under a high vacuum (10−5 mbar) for 15 h to give a yellow solid, noted as CeO2–200.Tungsten complexes (W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3,56 as well as 30% labelled W(
CtBu)(CH2tBu)3,56 as well as 30% labelled W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) *CtBu)(*CH2tBu)3)36 were prepared according to the described procedures.
*CtBu)(*CH2tBu)3)36 were prepared according to the described procedures.
Catalyst preparation: SOMC catalyst
A mixture of W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3 (1.6 g, 1.2 mmol) and CeO2–200 (7 g) was stirred in pentane (Carlo Erba, 99%) for 4 h at room temperature. The W(
CtBu)(CH2tBu)3 (1.6 g, 1.2 mmol) and CeO2–200 (7 g) was stirred in pentane (Carlo Erba, 99%) for 4 h at room temperature. The W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)(CH2tBu)3/CeO2–200 material was washed three times with pentane via filtration–condensation cycles. Then, all the volatiles were condensed into a 6 L vessel in order to quantify neopentane evolved during the grafting reaction. After evaporation of the solvent, the resulting grey powder was dried under a vacuum (10−5 mbar). The material was calcined under dry air at 500 °C for 16 h before the catalytic tests.
CtBu)(CH2tBu)3/CeO2–200 material was washed three times with pentane via filtration–condensation cycles. Then, all the volatiles were condensed into a 6 L vessel in order to quantify neopentane evolved during the grafting reaction. After evaporation of the solvent, the resulting grey powder was dried under a vacuum (10−5 mbar). The material was calcined under dry air at 500 °C for 16 h before the catalytic tests.
Catalyst preparation: conventional catalyst
For comparison, the impregnated catalyst based on tungsten (W) supported on ceria was prepared by a conventional wet impregnation. In a typical reaction, at room temperature, a water-based solution of ammonium metatungstate (Aldrich, 99.99%) ((NH4)6H2W12O40, 100 mg in 5 mL of water) was added to 2 g of ceria. The suspension was stirred overnight at room temperature and then water was evacuated in a rotary evaporator at 50 °C. The material obtained was then calcined under dry air at 500 °C for 16 h. The elemental analysis indicated the presence of ∼3.5 wt% W.NH3-SCR catalytic performance tests
The NH3-SCR catalytic evaluations were performed in a quartz fixed-bed reactor. A total gas flow rate of 300 mL min−1 (hourly space velocity: 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1) at atmospheric pressure, typically composed of 300 ppm NO (air liquid, crystal grade), 350 ppm NH3 (air liquid, crystal grade), 10 vol% O2 (air liquid, crystal grade), and 5% H2O (when used), was sent through 30 mg of the presented catalyst diluted with 500 mg of silicon carbide in order to ensure a volume of 1 cm3. The reactor equipped with an internal thermometer was heated from room temperature to 600 °C at a heating rate of 10 °C min−1. The system was maintained at 600 °C for 10 min before cooling down. Analyses of the gas concentrations of NO, NO2, N2O, and NH3 were carried out using Antaris IGS FTIR (Thermo Fisher) equipped with a gas cell of 200 mL. N2 selectivity was evaluated assuming that the outlet gas contains no N compounds other than NO, NO2, N2O, and NH3 (Scheme 2).
000 h−1) at atmospheric pressure, typically composed of 300 ppm NO (air liquid, crystal grade), 350 ppm NH3 (air liquid, crystal grade), 10 vol% O2 (air liquid, crystal grade), and 5% H2O (when used), was sent through 30 mg of the presented catalyst diluted with 500 mg of silicon carbide in order to ensure a volume of 1 cm3. The reactor equipped with an internal thermometer was heated from room temperature to 600 °C at a heating rate of 10 °C min−1. The system was maintained at 600 °C for 10 min before cooling down. Analyses of the gas concentrations of NO, NO2, N2O, and NH3 were carried out using Antaris IGS FTIR (Thermo Fisher) equipped with a gas cell of 200 mL. N2 selectivity was evaluated assuming that the outlet gas contains no N compounds other than NO, NO2, N2O, and NH3 (Scheme 2).
