Carbon quantum dots as a dual platform for the inhibition and light-based destruction of collagen fibers: implications for the treatment of eye floaters†
Alexandre
Barras‡
a,
Félix
Sauvage‡
b,
Inès
de Hoon
ab,
Kevin
Braeckmans
 b,
Dawei
Hua
b,
Gaëtan
Buvat
a,
Juan C.
Fraire
b,
Christophe
Lethien
a,
J.
Sebag
cd,
Michael
Harrington
e,
Amar
Abderrahmani
a,
Rabah
Boukherroub
a,
Stefaan
De Smedt
*b and
Sabine
Szunerits
b,
Dawei
Hua
b,
Gaëtan
Buvat
a,
Juan C.
Fraire
b,
Christophe
Lethien
a,
J.
Sebag
cd,
Michael
Harrington
e,
Amar
Abderrahmani
a,
Rabah
Boukherroub
a,
Stefaan
De Smedt
*b and
Sabine
Szunerits
 *a
*a
aUniv. Lille, CNRS, Centrale Lille, Univ. Polytechnique Hauts-de-France, UMR 8520 – IEMN, F-59000 Lille, France. E-mail: sabine.szunerits@univ-lille.fr
bLaboratory of General Biochemistry and Physical Pharmacy, Faculty of Pharmaceutical Sciences, Ghent University, 9000 Ghent, Belgium. E-mail: stefaan.desmedt@ugent.be
cVMR Institute for Vitreous Macula Retina, Huntington Beach, California 92647, USA
dDoheny Eye Institute/UCLA, Los Angeles, California 90033, USA
eHuntington Memorial Research Institute, Pasadena, CA, USA
First published on 17th April 2021
Abstract
Common in myopia and aging, vitreous opacities arise from clumped collagen fibers within the vitreous body that cast shadows on the retina, appearing as ‘floaters’ to the patient. Vitreous opacities degrade contrast sensitivity function and can cause significant impairment in vision-related quality-of-life, representing an unmet and underestimated medical need. One therapeutic approach could be the use of versatile light-responsive nanostructures which (i) interfere with the formation of collagen fibers and/or (ii) destroy aggregates of vitreous collagen upon pulsed-laser irradiation at low fluences. In this work, the potential of positively and negatively charged carbon quantum dots (CQDs) to interfere with the aggregation of type I collagen is investigated. We demonstrate that fibrillation of collagen I is prevented most strongly by positively charged CQDs (CQDs-2) and that pulsed-laser illumination allowed to destroy type I collagen aggregates and vitreous opacities (as obtained from patients after vitrectomy) treated with CQDs-2.
New conceptsThe utility of light together with nanomaterials in ophthalmology remains still rather unexploited. In this work, we show for the first time that carbon quantum dots (CQDs) can not only prevent the fibrillation of type I collagen, but are able to destroy collagen fibers. This concept was used to destroy sucessfully vitreous opacities in patient samples at low energy levels (1000 times inferior to the current laser therapy to treat floaters). This finding reinforces the potential of the utilisation of nanomaterials, notably CQDs, in ophthalmology as a versatile therapeutic platform. As vitreous opacities degrade contrast sensitivity function and cause significant impairment in vision-related quality-of-life, novel therapeutic approaches such as the utility of versatile light-responsive nanostructures are believed to be the concepts for the future. |
1. Introduction
Opacities in the vitreous body are one of the most common causes of severe impairment in ocular vision. Vitreous opacities are characterized by floating objects inside the vitreous body, which can result from several origins including myopia, infection, diabetic retinopathy, aging and eye injury. Vitreous is a highly hydrated gel-like structure (>98% water), which is maintained by a diluted network of long, thin collagen fibrils. Individual collagen fibrils are normally spaced apart by hyaluronan, an anionic, non-sulfated glycosaminoglycan (polysaccharide), which attracts water and generates a swelling pressure that inflates the gel. Vitreous collagen fibrils are heterotypic in composition, containing type IX collagen being regularly distributed along the fibril surfaces;1 rope-like fibrils of collagen types II surrounding a central core of hybrid V/XI, with type II collagen predominating.2 Type IX collagen has chondroitin sulfate glycosaminoglycan chains attached to it which extend away from the fibril surfaces and space apart the collagen fibrils, thereby preventing fibril aggregation.3 Vitreous opacities and the degeneration of the vitreous body result from several interconnected mechanisms including the progressive cross-linking of individual collagen fibrils and their aggregation, and finally, liquefaction.4 Liquefaction is a physicochemical degenerative change that disrupts the homogeneity of the gel vitreous and is characterized by the dissociation of hyaluronan from collagen. Thus, vitreous progressively liquefies as the collagen fibril aggregates and HA dissociates from collagen drawing water with it to form liquid vitreous. Collagen becomes concentrated in some areas of the gel, while other parts of the vitreous body are devoid of collagen fibrils forming liquid compartments.5 Another proposed mechanism for vitreous liquefaction is that collagen fibrils are fragmented.6 It is believed that type IX collagen shields type II collagen and prevents fusion between fibrils.3 Loss of type IX collagen results in type II collagen becoming increasingly exposed, predisposing to fusion of adjacent fibrils on contact. In both cases, when the aggregates are sufficiently large, they are visible as vitreous opacities resulting in degradation of contrast sensitivity function4,7 with a negative impact on the quality of life.8,9 Other mechanisms of vitreous liquefication can be considered, including enzymatic liquefaction and oxidative stress-induced liquefaction.10The treatment of vitreous opacification relies on its origin. However, in case of treatment failure, the only curative treatment for removing floaters remains vitrectomy (i.e. a surgical removal of vitreous through small openings in the pars plana)11,12 or by Nd:YAG laser treatment.13 However, treatment with the Nd:YAG laser has relatively little efficacy14 and sometimes can worsen the eye symptoms. In addition, if the opacities are located too close to the retina, for safety reasons, laser treatment cannot be achieved. There is a true medical need for searching for safe and non-invasive methods for treating vitreous floaters. Also, non-surgical therapies have been under development, but, pharmacologic vitreolysis has not been designed to address vitreous opacities.10,15,16 More recently, the use of light absorbing gold nanoparticles (Au NPs) was proposed by some of us as an alternative solution.17 When coated with hyaluronic acid, after injection in vitreous ex vivo, these particles diffuse through vitreous and cluster on the collagenous opacities. Thanks to their photothermal properties, Au NPs heat up in an ultrafast manner when illuminated by a nanosecond pulsed-laser; this leads to the evaporation of the water surrounding the Au NPs and the formation of vapor nanobubbles (VNBs). We showed that VNBs collapsing at the surface of the vitreous opacities provide sufficient mechanical force to ablate them. Importantly, the combined use of the Au NPs and nanosecond laser pulses allowed destruction of the opacities at a laser energy ∼1000 times lower than typically used in YAG laser therapy. Of note is that pulsed lasers are commonly used in ophthalmology for the treatment of cataract and in corneal surgery.
Carbon-based nanostructures, such as carbon quantum dots (CQDs), offer promising therapeutic potential as they can engage with biological molecules.18 These nanostructures have proven to be excellent drug carrier due to their good biocompatibility.19 The ease of introducing functional groups has enabled their application as medical countermeasure to enveloped viruses, including next to herpes simplex virus type 1,20 human coronavirus. The intrinsic fluorescence properties make them well-adapted for bio-imaging but allows as well their use in biosensing platforms.18 One of their applications is the treatment of eye infections with highly positively charged CQDs.21 CQDs have also been explored for the treatment of corneal neovascularization22 and to improve retinal angiography.23,24 Motivated by the reported ability of CQDs to prevent human insulin fibrillation,25–28 and the potential of CQDs in regulating the aggregation behavior of human islet amyloid polypeptide (hIAPP)29 and by their good light absorbing properties over a wide wavelength spectrum, we aim to investigate (i) the potential of CQDs to inhibit the formation of collagen aggregates and (ii) the combined use of CQDs and pulsed laser light to destroy collagen fibers and vitreous opacities through the generation of VNBs (Fig. 1).