The catalytic activity and N2 selectivity were calculated by the following equations:19
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support of Toyota Motor Corporation is gratefully acknowledged. CC thanks CSC (China Scholarship Council) for financial support. At ELETTRA synchrotron light source, in Basovizza, Italy, we would like to thank Dr Luca Olivi for his help during the recording of the X-Ray absorption spectra and for the use of the glovebox.Notes and references
- R. M. Doherty, M. R. Heal and F. M. O’Connor, Climate change impacts on human health over Europe through its effect on air quality, Environ. Health, 2017, 16, 33–44 Search PubMed.
- M. Fiebig, A. Wiartalla, B. Holderbaum and S. Kiesow, Particulate emissions from diesel engines: correlation between engine technology and emissions, J. Occup. Med. Toxicol., 2014, 9, 6 Search PubMed.
- N. Hooftman, M. Messagie, J. Van Mierlo and T. Coosemans, A review of the European passenger car regulations – Real driving emissions vs. local air quality, Renewable Sustanable Energy Rev., 2018, 86, 1–21 Search PubMed.
- S. Ramalingam, S. Rajendran and P. Ganesan, Performance improvement and exhaust emissions reduction in biodiesel operated diesel engine through the use of operating parameters and catalytic converter: a review, Renewable Sustanable Energy Rev., 2018, 81, 3215–3222 Search PubMed.
- F. Pischinger, The diesel engine for cars - Is there a future?, J. Eng. Gas Turbines Power, 1998, 120, 641–647 Search PubMed.
- S. Zhang, B. Zhang, B. Liu and S. Sun, A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: reaction mechanism and catalyst deactivation, RSC Adv., 2017, 7, 26226–26242 Search PubMed.
- J. Xu, H. Wang, F. Guo, C. Zhang and J. Xie, Recent advances in supported molecular sieve catalysts with wide temperature range for selective catalytic reduction of NOX with C3H6, RSC Adv., 2019, 9, 824–838 Search PubMed.
- W. Shan and H. Song, Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature, Catal. Sci. Technol., 2015, 5, 4280–4288 Search PubMed.
- J.-K. Lai and I. E. Wachs, A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5-WO3/TiO2 Catalysts, ACS Catal., 2018, 8, 6537–6551 Search PubMed.
- G. Busca, L. Lietti, G. Ramis and F. Berti, Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review, Appl. Catal., B, 1998, 18, 1–36 Search PubMed.
- Z. G. Liu, N. A. Ottinger and C. M. Cremeens, Vanadium and tungsten release from V-based selective catalytic reduction diesel aftertreatment, Atmos. Environ., 2015, 104, 154–161 Search PubMed.
- R. Mrad, A. Aissat, R. Cousin, D. Courcot and S. Siffert, Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR), Appl. Catal., A, 2015, 504, 542–548 Search PubMed.
- J. Xu, H. Yu, C. Zhang, F. Guo and J. Xie, Development of cerium-based catalysts for selective catalytic reduction of nitrogen oxides: a review, New J. Chem., 2019, 43, 3996–4007 Search PubMed.
- M. Moliner and A. Corma, From metal-supported oxides to well-defined metal site zeolites: the next generation of passive NOx adsorbers for low-temperature control of emissions from diesel engines, React. Chem., 2019, 4, 223–234 Search PubMed.
- L. Zhang, Q. Wu, X. Meng, U. Mueller, M. Feyen, D. Dai, S. Maurer, R. McGuire, A. Moini, A.-N. Parvulescu, W. Zhang, C. Shi, T. Yokoi, X. Pan, X. Bao, H. Gies, B. Marler, D. E. De Vos, U. Kolb and F.-S. Xiao, Recent advances in the preparation of zeolites for the selective catalytic reduction of NOx in diesel engines, React. Chem. Eng., 2019, 4, 975–985 Search PubMed.
- Z. Song, P. Liu, Y. Fu, H. Liu, Z. Huang, H. Kang, Y. Mao, B. Liu and Y. Guo, Promotional effect of acidic oxide on catalytic activity and N-2 selectivity over CeO2 for selective catalytic reduction of NOx by NH3, Appl. Organomet. Chem., 2019, 33, e4919 Search PubMed.
- G. He, Z. Lian, Y. Yu, Y. Yang, K. Liu, X. Shi, Z. Yan, W. Shan and H. He, Polymeric vanadyl species determine the low-temperature activity of V-based catalysts for the SCR of NOx with NH3, Sci. Adv., 2018, 4, eaau4637 Search PubMed.
- Z. Hu and R. T. Yang, 110th Anniversary: Recent Progress and Future Challenges in Selective Catalytic Reduction of NO by H-2 in the Presence of O-2, Ind. Eng. Chem. Res., 2019, 58, 10140–10153 Search PubMed.
- Y. Peng, K. Li and J. Li, Identification of the active sites on CeO2-WO3 catalysts for SCR of NOx with NH3: an in situ IR and Raman spectroscopy study, Appl. Catal., B, 2013, 140, 483–492 Search PubMed.
- C. Paolucci, I. Khurana, A. A. Parekh, S. Li, A. J. Shih, H. Li, J. R. Di Iorio, J. D. Albarracin-Caballero, A. Yezerets, J. T. Miller, W. N. Delgass, F. H. Ribeiro, W. F. Schneider and R. Gounder, Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction, Science, 2017, 357, 898–903 Search PubMed.
- N. Popoff, E. Mazoyer, J. Pelletier, R. M. Gauvin and M. Taoufik, Expanding the scope of metathesis: a survey of polyfunctional, single-site supported tungsten systems for hydrocarbon valorization, Chem. Soc. Rev., 2013, 42, 9035–9054 Search PubMed.
- A. Beniya and S. Higashi, Towards dense single-atom catalysts for future automotive applications, Nat. Catal., 2019, 2, 590–602 Search PubMed.
- F. Can, X. Courtois, S. Berland, M. Seneque, S. Royer and D. Duprez, Composition dependent performance of alumina-based oxide supported WO3 catalysts for the NH3-SCR reaction and the NSR plus SCR coupled process, Catal. Today, 2015, 257, 41–50 Search PubMed.
- Y. Xu, X. Wu, L. Cao, Y. Ma, R. Ran, Z. Si, D. Weng, Z. Ma and B. Wang, Crystal orientation-dependent activity of tungsten-based catalysts for selective catalytic reduction of NOx with NH3, J. Catal., 2019, 375, 294–303 Search PubMed.
- S. Lwin, Y. Li, A. I. Frenkel and I. E. Wachs, Nature of WOx Sites on SiO2 and Their Molecular Structure–Reactivity/Selectivity Relationships for Propylene Metathesis, ACS Catal., 2016, 6, 3061–3071 Search PubMed.
- A. Basrur, S. Patwardhan and S. Vyas, Propene metathesis over silica-supported tungsten-oxide catalyst. Catalyst induction mechanism, J. Catal., 1991, 127, 86–95 Search PubMed.
- C. Martin, P. Malet, G. Solana and V. Rives, Structural analysis of silica-supported tungstates, J. Phys. Chem. B, 1998, 102, 2759–2768 Search PubMed.
- A. Vanroomsmalen, D. Koster and J. Mol, Infrared-spectroscopy of some chemisorbed molecules on tungsten oxide-silica, J. Phys. Chem., 1980, 84, 3075–3079 Search PubMed.
- C. Larabi, K. C. Szeto, Y. Bouhoute, M. O. Charlin, N. Merle, A. De Mallmann, R. M. Gauvin, L. Delevoye and M. Taoufik, Solvent-Free Ring-Opening Metathesis Polymerization of Norbornene over Silica-Supported Tungsten-Oxo Perhydrocarbyl Catalysts, Macromol. Rapid Commun., 2016, 37, 1832–1836 Search PubMed.
- C. Larabi, N. Merle, F. Le Quemener, P. Rouge, E. Berrier, R. M. Gauvin, E. Le Roux, A. de Mallmann, K. C. Szeto and M. Taoufik, New synthetic approach towards well-defined silica supported tungsten bisoxo, active catalysts for olefin metathesis, Catal. Commun., 2018, 108, 51–54 Search PubMed.