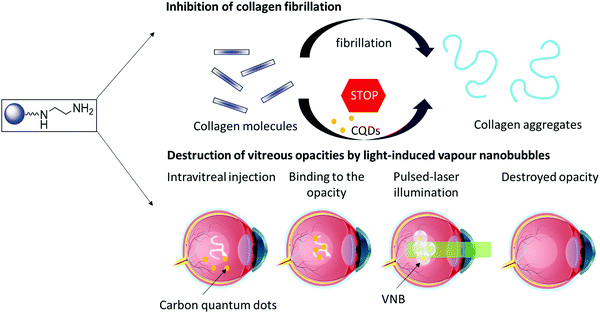 | ||
| Fig. 1 Carbon quantum dots (CQDs) to inhibit the formation of collagen aggregates (top) and to destroy collagen aggregates by light induced VNBs (bottom). | ||
2. Results and discussion
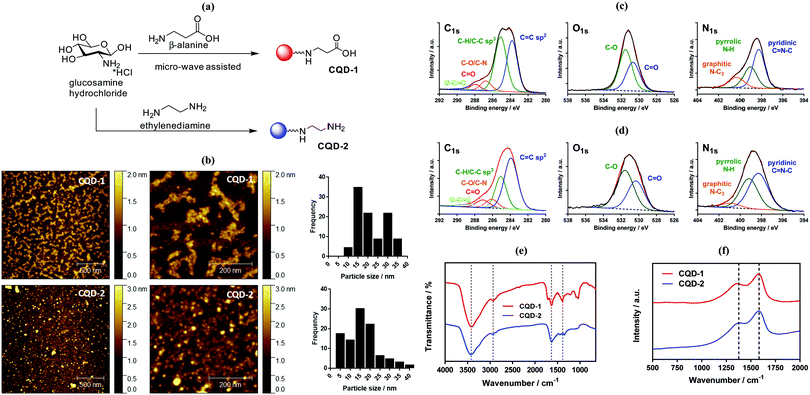
2.1. One-step microwave-assisted synthesis of aminated and carboxylated fluorescent carbon dots
We have shown previously that a large panel of differently functionalized CQDs can be produced by hydrothermal carbonization of different organic precursors.30 Hydrothermal techniques lead to highly fluorescent CQDs, but long reaction times and high temperature conditions using a close Teflon-line reactor lead too often to a lack of reproducibility and replicability. Alternatively, microwave-assisted synthesis of CQDs is a more reproducible and easier approach due to the on-line monitoring of temperature and pressure in the reaction vessel.18,31 To this end, a rapid synthesis of carboxylic acid-decorated CQDs (CQD-1) using glucosamine as a starting material and an amino acid (β-alanine) as surface a passivating agent was investigated.32 Aminated CQDs (CQD-2) were prepared in a comparable manner from glucosamine hydrochloride and ethylenediamine as a passivating agent (Fig. 2a).33 To remove larger precipitates, the CQDs suspension was centrifuged and then dialyzed against water with a final yield of 0.8% and 3.4% for CQD-1 and CQD-2, respectively. CQD-1 exhibit a spherical shape with a primary average diameter of 20.8 ± 6.6 nm (Table 1), as determined by AFM (Fig. 2b). CQD-1 form a “bunch of grapes” in solution and their size reaches up to 50–350 nm in good accordance with the hydrodynamic size recorded by DLS. CQD-2 display a spherical shape with an average diameter of 16.8 ± 6.7 nm (Table 1), as determined by AFM (Fig. 2b). Transmission electron microscopy (TEM) observations revealed that the CQDs were almost spherical with an average particle size of ∼26 and ∼19 nm, respectively, in a good concordance with sizes determined by AFM (Fig. S1, ESI†). The amorphous nature of the CQDs was concluded by the absence of clear lattice fringes as well as any clear pattern in the selected area electron diffraction in the TEM images (data not shown).| CQD-1 | CQD-2 | |
|---|---|---|
| DMEM = Dulbecco's Modified Eagle's. | ||
| ζ/mV | (−)24.0 ± 1.6 | (+)32.5 ± 0.8 |
| Size determined by AFM/nm | 20.8 ± 6.6 | 16.8 ± 6.7 |
| Hydrodynamic size (intensity)/nm | 389 ± 123 (100%) | 10.6 ± 2.3 (63%) |
| 265 ± 199 (29%) | ||
| O/at% | 22.8 | 14.4 |
| N/at% | 5.7 | 13.7 |
| I D/IG ratio | 1.94 | 1.56 |
| Colloidal stability in water | Excellent | Excellent |
| Colloidal stability in DMEM | Excellent | Excellent |
| Quantum yield (Φ) | 0.008 | 0.002 |
The chemical composition of the carbon dots was assessed by X-ray photoelectron spectroscopy (XPS, see survey in ESI,† Fig. S2). The C1s high resolution XPS spectra of the CQDs depict different carbon features (Fig. 2c). In the case of CQD-1, the high-resolution spectrum of the C1s reveals the presence of fives peaks ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (283.8 eV), C–C/C–H (285.0 eV), C–N/C–O (286.8 eV), C
C (283.8 eV), C–C/C–H (285.0 eV), C–N/C–O (286.8 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.9 eV) and –O–C
O (287.9 eV) and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.2 eV). The presence of carbonyl and carboxylic functions is additionally validated by the presence of a band at 530.7 eV (C
O (289.2 eV). The presence of carbonyl and carboxylic functions is additionally validated by the presence of a band at 530.7 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the O1s high resolution spectrum, next to 531.5 eV (C–O). The N1s high resolution spectrum can be curve-fitted with bands at 398.3 eV (pyridinic C
O) in the O1s high resolution spectrum, next to 531.5 eV (C–O). The N1s high resolution spectrum can be curve-fitted with bands at 398.3 eV (pyridinic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C), 399.1 eV (pyrrolic N–H) and 400.3 eV (graphite-like structure N–C3). In case of CQD-2 (Fig. 2d), the high-resolution spectrum of the C1s can be deconvoluted with fives peaks assigned to C
N–C), 399.1 eV (pyrrolic N–H) and 400.3 eV (graphite-like structure N–C3). In case of CQD-2 (Fig. 2d), the high-resolution spectrum of the C1s can be deconvoluted with fives peaks assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (283.9 eV), C–C/C–H (285.0 eV), C–N/C–O (286.1 eV), C
C (283.9 eV), C–C/C–H (285.0 eV), C–N/C–O (286.1 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.1 eV) and –O–C
O (287.1 eV) and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.4 eV). The presence of oxygen functions is also corroborated by the presence of the band at 530.3 eV (C
O (288.4 eV). The presence of oxygen functions is also corroborated by the presence of the band at 530.3 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the O1s high resolution spectrum, next to 531.6 eV (C–O). The N1s high resolution spectrum shows comparable bands as CQDs-1: 398.3 eV (pyridinic C
O) in the O1s high resolution spectrum, next to 531.6 eV (C–O). The N1s high resolution spectrum shows comparable bands as CQDs-1: 398.3 eV (pyridinic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C), 399.2 eV (pyrrolic N–H) and 400.9 eV (graphite-like structure N–C3). Both CQDs have a variety of polar groups (hydroxyl, carbonyl, carboxyl, amine) present on their surface. The presence of N–C3 moieties indicates that some nitrogen atoms were incorporated into the carbon framework. The oxygen content in the CQD-1 and CQD-2 was determined to be 22.8 at% and 14.4 at%, respectively. Higher nitrogen content (13.7 at%) was found in CQD-2 in comparison with CQD-1 (5.7 at%). Furthermore, the presence of primary amino groups was validated by the ninhydrin reaction test (see Fig. S3, ESI†), which allowed estimating a surface coverage of 0.24 ± 0.2 mmol g−1.
N–C), 399.2 eV (pyrrolic N–H) and 400.9 eV (graphite-like structure N–C3). Both CQDs have a variety of polar groups (hydroxyl, carbonyl, carboxyl, amine) present on their surface. The presence of N–C3 moieties indicates that some nitrogen atoms were incorporated into the carbon framework. The oxygen content in the CQD-1 and CQD-2 was determined to be 22.8 at% and 14.4 at%, respectively. Higher nitrogen content (13.7 at%) was found in CQD-2 in comparison with CQD-1 (5.7 at%). Furthermore, the presence of primary amino groups was validated by the ninhydrin reaction test (see Fig. S3, ESI†), which allowed estimating a surface coverage of 0.24 ± 0.2 mmol g−1.
The chemical composition of the CQDs was further investigated by Fourier transform infrared (FT-IR) spectroscopy (Fig. 2e). The FTIR spectrum of CQD-2 comprises a broad band between 3415–3425 cm−1 attributed to the O–H/N–H stretching vibrations, while the C–H stretching and bending vibrations are located at 2932 and at 1384 cm−1, respectively. The band at 1636 cm−1 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the aromatic structure, and C
C bonds of the aromatic structure, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (–NHCO–). Compared to CQD-2, the more intense peaks at 1083 and 1041 cm−1 in the FTIR spectrum of CQD-1 are associated with C–O stretching of either the residual carbohydrate or β-alanine. The sharp peak at 1710 cm−1 is assigned to the asymmetric stretching vibration of carboxylic acid moieties.34
O stretching vibration (–NHCO–). Compared to CQD-2, the more intense peaks at 1083 and 1041 cm−1 in the FTIR spectrum of CQD-1 are associated with C–O stretching of either the residual carbohydrate or β-alanine. The sharp peak at 1710 cm−1 is assigned to the asymmetric stretching vibration of carboxylic acid moieties.34
Typical Raman spectra of CQD-1 and CQD-2 are seen in Fig. 2f. The observed peaks are typical of sp2 carbon materials at 1580 cm−1 (G-band) and 1378 cm−1 (D band), indicating that the material is graphitic in nature. The fact that the D band is rather broad points towards a disordered structure, keeping in mind that the D-peak is not related to disorder but indirectly to the size of the sp2 coordinating ring domains. The ID/IG ratio is 1.94 for CQD-1 and 1.56 for CQD-2 suggesting large surface disorder and amorphous nature.35
Zeta potential analysis is a source of information in terms of surface functionalities (Fig. S4a, ESI†). CQD-1 display a negative zeta potential value (ζ = −24.0 ± 1.6 mV) in pure Milli-Q water. The negative surface charge is due to an abundance of deprotonated carboxyl groups on its surface. In contrast, CQD-2 exhibit a positive surface charge with ζ = +32.5 ± 0.8 mV, underlining the presence of NH3+ groups. Due to the presence of hydrophilic groups, CQDs were readily dispersible at high concentrations (>10 mg mL−1) in pure water or PBS. The dispersion of 250 μg mL−1 CQDs (Fig. S4b, ESI†) in pure water and Dulbecco's Modified Eagle's medium (DMEM) is excellent for both particles. In PBS, the colloidal stability of CQD-2 is reduced and a precipitate is formed after 1 h incubation at 37 °C.
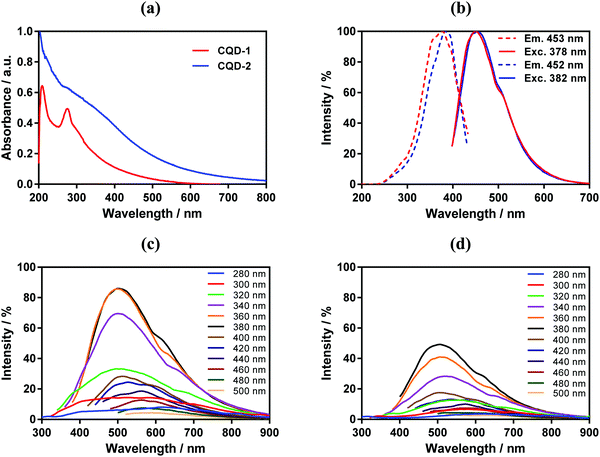
The CQDs absorb in the short-wavelength region with a maximum at around 220 nm due to π–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N carbon cores with a weak extending tail in the visible region (Fig. 3a). An additional shoulder at approximatively 280 nm is attributed to π–π* transition of different sp2 domains.36 The absorption shoulder at around 306 nm in CQD-1 is due to n–π* transitions of C
N carbon cores with a weak extending tail in the visible region (Fig. 3a). An additional shoulder at approximatively 280 nm is attributed to π–π* transition of different sp2 domains.36 The absorption shoulder at around 306 nm in CQD-1 is due to n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. This contribution is decreased in CQD-2 due to the high content of ethylenediamine;37 the strong absorption extending from 350 to 600 nm is related to surface passivation of CQDs-2 with ethylenediamine.38
O. This contribution is decreased in CQD-2 due to the high content of ethylenediamine;37 the strong absorption extending from 350 to 600 nm is related to surface passivation of CQDs-2 with ethylenediamine.38
As expected, CQD-1 and CQD-2 reveal photoluminescence characteristic (Fig. 3b) with a maximum fluorescence intensity at 450 nm under 380 nm excitation.39 The fluorescence emission is wavelength-dependent (Fig. 3c and d). CQD-1 display a broad (full width at half maximum of around 100 nm) and intense photon emission, while CQD-2 exhibit a lower fluorescence. Increasing the excitation wavelength to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm results in a red-shift of the emission band to 590 nm for CQD-1 and 580
nm results in a red-shift of the emission band to 590 nm for CQD-1 and 580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm for CQD-2, with the fluorescence emission intensity of CQD-1 being somehow higher than that of CQDs-2. Using quinine sulfate as reference with a quantum yield of 0.54, the fluorescent quantum yields of CDQ-1 and CQD-2 varied between 0.008 and 0.002, respectively (Table 1 and Fig. S5, ESI†). The long storage stability at 4 °C and effects of parameters such as prolonged UV exposure of CQDs were studied. The fluorescence intensity of CQD-1 had no obvious decrease (14%) after being stored 2 years at 4 °C (Fig. S6, ESI†). The fluorescence intensity of CQD-2 had higher decrease (48%). In contrast, the normalized fluorescence intensity before and after 2 years for both CQDs was almost unchanged, which suggest no chemical modification occurred over the storage period. CQDs displayed UV-dependent fluorescence behavior. CQD-2 present a relatively stable fluorescence emission intensity during 30 min exposure to UV irradiation (360 nm, 10 mW cm−2) in comparison to CQD-1.
nm for CQD-2, with the fluorescence emission intensity of CQD-1 being somehow higher than that of CQDs-2. Using quinine sulfate as reference with a quantum yield of 0.54, the fluorescent quantum yields of CDQ-1 and CQD-2 varied between 0.008 and 0.002, respectively (Table 1 and Fig. S5, ESI†). The long storage stability at 4 °C and effects of parameters such as prolonged UV exposure of CQDs were studied. The fluorescence intensity of CQD-1 had no obvious decrease (14%) after being stored 2 years at 4 °C (Fig. S6, ESI†). The fluorescence intensity of CQD-2 had higher decrease (48%). In contrast, the normalized fluorescence intensity before and after 2 years for both CQDs was almost unchanged, which suggest no chemical modification occurred over the storage period. CQDs displayed UV-dependent fluorescence behavior. CQD-2 present a relatively stable fluorescence emission intensity during 30 min exposure to UV irradiation (360 nm, 10 mW cm−2) in comparison to CQD-1.
2.2. Photothermal properties of CQD-1 and CQD-2
The photothermal properties of CQD-1 and CQD-2 (50 μg mL−1) were determined upon irradiation the aqueous solutions with a laser at 810 nm for 15 min. Fig. 4 indicates that in both cases a temperature plateau is attained after 10 min, reaching about 50 °C for a laser power of 1 W cm−2 and 66 °C for 2 W cm−2. However, the same temperature trend is observed with PBS solution, indicating poor photothermal properties of these CQDs under these experimental conditions.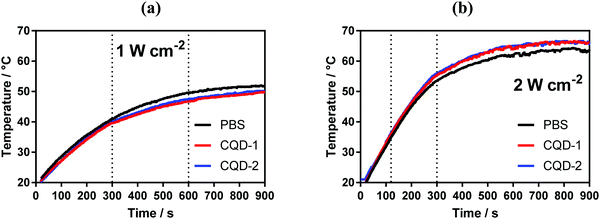 | ||
| Fig. 4 Photothermal properties of CQDs. Temperature profiles of CQDs (50 μg mL−1) upon laser irradiation at 810 nm laser at two different power densities: 1 W cm−2 (a) and 2 W cm−2 (b). | ||
2.3. Metabolic activity and cellular uptake of CQDs
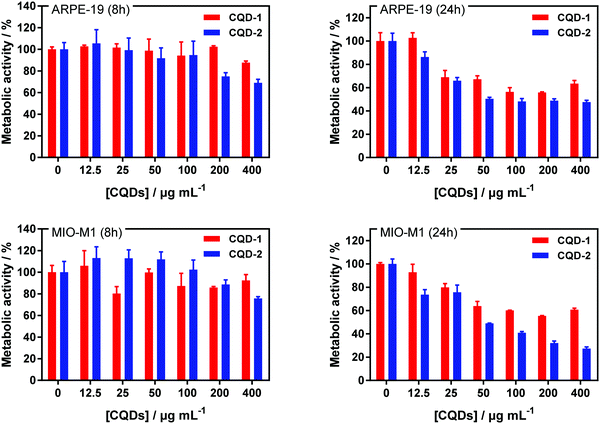
The cell toxicity of CQD-1 and CQD-2 was established on ARPE-19 and MIO-M1 cell lines after 8 h and 24 h incubation (Fig. 5). The CQDs toxicity was evaluated using the resazurin assay, based on the conversion of non-fluorescent dye to a fluorescent molecule by mitochondrial and cytosolic enzymes. All CQDs are non-toxic to ARPE-19 and MIO-M1 cells up to 100 μg mL−1 for 8 h incubation. For longer incubation times, the metabolic activities decrease from 25 μg mL−1 onwards.Furthermore, the cell toxicity of CQD-1 and CQD-2 was established on HeLa and U-87 MG cell lines (Fig. S7a, ESI†). All CQDs are non-toxic to HeLa and U-87 MG cells up to 100 μg mL−1 for both incubation times. HeLa and U-87 MG cells were incubated with CQDs at 100 μg mL−1 during 24 h and then, nuclei were stained with Hoechst 33258, a fluorescent dye for labeling DNA in fluorescence microcopy (Fig. S7b, ESI†). The green fluorescence, which is attributed to the CQDs, is homogeneously distributed in the cytoplasm after 24 h when incubated at 37 °C, which confirms the adsorption and internalization of positively charged CQD-2 inside the cells, while there is almost no fluorescence for CQD-1.
2.4. Inhibition of type I collagen aggregation with CQD-1 and CQD-2
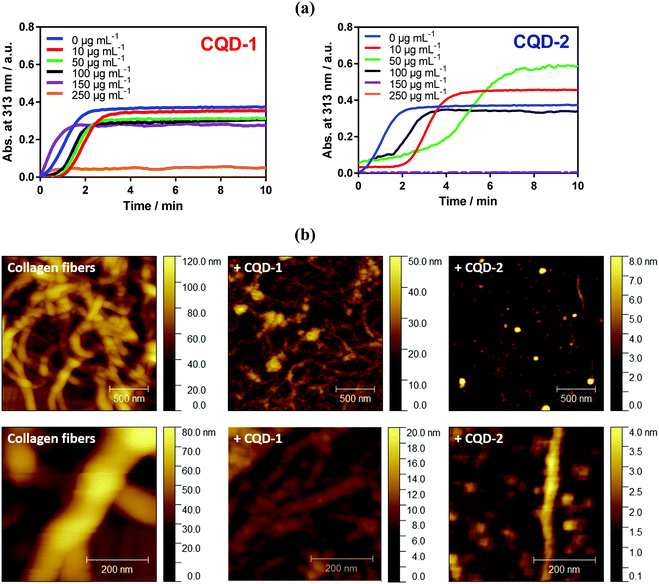
Fibril formation of type I collagen has been widely studied by some research teams.40–46 Therefore, type I collagen was investigated in this present work to assess to what extent CQDs may inhibit its fibrillation. As explained in the experimental section, fibril formation of type I collagen was initiated by incubating collagen I solution at 37 °C. The change in the optical density of the sample at 313 nm as a function of time was recorded (Fig. 6a). After a lag time of about 2 min, a collagen self-assembly growth phase was observed, reaching a plateau after about 3 min which is in line with other reports.45 AFM imaging (Fig. 6b) confirmed the formation of collagen fibers, with a typical diameter of ∼150 nm.The presence of a low amount of negatively charged CQD-1 (<100 μg mL−1) mainly influences the lag time i.e. the onset of collagen fibrillation. Through AFM imaging, aggregate formation is observed in the presence of 100 μg mL−1 of CQD-1, with fibers with a diameter of 40–50 nm, much smaller compared with control collagen fibers. A higher CQD-1 concentration of 250 μg mL−1 resulted in complete inhibition of the collagen fibrillation. In contrast, the presence of a low amount of positively charged CQD-2 induced an acceleration of type I collagen fibril formation (Fig. 6a). The AFM image of collagen in the presence of 100 μg mL−1 CQD-2 (Fig. 6b) indicates a quasi-complete inhibition of fibrillation with isolated aggregates of 1.5 μm in length and a diameter of ∼50 nm. Note that a complete inhibition of collagen fibrillation was already observed at a concentration of 150 μg mL−1.
At low CQDs concentration, some collagen fibril assembly remains. This indicates that the inhibitory effect of CQDs on the nucleation step and intermolecular interactions is concentration dependent. CQDs inhibits fibrillation in a mechanism involving an endothermic reaction along with electrostatic and hydrophobic interactions between neighboring molecules. Hydrogen bonds are believed to be the most stabilizing forces prevailing in these proteins. It is, thus, hypothesized that the hydrogen bonds may be formed between carbonyl and carboxylic acid groups present on the surface of CQD-1 and amine and hydroxyl groups of type I collagen.47 This might alter the interactive forces between collagen molecules. The main differences between both type of particles is their surface charge and functionalization. Note that physiochemical properties of nanoparticles such as size and surface charge, have been considered as critical factors for their antifibrotic properties.48,49
2.5. Destruction of type I collagen fibers under pulsed-laser irradiation in the presence of CQDs
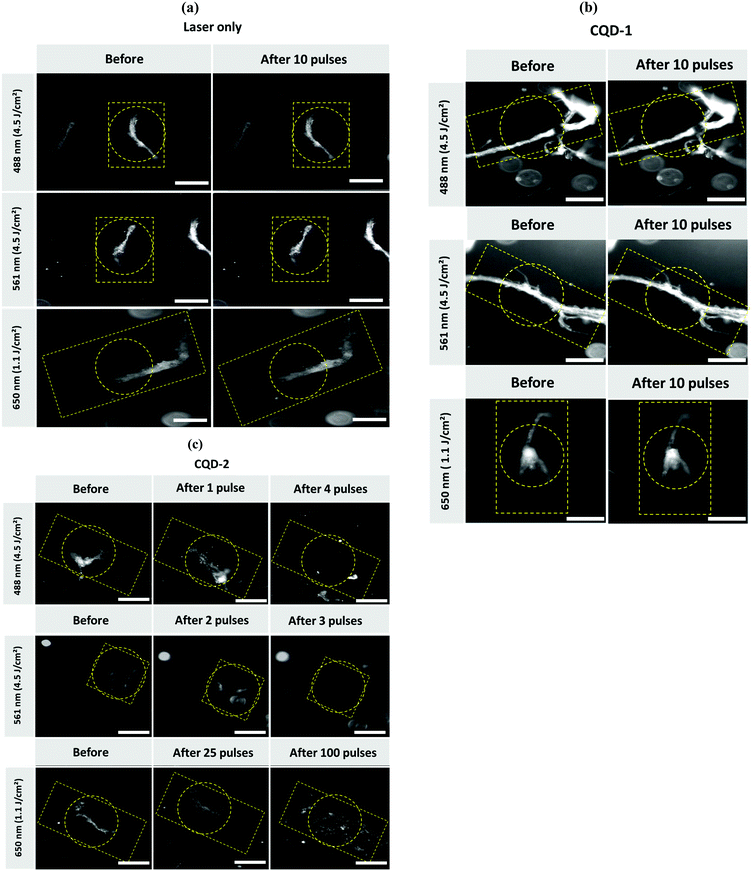
We have recently shown that Au NPs, able to trigger the formation of vapor nanobubbles (VNBs) under pulsed-laser irradiation, adhere to type I collagen fibers and can destroy vitreous opacities.17 Though, different to CQDs, Au NPs do not seem to interfere with collagen fibrillation (Fig. S8, ESI†). The destruction of the type I collagen fibers/vitreous opacities is believed to be mechanical: collapsing VNBs induce a mechanical force which breaks down the fibers. To investigate whether a similar effect could be observed with CQD-1 and CQD-2, type I collagen fibers were mixed with CQD-1 or CQD-2 and irradiated with a pulsed-laser at different fluences and wavelengths. Dark field microscopy was used to visualize the collagen fibers before and after laser irradiation.As seen in Fig. 7a, applying light fluences of 1.1 and 4.5 J cm−2 and laser irradiation wavelengths of 488, 561 and 650 nm, without the use of CQDs, did not destroy the type I collagen fibers. Irradiation of the collagen fibers in the presence of CQD-1 showed the same result: the collagen fibers remained intact (Fig. 7b). This contrasts to the use of CQD-2 (Fig. 7c). Light pulses of <3 ns at 488 and 561 nm (4.5 J cm−2) clearly destroyed the collagen fibers. Using a higher wavelength of 650 nm did no longer allow the complete destruction of the fibers, even if 100 pulses were applied (Fig. 7c). This could be expected as CQD-2 hardly absorb at 650 nm (Fig. 3a). Given that the 488 nm laser light might be (more) harmful for ocular tissues (if compared to 561 nm light), we selected 561 nm to continue our experiments on human opacities.
While we find that the pulsed-laser irradiation in the presence of CQD-2 totally destroys the aggregates, the same approach is inefficient using CQD-1. This difference may rely on the surface charge of the two nanoparticles, which is negative for CQD-1 and positive for CQD-2 (Table 1). The difference in charges could lower the interaction of CQD-1 with the collagen fibers, when compared to CQD-2. Temperature effects could be also involved. However, CQDs show no photothermal activity (Fig. 4). In spite one can expect higher photothermal effects with gold nanoparticles, no differences in terms of fluence was observed for fiber destruction: in both cases fluence of 4.5 J cm−2 was required for efficient fiber demolition. This can be explained by the relatively small size of both AuNPs and CQDs which cannot trigger formation of vapor nanobubbles in solution but only when they form clusters or in situation where the distance between particles is reduced (as it is likely the case when both AuNPs or CQDs are bound to fibers).
However, inhibition of the fibrillation with both types of CQDs clearly suggests that they can both interact with collagen molecules (Fig. 7a).
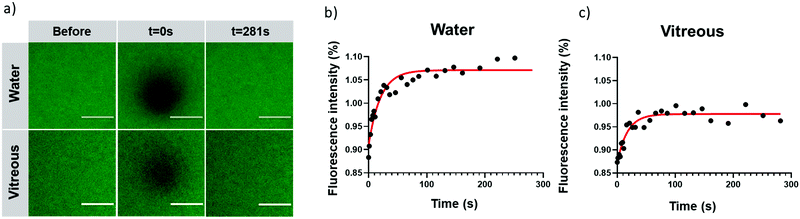
2.6. Assessment of the mobility of CQDs in vitreous by fluorescence recovery after photobleaching (FRAP)
After intravitreal injection, CQDs must keep their mobility to diffuse and their capacity to bind to the target, the vitreous opacities. Although we expected that CQDs, due to their relatively small size (around 10 nm), could diffuse in the bovine vitreous,50 we decided to perform FRAP experiments to ensure they were mobile and could therefore reach the opacities after intravitreal injection. Indeed, while CQDs are positively charged and the vitreous net charge is negative, one can expect that they will interact with the glycosaminoglycans and collagen network of the vitreous thus forming aggregates. FRAP is a technique allowing to measure the diffusion coefficients of fluorescent molecules or nanoparticles. Briefly, a laser of strong intensity is applied on the sample and induces its photobleaching. The diffusion of the surrounding (fluorescent) molecules (or particles) into the bleached area can be measured and observed by a progressive increase of fluorescence intensity. Recovery curves can, therefore, be obtained from which diffusion coefficients can be calculated.As observed in confocal images (Fig. 8a), fluorescence recovery curves in water and in vitreous (Fig. 8b and c) indicate that a complete fluorescence recovery occurs in the bleached area. This observation attests that cationic CQDs can diffuse both in water and in bovine vitreous. Their diffusion coefficients in water and vitreous are comparable (31.4 ± 7.4 μm2 s−1 and 32.9 ± 13.2 μm2 s−1, respectively). Our observation suggests that, despite a positive charge, CQDs keep their mobility in vitreous. The diffusion of CQDs can be due to their small size (around 10 nm) allowing diffusion through the meshes of the collagen network whose size are comprised between 500 and 1000 nm.50 This result also indicates that, after intravitreal injection, CQD-2 can sufficiently diffuse to bind the opacities.
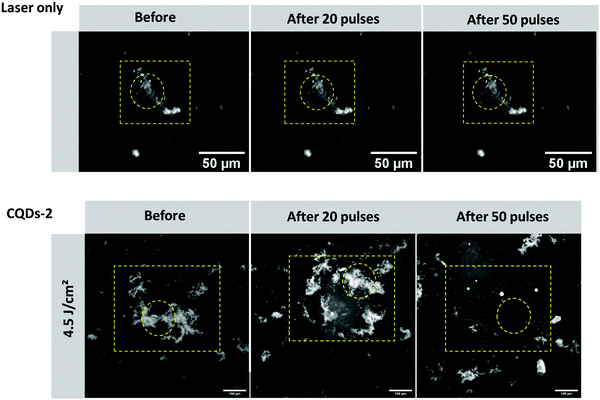
2.7. Destruction of human vitreous opacities treated with CQD-2 under pulsed-laser irradiation
Type I collagen fibers were previously used to attest the efficacy of their destruction after treatment with CQD-2 and upon pulsed-laser irradiation. Since the collagen composition, size and shape of vitreous opacities differ from type I collagen fibers, we therefore decided to test our concept on real opacities from human vitreous obtained from patients after vitrectomy. The first observation is that, without treatment with CQDs, no destruction or change in opacities could be observed after 50 pulses at 561 nm using a fluence of 4.5 J cm−2, suggesting the laser itself cannot destroy the opacities. However, as shown in Fig. 9, when vitreous samples were mixed with CQD-2 (0.4 mg mL−1) a clear destruction of the opacities could be observed after 50 pulses (561 nm; 4.5 J cm−2). This result is in line with previous observations from some of us at a same wavelength and fluence using 10 nm hyaluronan-coated Au NPs and suggests a similar efficacy.173. Conclusion
In this work, we have shown that cationic CQDs could inhibit the fibrillation of type I collagen in vitro and destroy type I collagen fibers and human vitreous opacities through the generation of light-induced VNBs at a low fluence (4.5 J cm−2). While negatively charged CQDs can inhibit collagen fibrillation, they were unable to destroy the collagen fibers at the studied fluences and wavelengths. Interestingly, while Au NPs can generate VNBs and have been reported to destroy vitreous opacities, they did not impact collagen fibrillation. CQDs can promote the destruction of vitreous opacities in a similar manner (fluence, number of pulses) with a capacity to inhibit collagen fibrillation. Thanks to this dual effect (i.e. inhibition and destruction), cationic CQDs become attractive therapeutic tools for the treatment of vitreous opacities by avoiding or postponing their reformation after laser treatment.Taken together, our results suggest that this dual effect provided by CQDs could be further explored in the context of fibrotic diseases and more generally for diseases involving protein aggregation.
4. Experimental section
4.1. Materials
Glucosamine hydrochloride, ethylenediamine and β-alanine were purchased from Sigma Aldrich Chimie (France) and used as-received.Samples of human vitreous containing opacities were collected at the VMR Institute for Vitreous Macula Retina (Huntington Beach, CA, USA) from patients undergoing vitrectomy for the treatment of vision-degrading myodesopsia. The study protocol adhered to the Declaration of Helsinki. Prior to surgery, patients gave a written informed consent that has been reviewed and accepted by the ethical committee of Saint Joseph Health Center for clinical research (Irvine, CA, USA). After vitrectomy, the (undiluted) samples were frozen and stored at −80 °C until further use.
4.2. Synthesis of carbon quantum dots (CQDs) and gold nanoparticles (AuNPs)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min) in order to remove big particles. The supernatant was dialyzed against water during 24 h (SpectraPor RC membranes, 1 kDa) and then kept (or lyophilized) to yield a brown solution.
000g, 10 min) in order to remove big particles. The supernatant was dialyzed against water during 24 h (SpectraPor RC membranes, 1 kDa) and then kept (or lyophilized) to yield a brown solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min) in order to remove big particles. The supernatant was dialyzed against water during 24 h (SpectraPor RC membranes, 1 kDa) and then kept (or lyophilized) to yield a brown solution.
000g, 10 min) in order to remove big particles. The supernatant was dialyzed against water during 24 h (SpectraPor RC membranes, 1 kDa) and then kept (or lyophilized) to yield a brown solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g), resuspended in an aqueous solution of HA (2–4 pM), and kept under rotation overnight. HA-Coated AuNPs were finally washed by centrifugation, resuspended in water, and stored for further use. The synthetized NPs were characterized by DLS and zeta potential (Zetasizer Nano, Malvern) measurements. The final concentration of the AuNPs was estimated from the extinction of the dispersions as measured at the maximal extinction wavelength (Ext) and the theoretical extinction cross section σEXT (cm2), as previously reported.17
000g), resuspended in an aqueous solution of HA (2–4 pM), and kept under rotation overnight. HA-Coated AuNPs were finally washed by centrifugation, resuspended in water, and stored for further use. The synthetized NPs were characterized by DLS and zeta potential (Zetasizer Nano, Malvern) measurements. The final concentration of the AuNPs was estimated from the extinction of the dispersions as measured at the maximal extinction wavelength (Ext) and the theoretical extinction cross section σEXT (cm2), as previously reported.17
4.3. Primary amine quantification using a modified Kaiser test
The amount of primary amine groups on the CQDs was calculated using a spectrophotometric assay based on the modified Kaiser test with the following modifications.51 Briefly, CQDs-2 were diluted to 0.1 mg mL−1 in Milli-Q water. To this solution was added 50 μL of phenol solution (40 g in 10 mL EtOH), 50 μL of potassium cyanide (2 mL of KCN solution – 65 mg in 100 mL water – in 100 mL pyridine) and 50 μL of ninhydrin solution (2.5 g in 50 mL EtOH). The mixture was heated at 100 °C during 10 min, cooled down on ice and further diluted with 1 mL of ethanolic solution (EtOH/water = 60/40). The absorption of the mixture was determined in triplicate at 570 nm using a PerkinElmer Lambda UV/Vis 950 dual-beam spectrophotometer in 10 mm quartz cuvette. Benzylamine solution was used from 10 to 100 μM to generate the calibration curve.4.4. Characterizations
4.5. Measurement of the photothermal properties
The photothermal properties of 50 μg mL−1 solutions of CQDs in PBS (pH 7.4, 0.01 M, Gibco®) were determined by using a continuous laser beam at 810 nm (Gbox model, Fournier Medical Solution) at two power densities (1 and 2 W cm−2). The temperature changes were recorded by an infrared camera (FLIR A655sc, IR lens: f = 24.6 mm and 25°) and an image analysis software FLIR ResearchIR Max4.4.6. Biological evaluations
4.7. Collagen fibril formation
Collagen I from rat tail (A10483-01, Gibco®) was dissolved in PBS (pH 7.4, 0.01 M, Gibco®) at 0 °C (ice bath) to obtain a final concentration of 0.3 mg mL−1. CQDs at different concentrations were added. Subsequently, the collagen/CQDs solution was homogenized and incubated without stirring during 1 h at 37 °C. The fibrillation process was followed by measuring the absorbance/turbidity of the solutions at 313 nm by using a NanoDrop™ One C spectrophotometer (Thermo Scientific). The baseline correction was done just before to start each experiment (t = 0 min).4.8. Photoablation of collagen fibers and human vitreous opacities
Photoablation experiments were performed on collagen fibers in water and human vitreous containing opacities. Collagen fibers (0.02 mg mL−1) were mixed with CQDs in water (0.4 mg mL−1) and allowed to equilibrate for 30 min before the experiment. After thawing the human vitreous samples, they were directly mixed with an equal volume of a dispersion of CQDs (typically 0.4 mg mL−1 in water). The samples were then allowed to equilibrate for 30 min at room temperature prior to applying (nanosecond) laser pulses.First, dark-field microscopy imaging was performed to locate and align the nanosecond laser on the collagen fibers or human vitreous opacities in the sample. To generate vapor nanobubbles, 7 ns laser pulses were used. The wavelengths of the laser light were 488 nm, 561 nm or 650 nm. A beam expander (#GBE05-A, Thorlabs) combined with an iris diaphragm (#D37SZ, Thorlabs) was used to adjust the diameter of the laser beam to 150 μm. The laser pulse energy was monitored by an energy meter (J-25MB-HE&LE, Energy Max-USB/RS sensors, Coherent) synchronized with the pulsed laser.
4.9. Diffusion experiments in water and bovine vitreous by photobleaching
Fluorescence recovery after photobleaching (FRAP) experiments were performed to determine the mobility of the fluorescent CQDs in water and bovine vitreous. FRAP experiments were performed using a confocal scanning laser microscope (Nikon A1R) equipped with a 10× lens (CFI Plan Apochromat; Nikon, Badhoevedorp, The Netherlands). In a FRAP experiment, fluorescent nanoparticles in a (vitreous) sample are irreversibly bleached in a small area (100 μm) by an intense laser beam. The surrounding particles (i.e. outside of the bleached zone) will diffuse into the photobleached area, leading to a fluorescence recovery over time. Diffusion coefficients (D) can be derived from the rate and amount of recovery of fluorescence in the bleached zone by fitting the data points to appropriate equations.52For FRAP experiments in water, 5 μL of a water solution of CQD-2 (2.2 mg mL−1) was applied between a microscope slide and a coverslip which were separated by a 120 μm-thick double adhesive spacer (Secure-Seal Spacer; Molecular Probes, Leiden, The Netherlands). For FRAP experiments in bovine vitreous, bovine vitreous was carefully removed from the eye ball (to avoid undesired liquefaction) and 400 μL were carefully cut and transferred into a glass-bottomed dish. 40 μL of a solution of CQD-2 (2.2 mg mL−1) were then injected in the vitreous using a 1 mL syringe equipped with a 21.5G needle and allowed to equilibrate at room temperature for 30 min before performing FRAP experiments.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Centre National de la Recherche Scientifique (CNRS), the University of Lille is acknowledged. This work was partly supported by the French Renatech network. The authors would also like to acknowledge funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 810685 (DelNam project) and the Marie Sklodowska-Curie grant agreement No 847568, and CPER “Photonics for Society”.References
- L. Vaughan, M. Mendler, S. Huber, P. Bruckner, K. H. Winterhalter, M. I. Irwin and R. Mayne, J. Cell Biol., 1988, 106, 991–997 CrossRef CAS.
- P. N. Bishop, Prog. Retinal Eye Res., 2000, 19, 323–344 CrossRef CAS.
- P. N. Bishop, D. F. Holmes, K. E. Kadler, D. McLeod and K. J. Bos, Invest. Ophthalmol. Visual Sci., 2004, 45, 1041–1046 CrossRef PubMed.
- J. Sebag, Prog. Retinal Eye Res., 2020, 100847 CrossRef CAS PubMed.
- J. Sebag and E. Balazs, Invest. Ophthalmol. Visual Sci., 1989, 30, 1867–1871 CAS.
- L. Los, R. van der Worp, M. van Luyn and J. Hooymans, Invest. Ophthalmol. Visual Sci., 2003, 44, 2828–2833 CrossRef PubMed.
- G. Garcia, M. Khoshnevis, J. Nguyen-Cuu, K. M. P. Yee, J. H. Nguyen, A. A. Sadun and J. Sebag, Graefe's Arch. Clin. Exp. Ophthalmol., 2018, 256, 919–925 CrossRef PubMed.
- J. Sebag, Am. J. Ophthalmol., 2011, 152, 3–4 CrossRef PubMed.
- J. Mamou, C. A. Wa, K. M. Yee, R. H. Silverman, J. A. Ketterling, A. A. Sadun and J. Sebag, Invest. Ophthalmol. Visual Sci., 2015, 56, 1611–1617 CrossRef PubMed.
- E. Ankamah, J. Sebag, E. Ng and J. M. Nolan, Antioxidants, 2020, 9, 7 CrossRef PubMed.
- J. Sebag, K. M. P. Yee, L. Huang, C. Wa and A. A. Sadun, Retina, 2014, 34, 1062–1068 CrossRef PubMed.
- J. Sebag, K. M. P. Yee, J. H. Nguyen and J. Nguyen-Cuu, Ophthalmol. Retina, 2018, 2, 881–887 CrossRef CAS PubMed.
- R. Milston, M. Optoma and M. C. Madigan, Surv. Ophthalmol., 2016, 61, 211–227 CrossRef PubMed.
- J. H. Nguyen, J. Nguyen-Cuu, F. Yu, K. M. Yee, J. Mamou, R. H. Silverman, J. Ketterling and J. Sebag, Ophthalmol. Retina, 2019, 126, 1517–1526 CrossRef PubMed.
- P. Stalmans, M. S. Benz, A. Gandorfer, A. Kampik, S. Pakola and J. A. Haller, N. Engl. J. Med., 2012, 367, 606–615 CrossRef CAS PubMed.
- J. Sebag, Retina, 2009, 29, 871–874 CrossRef CAS PubMed.
- F. Sauvage, J. C. Fraire, K. Remaut, J. Sebag, K. Peynshaert, M. Harrington, F. Van de Velde, R. Xiong, M.-J. Tassignon, T. Brans, K. Braeckmans and S. De Smedt, ACS Nano, 2019, 13, 8401–8416 CrossRef CAS.
- K. Nekoueian, M. Amiri, M. Sillanpää, F. Marken, R. Boukherroub and S. Szunerits, Chem. Soc. Rev., 2019, 48, 4281–4316 RSC.
- M.-X. Zhao and B.-J. Zhu, Nanoscale Res. Lett., 2016, 11, 207 CrossRef PubMed.
- A. Barras, Q. Pagneux, F. Sane, Q. Wang, R. Boukherroubs, H. Didier and S. Szunerits, ACS Appl. Mater. Interfaces, 2016, 8, 9004–9013 CrossRef CAS PubMed.
- H.-J. Jian, R.-S. Wu, T.-Y. Lin, Y.-J. Li, H.-J. Lin, S. G. Harroun, J.-Y. Lai and C.-C. Huang, ACS Nano, 2017, 11, 6703–6716 CrossRef CAS PubMed.
- A. Shoval, A. Markus, Z. Zhou, X. Liu, R. Cazelles, I. Willner and Y. Mandel, Small, 2019, 15, 1902776 CrossRef PubMed.
- D. Qu, X. Miao, X. Wang, C. Nie, Y. Li and L. Luo, et al. , J. Mater. Chem. B, 2017, 5, 4988–4992 RSC.
- B. B. Karakoçak, J. Liang, S. Kavadiya, M. Y. Berezin, P. Biswas and N. Ravi, ACS Appl. Nano Mater., 2018, 1, 3682–3692 CrossRef.
- C.-Q. Li, X.-Y. Liu, S.-L. Li, P. Jiang, F.-J. Jiang and Y. Liu, ACS Appl. Bio Mater., 2019, 2, 4067–4076 CrossRef CAS.
- X. Han, Z. Jing, W.-C. Wu, B. Zou, Z. Peng, P. Ren, A. Wikramanayake, Z. Lu and R. M. Leblanc, Nanoscale, 2017, 9, 12862 RSC.
- S. Li, L. Wang, C. C. Chusuei, V. M. Suarez, P. L. Blackwelder, M. Micic, J. Orbulesu and R. M. Leblanc, Chem. Mater., 2015, 27, 1764–1771 CrossRef CAS.
- Q. Q. Yang, J. C. Jin, Z. Q. Xu, J. Q. Zhang, B. B. Wang, F. L. Jiang and Y. Liu, J. Mater. Chem. B, 2017, 5, 2010–2018 RSC.
- L. Wang, S. Zhu, T. Lu, G. Zhang, J. Xu, Y. Song, Y. Li, L. Wang, B. Yang and F. Li, J. Mater. Chem. B, 2016, 4, 4913–4921 RSC.
- Al. Łoczechin, K. Séron, A. Barras, E. Giovanelli, S. Belouzard, Y.-T. Chen, N. Metzler-Nolte, R. Boukherroub, J. Dubuisson and S. Zunerits, ACS Appl. Mater. Interfaces, 2019, 11(46), 42964–42974 CrossRef PubMed.
- T. V. de Medeiros, J. Manioudakis, F. Noun, J.-R. Macairan, F. Victoria and R. Naccache, J. Mater. Chem. C, 2019, 7, 7175–7195 RSC.
- S. A. Hill, D. Benito-Alifonso, S. A. Davis, D. J. Morgan, M. Berry and M. C. Galan, Sci. Rep., 2018, 8, 12234 CrossRef PubMed.
- S. K. Tammina and Y. Yang, J. Photochem. Photobiol., A, 2020, 387, 112134 CrossRef CAS.
- S. Chandra, S. Pradhan, S. Mitra, P. Patra, A. Bhattacharya, P. Pramanik and A. Goswami, Nanoscale, 2014, 6, 3647–3655 RSC.
- J.-B. Wu, M.-L. Lin, X. Cong, H.-N. Liu and P.-H. Tan, Chem. Soc. Rev., 2018, 47, 1822–1873 RSC.
- P. Zhu, K. Tan, Q. Chen, J. Xiong and L. Gao, Chem. Mater., 2019, 31, 4732–4742 CrossRef CAS.
- W. Zhang, Y. Wang, X. Liu, X. Meng, H. Xu, Y. Xu, B. Liu, X. Fang, H.-B. Li and T. Ding, Phys. Chem. Chem. Phys., 2017, 19, 28653–28665 RSC.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563–5565 CrossRef CAS.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- H.-J. Cjung, A. Steplewski, K. Y. Chung, J. Uitto and A. Fertala, J. Biol. Chem., 2008, 283, 25879–25889 CrossRef PubMed.
- X. Yin, L. Zhao, S.-G. Kang, J. Pan, Y. Song, M. Zhang, G. Xing, F. Wang, J. Li, R. Zhou and Y. Zhao, Nanoscale, 2013, 5, 7341 RSC.
- K. Rasheeda and N. Fathima, Colloids Surf., B, 2018, 170, 273–279 CrossRef CAS PubMed.
- H. Bharathy and N. Nishad Fathima, Int. J. Biol. Macromol., 2017, 10, 179–186 CrossRef.
- J. Jayamania, R. Ravikanth Reddy, B. Madhand and G. Shanmugama, Int. J. Biol. Macromol., 2018, 107, 175–185 CrossRef PubMed.
- B. G. Anand, K. Dubey and D. S. Shekhawat, Langmuir, 2017, 33, 13252–13261 CrossRef CAS PubMed.
- S. Perumal, K. Dubey, R. Badhwar, K. J. George, R. K. Sharma, G. Bagler, B. Madhan and K. C. Kar, Eur. Biophys. J., 2015, 44, 69–76 CrossRef CAS PubMed.
- H. S. Frederick, W. F. Joseph and P. S. Gurinder, J. Biomech., 2003, 36, 1529–1553 CrossRef.
- Y. Kim, J.-H. Park, H. Lee and J.-M. Nam, Sci. Rep., 2016, 6, 1 CrossRef PubMed.
- F. Sauvage, J. Schymkowitz, F. Rousseau, B. Z. Schmidt, K. Remaut, K. Braeckmans and S. C. De Smedt, Nano Today, 2020, 31, 100837 CrossRef CAS.
- S. Xu, N. J. Boylan, J. S. Suk, Y.-Y. Wang, E. A. Nance, J.-C. Yang, P. J. McDonnell, R. A. Cone, E. J. Duh and J. Hanes, J. Controlled Release, 2013, 167, 76–84 CrossRef PubMed.
- G. Jarre, S. Heyer, E. Memmel, T. Meinhardt and A. Krueger, Beilstein J. Org. Chem., 2014, 10, 2729–2737 CrossRef PubMed.
- T. K. L. Meyvis, S. S. De-Smedt, P. Van-Oostveldt and J. Demeester, Pharm. Res., 1999, 16, 1153–1162 CrossRef CAS PubMed.
Footnotes |
† Electronic supplementary information (ESI) available: Fig. S1: TEM images of CQD-1 (a) and CQD-2 (b). Fig. S2: XPS high resolution spectra of CQD-1 and CQD-2. Fig. S3: Kaiser test for the determination of the presence of primary amine groups: (a) calibration curve (benzylamine solution from 10 to 100 μM). (b) UV-vis absorption spectra of CQD-2, heated at 100 °C during 10 min, cooled down on ice and further diluted with 1 mL of ethanolic solution (EtOH/water = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). Fig. S4: (a) Zeta potential analysis. (b) Photographs of CQDs suspensions (250 μg mL−1) after 1 h incubation at 37 °C in water (W), PBS (0.01 M, P) and Dulbecco's Modified Eagle's medium (M). Fig. S5: The relationship between the integrated fluorescence intensity and the absorbance of quinine sulfate (QS), CQD-1 and CQD-2. Fig. S6: Optical stability of CQD-1 (red) and CQD-2 (blue) in various conditions: fluorescence emission after 2 years, normalized fluorescence emission after 2 years and relative fluorescence emission of CQDs solution (20 μg mL−1 in PBS) during UV light irradiation at 360 nm (10 mW cm−2) during 3 hours. Fig. S7: Metabolic activity and CQDs uptake: (a) metabolic activity of HeLa and U-87 MG cells treated with CQDs: cells were grown in 96-well plates (104 cells per well) with 100 μL of culture medium containing increasing concentration of CQDs for 8 h (left) and 24 h (right). The results, expressed as percentage of viability, are the mean value of two independent experiments with each treatment performed in triplicate. Negative control: without CQDs. (b) Fluorescence microscopy of HeLa and U-87 MG cells treated with 400 μg mL−1 of CQDs for 24 h. The blue signal corresponds to the nuclei stained with Hoechst 33258, while the green signal is attributed to CQDs. Scale bars = 100 μm. Fig. S8: Fibril formation study of type I collagen using turbidity data: UV-vis absorption evolution of collagen (0.3 mg mL−1) at 313 nm in PBS buffer (pH 7.4) at 37 °C under static condition (blue) and in the presence of increasing concentrations of gold nanoparticles (AuNPs) (1011–1013 AuNPs mL−1). See DOI: 10.1039/d1nh00157d 40). Fig. S4: (a) Zeta potential analysis. (b) Photographs of CQDs suspensions (250 μg mL−1) after 1 h incubation at 37 °C in water (W), PBS (0.01 M, P) and Dulbecco's Modified Eagle's medium (M). Fig. S5: The relationship between the integrated fluorescence intensity and the absorbance of quinine sulfate (QS), CQD-1 and CQD-2. Fig. S6: Optical stability of CQD-1 (red) and CQD-2 (blue) in various conditions: fluorescence emission after 2 years, normalized fluorescence emission after 2 years and relative fluorescence emission of CQDs solution (20 μg mL−1 in PBS) during UV light irradiation at 360 nm (10 mW cm−2) during 3 hours. Fig. S7: Metabolic activity and CQDs uptake: (a) metabolic activity of HeLa and U-87 MG cells treated with CQDs: cells were grown in 96-well plates (104 cells per well) with 100 μL of culture medium containing increasing concentration of CQDs for 8 h (left) and 24 h (right). The results, expressed as percentage of viability, are the mean value of two independent experiments with each treatment performed in triplicate. Negative control: without CQDs. (b) Fluorescence microscopy of HeLa and U-87 MG cells treated with 400 μg mL−1 of CQDs for 24 h. The blue signal corresponds to the nuclei stained with Hoechst 33258, while the green signal is attributed to CQDs. Scale bars = 100 μm. Fig. S8: Fibril formation study of type I collagen using turbidity data: UV-vis absorption evolution of collagen (0.3 mg mL−1) at 313 nm in PBS buffer (pH 7.4) at 37 °C under static condition (blue) and in the presence of increasing concentrations of gold nanoparticles (AuNPs) (1011–1013 AuNPs mL−1). See DOI: 10.1039/d1nh00157d |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2021 |