- N. Merle, F. Le Quemener, Y. Bouhoute, K. C. Szeto, A. De Mallmann, S. Barman, M. K. Samantaray, L. Delevoye, R. M. Gauvin, M. Taoufik and J.-M. Basset, Well-Defined Molybdenum Oxo Alkyl Complex Supported on Silica by Surface Organometallic Chemistry: A Highly Active Olefin Metathesis Precatalyst, J. Am. Chem. Soc., 2017, 139, 2144–2147 Search PubMed.
- N. Merle, F. Le Quemener, S. Barman, M. K. Samantaray, K. C. Szeto, A. De Mallmann, M. Taoufik and J.-M. Basset, Well-defined silica supported bipodal molybdenum oxo alkyl complexes: a model of the active sites of industrial olefin metathesis catalysts, Chem. Commun., 2017, 53, 11338–11341 Search PubMed.
- F. Zhang, K. C. Szeto, M. Taoufik, L. Delevoye, R. M. Gauvin and S. L. Scott, Enhanced Metathesis Activity and Stability of Methyltrioxorhenium on a Mostly Amorphous Alumina: Role of the Local Grafting Environment, J. Am. Chem. Soc., 2018, 140, 13854–13868 Search PubMed.
- A. Badri, C. Binet and J. Lavalley, An FTIR study of surface ceria hydroxy groups during a redox process with H-2, J. Chem. Soc., Faraday Trans., 1996, 92, 4669–4673 Search PubMed.
- B. Beck, M. Harth, N. G. Hamilton, C. Carrero, J. J. Uhlrich, A. Trunschke, S. Shaikhutdinov, H. Schubert, H.-J. Freund, R. Schloegl, J. Sauer and R. Schomaecker, Partial oxidation of ethanol on vanadia catalysts on supporting oxides with different redox properties compared to propane, J. Catal., 2012, 296, 120–131 Search PubMed.
- M. Taoufik, E. Le Roux, J. Thivolle-Cazat, C. Coperet, J.-M. Basset, B. Maunders and G. J. Sunley, Alumina supported tungsten hydrides, new efficient catalysts for alkane metathesis, Top. Catal., 2006, 40, 65–70 Search PubMed.
- E. Mazoyer, J. Trebosc, A. Baudouin, O. Boyron, J. Pelletier, J.-M. Basset, M. J. Vitorino, C. P. Nicholas, R. M. Gauvin, M. Taoufik and L. Delevoye, Heteronuclear NMR Correlations To Probe the Local Structure of Catalytically Active Surface Aluminum Hydride Species on gamma-Alumina, Angew. Chem., Int. Ed., 2010, 49, 9854–9858 Search PubMed.
- C. Binet, A. Badri and J. Lavalley, A spectroscopic characterization of the reduction of ceria fraom electronic-transitions of intrinsic point-defects, J. Phys. Chem., 1994, 98, 6392–6398 Search PubMed.
- J. Joubert, F. Delbecq, P. Sautet, E. Le Roux, M. Taoufik, C. Thieuleux, F. Blanc, C. Coperet, J. Thivolle-Cazat and J.-M. Basset, Molecular understanding of alumina supported single-site catalysts by a combination of experiment and theory, J. Am. Chem. Soc., 2006, 128, 9157–9169 Search PubMed.
- S. A. Ansari, M. M. Khan, M. O. Ansari, S. Kalathil, J. Lee and M. H. Cho, Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications, RSC Adv., 2014, 4, 16782–16791 Search PubMed.
- R. M. Rakhmatullin, V. V. Pavlov and V. V. Semashko, EPR study of nanocrystalline CeO2 exhibiting ferromagnetism at room temperature, Phys. Status Solidi B, 2016, 253, 499–503 Search PubMed.
- R. M. Rakhmatullin, V. V. Semashko, S. L. Korableva, A. G. Kiiamov, A. A. Rodionov, R. Tschaggelar, J. A. van Bokhoven and C. Paun, EPR study of ceria nanoparticles containing different concentration of Ce3+ ions, Mater. Chem. Phys., 2018, 219, 251–257 Search PubMed.
- C. Anandan and P. Bera, XPS studies on the interaction of CeO2 with silicon in magnetron sputtered CeO2 thin films on Si and Si3N4 substrates, Appl. Surf. Sci., 2013, 283, 297–303 Search PubMed.
- Y. Zhu, N. Jain, M. K. Hudait, D. Maurya, R. Varghese and S. Priya, X-ray photoelectron spectroscopy analysis and band offset determination of CeO2 deposited on epitaxial (100), (110), and (111)Ge, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2014, 32, 011217 Search PubMed.
- X. Yao, Z. Wang, S. Yu, F. Yang and L. Dong, Acid pretreatment effect on the physicochemical property and catalytic performance of CeO2 for NH3-SCR, Appl. Catal., A, 2017, 542, 282–288 Search PubMed.
- W. Shan, F. Liu, H. He, X. Shi and C. Zhang, A superior Ce–W–Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3, Appl. Catal., B, 2012, 115, 100–106 Search PubMed.
- X. Dong, J. Wang, H. Zhao and Y. Li, The promotion effect of CeOx on Cu-SAPO-34 catalyst for selective catalytic reduction of NOx with ammonia, Catal. Today, 2015, 258, 28–34 Search PubMed.
- X. Yao, T. Kong, L. Chen, S. Ding, F. Yang and L. Dong, Enhanced low-temperature NH3-SCR performance of MnOx/CeO2 catalysts by optimal solvent effect, Appl. Surf. Sci., 2017, 420, 407–415 Search PubMed.
- H. Xu, M. Sun, S. Liu, Y. Li, J. Wang and Y. Chen, Effect of the calcination temperature of cerium-zirconium mixed oxides on the structure and catalytic performance of WO3/CeZrO2 monolithic catalyst for selective catalytic reduction of NOx with NH3, RSC Adv., 2017, 7, 24177–24187 Search PubMed.
- S. Liu, R. Zhang, P. Li, H. Chen, Y. Wei and X. Liang, Morphology effect of diverse ceria with active tungsten species on NH3-SCR behaviors, Catal. Today, 2020, 339, 241–253 Search PubMed.
- S. Kuba, P. Heydorn, R. Grasselli, B. Gates, M. Che and H. Knozinger, Redox properties of tungstated zirconia catalysts: relevance to the activation of n-alkanes, Phys. Chem. Chem. Phys., 2001, 3, 146–154 Search PubMed.
- Z. Liu, W. Xu, S. Yao, A. C. Johnson-Peck, F. Zhao, P. Michorczyk, A. Kubacka, E. A. Stach, M. Fernandez-Garcia, S. D. Senanayake and J. A. Rodriguez, Superior performance of Ni–W–Ce mixed-metal oxide catalysts for ethanol steam reforming: Synergistic effects of W- and Ni-dopants, J. Catal., 2015, 321, 90–99 Search PubMed.
- M. Churchill and W. Youngs, Crystal-structure and molecular-geometry of W(
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCMe3)(
CCMe3)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCMe3)(CH2CMe3)(DMPE), a mononuclear tungsten(VI) complex with metal-alkylidyne, metal-alkylidene, and metal-alkyl linkages, Inorg. Chem., 1979, 18, 2454–2458 Search PubMed.
CHCMe3)(CH2CMe3)(DMPE), a mononuclear tungsten(VI) complex with metal-alkylidyne, metal-alkylidene, and metal-alkyl linkages, Inorg. Chem., 1979, 18, 2454–2458 Search PubMed. - E. Kummerle and G. Heger, The structures of C-Ce2O3 + delta, Ce7O12, and Ce11O20, J. Solid State Chem., 1999, 147, 485–500 Search PubMed.
- A. Di Cicco and A. Filipponi, 14TH INTERNATIONAL CONFERENCE ON X-RAY ABSORPTION FINE STRUCTURE (XAFS14), PROCEEDINGS, ed. DiCicco, A. and Filipponi, A., 2009, vol. 190 Search PubMed.
- D. Clark and R. Schrock, Mulitple metal-carbon bonds.12. Tungsten and molybdenum neopentylidyne and some tungsten neopentylidene complexes, J. Am. Chem. Soc., 1978, 100, 6774–6776 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nj02146f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |