A nanoimprinted artificial engram device†
Xuesong
Li
ab,
Pan
Zeng
a,
Feilong
Wang
a,
Dai
Zhang
 ab,
Yi
Zhou
c,
Rongqing
Liang
a,
Qiongrong
Ou
a,
Xiang
Wu
c and
Shuyu
Zhang
ab,
Yi
Zhou
c,
Rongqing
Liang
a,
Qiongrong
Ou
a,
Xiang
Wu
c and
Shuyu
Zhang
 *a
*a
aInstitute for Electric Light Sources, Department of Light Sources and Illuminating Engineering, School of Information Science and Technology, Fudan University, Shanghai 200433, P. R. China. E-mail: zhangshuyu@fudan.edu.cn
bInstitute of Future Lighting, Academy for Engineering and Technology, Fudan University, Shanghai 200433, P. R. China
cKey Laboratory of Micro and Nano Photonic Structures (Ministry of Education), Department of Optical Science and Engineering, School of Information Science and Technology, Shanghai Engineering Research Centre of Ultra Precision Optical Manufacturing, Fudan University, Shanghai 200433, P.R. China
First published on 8th July 2021
Abstract
At present, mainstream neuromorphic hardware is based on artificial synapses; however, an engram, instead of a synapse, has recently been confirmed as the basic unit of memory, which verifies the engram theory proposed by Richard Semon in 1904. Here, we demonstrate an artificial engram device based on a nanoimprinted curable resin. The variation in the relative diffraction efficiency based on the asymmetric reversible topological change of the nanoimprinted resin enables the device to meet all the requirements for artificial engrams, including synaptic plasticity, long memory storage time, asymmetric memorizing–forgetting behaviour and measurable changes and responses. On this basis, we demonstrate the concept of realizing memory formation, memory manipulation and implantation, and memory consolidation using our artificial engram device in comparison with its biological counterpart.
New conceptsThe corner stone of current non-von Neumann architecture-based neuromorphic hardware is artificial synapse, however, proposed by Richard Semon in 1904 and experimentally verified by Tonegawa's research team in 2012, the engram theory points out an engram, instead of a synapse, is the basic unit of memory. So, the question is “Can an artificial engram be realized?”, which has not been addressed so far. Here we develop an artificial engram device based on a nanoimprinted curable resin, which meets all the requirements for engrams including synaptic plasticity, long memory storage time, asymmetric memorizing–forgetting behaviour and measurable changes and responses. Using this device, we demonstrate the concept of realizing memory formation, memory manipulation and implantation, and memory consolidation. We believe the development of artificial engram devices may open a new direction parallel to the current study of artificial synapses, and it will be interesting to see how this sort of devices work synergistically with artificial synaptic devices to promote the realization of high-efficiency brain-like computing and storage in the future. Also, the use of curable resin in this work may initiate the development of materials alike including phase change materials, photoswitch materials and hydrogels in the field of artificial engrams. |
Introduction
The era of artificial intelligence and big data has created a great need for high-speed data analysis. However, due to the problem of “memory walls” and “power walls” caused by the separation of processing and memory units, the conventional von Neumann architecture-based computing system is facing huge challenges to achieve fast and energy-efficient processing.1,2 Innovative brain-like computers that attempt to emulate the function of the biological synapse have shown outstanding characteristics, such as massive parallelism, distributed storage and processing, self-learning, and low power consumption.3–8 Originating from Donald O. Hebb's theory of synaptic plasticity,9 the current focus of neuromorphic hardware is mainly on artificial synapses, including a variety of complementary metal oxide semiconductors (CMOSs) and photonic memristors.10–15Fascinatingly, recent studies have confirmed Richard Semon's engram theory proposed in 1904,16 which highlights that an engram, instead of a synapse, is the basic unit of memory. These findings suggest that the linked engram cell ensembles distributed over various brain regions, namely, the so-called engram complex, support the overall process of memory encoding, storage and retrieval.17–20 In 2012, Tonegawa et al. identified engram cells and demonstrated specific memory recall by activating specific memory engram cells.21 Interestingly, these cells, which serve as an index of memory content, can be artificially updated with tempered information to create a false memory.22,23 Although synaptic plasticity contributes to the formation of engrams,24 memory consolidation is not determined by synaptic plasticity in single engram cells but rather the connectivity pattern of engram cells established during encoding, the complexity of which increases as the brain anatomy evolves,25,26 challenging the widely circulated conceptions of memory consolidation. The discovery of silent engrams further suggests that there are multiple states of an engram, depending on their retrievability.24,27
With ongoing comprehensive investigations of engrams in the field of brain science, the engram has been found to be the basis and key of memory coding, storage and retrieval. Therefore, we aim to answer the following question: can we learn from the functional correlation between engrams and dynamic memories and develop artificial engrams emulating biological functions, especially in the form of a highly integrated and flexible optoelectronic device?
An artificial engram state that can last for days
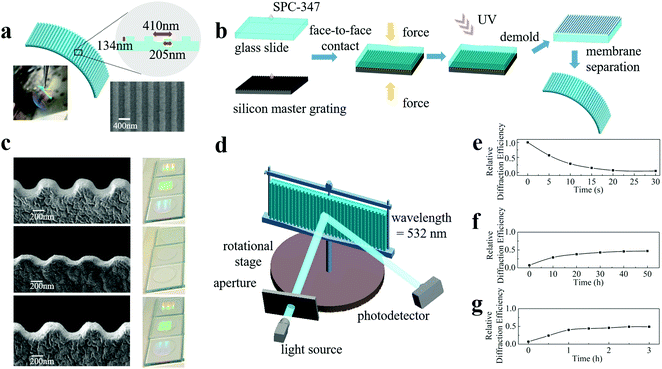
From a cognitive view, ideal artificial engrams need to reflect all three major steps of learning and memory: encoding events, storing encoded information, and retrieving this information for recall. To meet this goal, the physical or chemical changes in artificial engrams need to be triggered instantly by learning. Such a change needs to be retained for several days24 before restoration, and the length of time is learning-strength-dependent. The retrieval step requires artificial engrams to be detectable and identifiable. Therefore, the requirements for artificial engrams are synaptic plasticity, long memory storage time, asymmetric memorizing–forgetting behaviour and measurable changes and responses. Here, we propose a strategy for developing artificial engrams based on nanoimprinting technology with an ultraviolet-curable resin (UVCR), which is capable of satisfying the requirements for emulating biological engrams.Fig. 1(a) shows a schematic diagram of our artificial engram device, which is a one-dimensional nanoimprinted submicrometre grating composed of a SPC-347 UVCR (FOSPIA EFiRON, Ansan, Korea), a photoresistor for UV nanoimprinting. The photograph in Fig. 1(a) shows the self-supported flexible membrane of the nanoimprinted SPC-347 UVCR. The grating period of the device was 410 nm with a duty cycle of 50% (as seen in the top view image from scanning electron microscopy (SEM)) and a groove depth of 134 nm. The process of device fabrication is illustrated in Fig. 1(b). A small amount of SPC-347 UVCR was drop-cast on both a 25 mm × 25 mm silicon master grating and a 25 mm × 25 mm clean glass slide. The silicon master grating had a grating period of 410 nm, a duty cycle of 50% and a groove depth of 150 nm, and an anti-sticking layer (trichlorosilane, Sigma Aldrich, St. Louis, USA) was deposited on its surface before nanoimprinting. The master grating and glass slide were then held together face-to-face for 2 minutes to allow the SPC-347 UVCR to spread via the capillary effect and fill in all the air gaps between the grating and slide. A UV lamp was applied for 2 minutes to cure the SPC-347 UVCR. The glass slide was then separated from the silicon master grating using a blade, and the nanoimprinted SPC-347 UVCR was left on the glass slide. The nanoimprinted SPC-347 UVCR was then detached from the glass slide using a blade or ethanol and formed a nanoimprinted flexible membrane. The UV nanoimprint lithography applied here is a mature technology that offers rapid and reliable nanopattern duplication with upscaling compatibility (mass production can be realized with the aid of step-and-flash imprint lithography and roll-to-roll printing).28–30
Our artificial engram device can respond to both heating and UV irradiation but in very different ways. The memorizing process can be triggered by heating, which leads to a collapse of the grating ridges, as shown in the side view SEM image in Fig. 1(c). For instance, the groove depth decreased from 134 nm to 68 nm after a heating treatment of 120 °C for 30 seconds. This degree of topological change is heating-strength dependent, and this process can finish within several seconds. The heating process in our experiment was carried out using a hotplate, but in theory, heating can also be performed using an infrared beam to selectively choose a target heating area. The forgetting process can be carried out by natural restoration, during which the grating ridges are recovered. As shown in Fig. 1(c), the groove depth recovered to 109 nm after natural restoration for 2 days. Clearly, this process takes a much longer time than the memorizing process, therefore leading to an asymmetric memorizing–forgetting behaviour. Photographs of the SPC-347 UVCR grating after the heating and natural restoration processes as well as the pristine sample are shown in Fig. 1(c), and the disappearance and reappearance of the diffracted emission of ambient light reflects the topological change in the grating surface. Apart from the natural restoration-based forgetting process, which is regarded as a passive forgetting process, an active forgetting process can be triggered by UV irradiation. UV irradiation at a dose of approximately 100 mW cm−2 from a UV lamp can recover the grating ridges to the same extent as natural restoration but within a much shorter time length (∼3 hours). This active forgetting process plays an important role in imitating the valence switch of biological memory (described later in detail). This asymmetric memorizing–forgetting behaviour, the self-restorable capability without external stimuli and the active forgetting capability make the device a promising candidate for artificial engrams and also indicates that phase change materials,15,31–34 photoswitch materials35 and hydrogels36,37 may be able to realize more functions when mimicking neural networks than what has been achieved at present. For example, a recent study demonstrated a soft thermal swelling hydrogel with dynamic memorizing–forgetting behaviour.36
To quantify the degrees of the memorizing and forgetting processes, we measured the relative diffraction efficiencies and correlated the degrees of memorizing and forgetting with the change in efficiencies. As shown in Fig. 1(d), the device was vertically mounted on a rotational stage with the grating grooves aligned perpendicularly. A continuous-wave solid-state laser with a wavelength of 532 nm was used, and the laser emission was incident on the device at an angle of 25° to the surface normal of the device. A photodetector (PDA100A, THORLABS) was placed at an angle of 60° (-1st order) to the surface normal to measure the intensity of the diffracted emission. The diffraction efficiency of the intact device was normalized to 1, and the relative diffraction efficiencies of the stimulated devices were recorded.
Fig. 1(e–g) show the relative diffraction efficiency curves of our artificial engram device after heating, natural restoration and UV irradiation, respectively. When the device was heated at a temperature of 120 °C for 30 seconds, the relative diffraction efficiency showed a substantial decrease from 100% to 7 ± 1%. Approximately 50 hours under natural restoration conditions were required to recover the ridges, but the maximum efficiency of achievable recovery was approximately 47 ± 1%, as expected from the SEM images, which corresponds to the “silent” state of an engram.24 UV irradiation can accelerate the recovery and reach the same recovered efficiency in approximately 3 hours. The recovery effect of natural restoration and UV irradiation is the same, and the only difference is the length of time required. To confirm the device uniformity, we measured the relative diffraction efficiencies at different locations after heating and UV irradiation. The values are almost identical under the same stimulus, as shown in Fig. S1 (ESI†). To confirm the rationality of the efficiency values, we also carried out a numerical simulation of the change in diffraction efficiency based on the SEM results. The calculated efficiency values, which are in good agreement with the experimental results, are shown in Fig. S2 (ESI†).
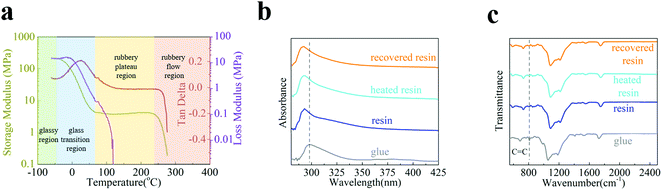
The above-mentioned intriguing characteristics originate from the intrinsic material properties of the SPC-347 UVCR. Fig. 2(a) shows the viscoelastic properties of the UV-cured SPC-347 UVCR using dynamic mechanical analysis. Clearly, room temperature (∼20 °C) is in the glassy transition region and the operating temperature of 120 °C is in the rubbery plateau region. The large width of the rubbery plateau is due to the molecular cross-linking of the UV-cured SPC-347 UVCR. When the grating structure was fabricated at room temperature, structural stress also formed. When the temperature surpassed the dividing point of the two regions (70–80 °C), the UV-cured SPC-347 UVCR became highly elastic and thus released structural stress by increasing its entropy,38,39 leading to a morphological change in the grating. When the SPC-347 UVCR was cooled to room temperature, the enthropic restoring force stored in the less randomly coiled molecular chains recovered the grating structure, and the molecular chains then reverted to a randomly coiled state; however, this process occurred at a very slow pace due to intermolecular friction. UV irradiation can increase the molecular vibration so that the impacts of the surrounding molecules can accelerate the randomizing process of the molecular chains, therefore recovering the grating structure at a much faster pace.
Fig. 2(b and c) show the absorption and Fourier transform infrared (FTIR) spectra, respectively, of the SPC-347 UV glue before UV curing (termed “glue”), the UV-cured SPC-347 UVCR (termed “resin”), the cured SPC-347 UVCR after heating treatment (120 °C for 30 seconds) (termed “heated resin”), and the heated SPC-347 UVCR after UV irradiation (100 mW cm−2 for 3 hours) (termed “recovered resin”). Clearly, chemical changes are not involved in the morphological change of the SPC-347 UVCR grating, as the spectra of the “resin”, the “heated resin” and the “recovered resin” are highly consistent for both absorption and FTIR. The spectral difference occurs only during the initial UV curing stage (from a glue to a cured resin), as the absorption peak is blue-shifted from 298 nm to 293 nm and the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks at 809 cm−1 decreases due to polymerization40 (since π-bonds were involved in the polymerization process). Thus, the morphological change in the grating after the heating treatment is not due to the change in cross-linking since the heating treatment does not jeopardize the cross-linking. Instead, the absorption and FTIR results further confirm that the morphological change in the grating during heating and its structural recovery are, in essence, attributed to pure physical changes.
C peaks at 809 cm−1 decreases due to polymerization40 (since π-bonds were involved in the polymerization process). Thus, the morphological change in the grating after the heating treatment is not due to the change in cross-linking since the heating treatment does not jeopardize the cross-linking. Instead, the absorption and FTIR results further confirm that the morphological change in the grating during heating and its structural recovery are, in essence, attributed to pure physical changes.
Synaptic plasticity of our artificial engram device
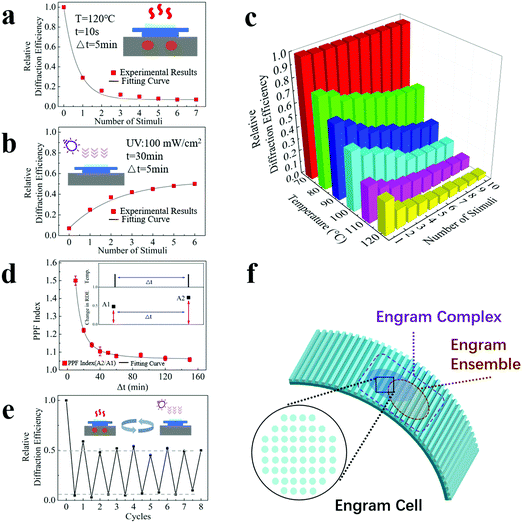
Apart from the characteristics of long memory storage time, asymmetric memorizing–forgetting behaviour and measurable changes and responses, excitingly, our device also possesses synaptic plasticity. Here, we investigated the performance of synaptic potentiation and depression and the performance of short-term potentiation, which have commonly been evaluated in studies of artificial synapses.To investigate the performance of synaptic potentiation and depression, our device was heated repetitively at 120 °C for 10 seconds with an interval of 5 minutes. The interval duration is set to dissipate the heat of the sample, and natural restoration has a minimum effect on the device due the short interval. As shown in Fig. 3(a), the relative diffraction efficiency continues to decrease at a gradually slowing pace, and it eventually becomes steady. An ascending curve with a slow-down pace covering a much longer time scale was observed when the device was treated with UV irradiation repetitively at a dose of 100 mW cm−2 for 30 minutes with an interval of 5 minutes (Fig. 3(b)). These results indicate that similar to a biological engram, our artificial engram device shows an accumulation effect during the memorizing and forgetting processes, and these processes obey the law of diminishing marginal utility and, consequently, arrive at a stable state.
To further investigate the temperature effect, Fig. 3(c) shows the relative diffraction efficiency as a function of both temperature and the number of heating stimuli. For each heating stimulus, our device was heated repetitively at the target temperature for 10 seconds with an interval of 5 minutes. The results show a distinctive stimulus threshold between 70 °C and 80 °C for the heating process, which matches the dividing point of the glassy transition region and the rubbery plateau region in Fig. 2(a). The relative diffraction efficiency of our device exhibits a strong and nonlinear dependence on the temperature and number of stimuli. A higher temperature and a larger number of stimuli lead to a more pronounced change in synaptic weight.
Our device has a property similar to the Ebbinghaus forgetting curve; that is, a timely review will strengthen its memory. Such behaviour was analysed by measurement of paired-pulse facilitation (PPF), a typical form of short-term potentiation caused by two closely occurring stimuli on the time scale. Here, a PPF index, which refers to the changing ratio of two adjacent relative diffraction efficiencies to the initial value, was calculated, and the dependence of the PPF index on the intermediate time Δt between two stimuli is shown in Fig. 3(d). The heating conditions were 100 °C for 10 seconds, and UV irradiation was applied during the intermediate time. The inset shows that the change in the relative diffraction efficiency increases from A1 (under the first stimulus) to A2 (under the second stimulus after Δt), and the PPF index equals the value of A2/A1. The longer Δt is, the lower the PPF index is, which means that a delayed stimulus has a reduced effect, corresponding to a less effective memorizing result when the time gap between the initial learning and subsequent review processes is large.
Furthermore, our device shows a reasonable cycling capability, which enables repetitive use. Fig. 3(e) shows the relative diffraction efficiencies under the cycling test. For each heating period, the temperature was set to 120 °C and the duration was 30 seconds. For each UV irradiation period, the dose was set to 100 mW cm−2 and the duration was 3 hours. The set duration for the restoration process was sufficient so that the sample was fully recovered after each UV irradiation period. The efficiency at the end point of each heating period is consistent (approximately 7 ± 2%), as is the efficiency at the end point of each UV irradiation period (approximately 51 ± 4%), clearly showing the repeatability of the sample under the same stimulus. Although Fig. 3(e) only shows 8 cycling operations, the maximum cycle number that our device can theoretically withstand is expected to be more than tens of thousands, based on the typical fatigue properties of acrylates and fluorine-containing polymers (SPC-347 UVCR is a fluorine-containing acrylate).
According to all the above-mentioned findings, our device meets all the requirements for artificial engrams, including synaptic plasticity, long memory storage time, asymmetric memorizing–forgetting behaviour and measurable changes and responses, and thus can realize the fundamental functions of a biological engram for information encoding, storage and retrieval.
From the biological perspective, an engram is only the first step of memory formation. Strictly speaking, an engram is not yet a memory but rather provides the necessary physical conditions for a memory to emerge.24,41 In 2015, Tonegawa et al. proposed normative usage of engram-related terminologies, including engrams, engram cells, engram cell ensembles, and engram complexes.20,24 The concepts of these biological terminologies and the functions they have can be properly reflected in our artificial engram device, as illustrated in Fig. 3(f). An engram refers to the enduring physical and/or chemical changes triggered by learning, which is reflected by the topological changes of our device under external stimuli. An engram cell is the carrier of a given engram and can support more than one memory. An engram cell is the basic unit of an engram system, so for our device, the minimal stimulable area is defined as a pixel, which corresponds to an engram cell. An important property of engram is that engram cells can support more than one experience, and the co-allocated engram cells make the two experiences linked or integrated. This is due to the nature of competition for allocation to an engram. Within a given brain region, engram cells that have been allocated to a previous experience are more likely to be co-allocated to the engram which supports the memory of a related experience that occurs close in time.24 The engram cells localized within a brain region work together and form an engram cell ensemble, and ensembles in different brain regions, i.e., the hippocampus and amygdala, can be further connected via engram cell pathways and form an engram complex that supports a memory stored in the ensembles distributed throughout the brain. The working mechanism of memory storage and retrieval is based on the specific connectivity patterns of engram ensembles at different brain regions. For our device, a certain combination of activated pixels constitutes a pattern that corresponds to a particular ensemble. Different patterns can overlap and form a pattern complex, mimicking the structure and functions of an engram complex. In the following sections, we will demonstrate how our artificial engram device realizes the functions of engram complexes and fulfils the targets of engram complex-involving memory formation and retrieval, memory manipulation and implantation, and memory consolidation.
Memory formation and retrieval
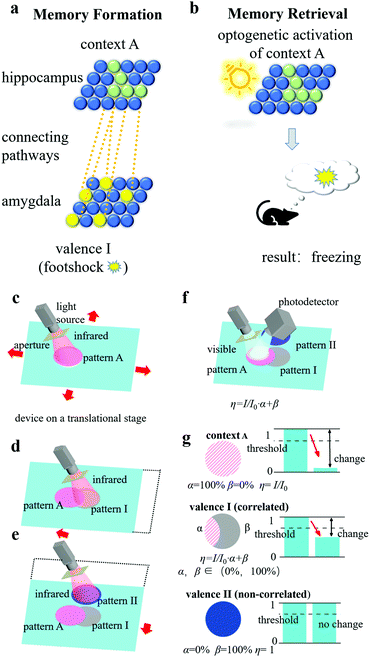
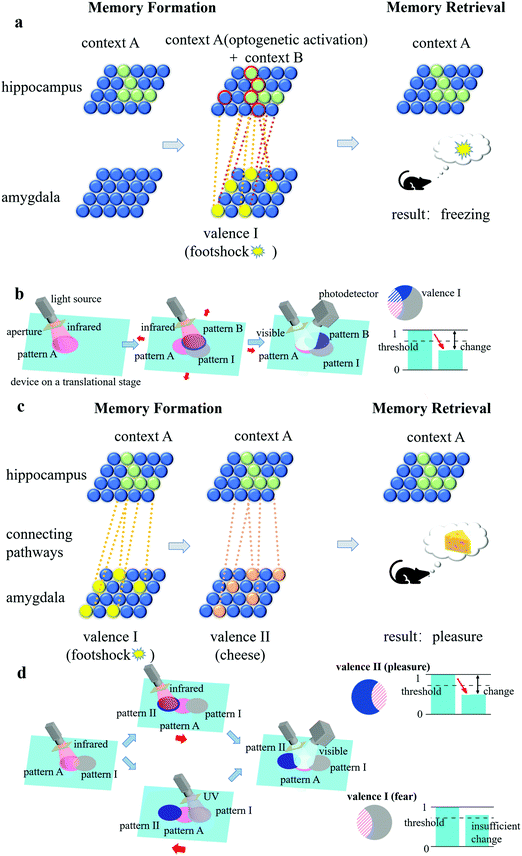
From the perspective of a biological engram complex, the formation and retrieval of memory is based mainly on the following three properties: intrinsic excitability, physiological and structural plasticity, and ensemble connectivity.19,24 Intrinsic excitability determines which neurons become engram cells, physiological plasticity (synaptic strength) and structural plasticity (density of dendritic spines) are responsible for storing long-term memories, and ensemble connectivity determines the connections and pathways between ensembles. For our artificial engram device, we use the shape of the pattern, the changing degree of the grating topology, and the overlapping degree of patterns to reflect the intrinsic excitability, physiological and structural plasticity, and ensemble connectivity, respectively.The artificially defined pattern is the first step of operations related to memory, which are designed to quickly reflect a contextual identity. For example, patterns A, B, C, etc. can be regarded as engram cell ensembles in the hippocampus that are related to different contexts, including places, light, odours, etc. (termed the conditioned stimulus), and patterns I, II, III, etc. can be regarded as engram cell ensembles in the amygdala that are related to different feelings and emotions (termed the unconditioned stimulus). The topological change of the grating in the patterns is determined by the heating strength, and thus, the overlapping area of the patterns has an enhanced effect. The overlapping degree depends on the connectivity of two events. For example, if a conditioned stimulus and an unconditioned stimulus are strongly correlated, then pattern A and pattern I have a large overlapping area, and a memory linking the context and the feeling is established.
Here, we compare memory formation and retrieval in a biological engram system and an artificial engram system. In the biological system (Fig. 4(a)), when a mouse was exposed to context A, neurons with higher intrinsic excitability transformed into engram cells (the green solid spheres in Fig. 4(a)), and the collection of these cells formed a hippocampal engram ensemble containing contextual information A. If, at the same time, a footshock stimulus was applied, then an amygdala engram ensemble (the group of yellow solid spheres in Fig. 4(a)) was formed and carried valence information I, which represents the fear emotion. Since the context and footshock stimulus were highly correlated in time, the connectivity between the hippocampal engram ensemble and the amygdala engram ensemble was high (as illustrated by the orange dotted lines in Fig. 4(a)). As a result, when the hippocampal engram ensemble was optogenetically activated by light (Fig. 4(b)), not only the context information A but also the valence information I would be read out so that the mouse would freeze as it recalled the experience of being footshocked.20
The same process can be realized in the artificial engram system, and the concept is illustrated as follows. As shown in Fig. 4(c), the system consists of an artificial engram device on a motor-controlled translational stage, a multi-wavelength switchable light source with an adjustable aperture, and a movable photodetector placed at the -1st order diffracted reflection angle. The light source can selectively emit infrared irradiation for heating, UV irradiation for active restoration, and visible light for diffraction efficiency measurement. The aperture is mounted at the outlet of the light source and used to define the shape and area of the pattern. The translational stage can move the device in a plane so that the pattern can be allocated to a target area of the device using (x, y) coordinates. If a conditioned stimulus and an unconditioned stimulus are highly correlated, then the translational stage will allocate close (x, y) coordinates to the pattern of contextual information (i.e., pattern A) and the pattern of valence information (i.e., pattern I), so that the two patterns largely overlap. The photodetector records the light intensity and can distinguish the values from different patterns by moving itself to the -1st order diffracted reflection angle of the target pattern. Although we only demonstrate the practical fabrication of an artificial engram device instead of the entire system, the setup of such artificial engram system is theoretically feasible. For memory formation, contextual information A triggered the formation of pattern A using infrared irradiation (Fig. 4(c)), and highly correlated valence information I led to the formation of pattern I under infrared irradiation with slightly different (x, y) coordinates assisted by the translational stage (Fig. 4(d)). If the two events were not correlated, then the two patterns (i.e., pattern A and pattern II) had no overlapping area (Fig. 4(e)). When context A was recalled (Fig. 4(f)), the visible light for the measurement of diffractive efficiency was incident on pattern A, and the contextual information in pattern A and any correlated valence information could be read out by measuring the change in the relative diffraction efficiency of corresponding patterns. The relative diffraction efficiency η of the patterns was determined using the following equation:
| η = I/I0 × A1 + A2, | (1) |
Memory manipulation and implantation
The connectivity between engram ensembles is critical to retrieve a memory. If more than one pair of conditioned and unconditioned stimuli are in play, then it is possible to create a false memory by manipulating the strength of the connectivity between different pairs of ensembles. Fig. 5(a) shows a classic example of optogenetic manipulation of memory involving two conditioned stimuli (context A and B) and one unconditioned stimulus (footshock).20 A mouse was placed in context A to form a neutral memory, and the corresponding ensemble was molecularly labelled. The labelled ensemble was optogenetically activated when the mouse was transferred to context B and a footshock stimulus was simultaneously applied. Then, when the mouse was moved back to context A, it froze as if the fear emotion was triggered, although the footshock was only applied when it was placed in context B, not context A. Fig. 5(b) shows the corresponding route of realizing memory manipulation in the artificial engram system. Pattern A (context A) was first formed, followed by the formation of pattern B (context B) and pattern I (footshock). The formed pattern B and pattern I had a large overlapping area with pattern A due to the strong correlation (the engram ensemble of context A was active when the footshock stimulus was applied). When light is incident on pattern A, the valence information (fear) in pattern I can be successfully retrieved since the relative diffraction efficiency of pattern I fell below the threshold, and a false memory was therefore created.Researchers have also discovered that the memory valence switch is feasible in engrams.20 Here, two unconditioned stimuli (valences I and II) and one conditioned stimulus (context A) are in play, as shown in Fig. 5(c). With the aid of a protein synthesis inhibitor, the former connection between context A and valence I was weakened, and at the same time, the new connection between context A and valence II was built up, resulting in a manipulated valence switch from one to the other (e.g., from fear to pleasure) for context A. In 2019, Vetere et al. paired a genetically specific olfactory glomerulus with optogenetic stimulation of either appetitive or aversive neural pathways, and after this memory implantation process, mice reacted to the real odour that activated this olfactory glomerulus in a predicted manner.42 Such memory valence switching and implantation can also be realized in artificial engram systems. Here, UV irradiation works as a protein synthesis inhibitor and accelerates the weakening of the former connection through its “active forgetting” function (from several days to 3 hours). As shown in Fig. 5(d), the topological change of the grating in the overlapping area of patterns A and I was weakened by UV irradiation and, in the overlapping area of patterns A and II, was strengthened by infrared irradiation. Thus, when light is incident on pattern A, the manipulated valence information in pattern II (pleasure instead of fear) can be retrieved successfully.
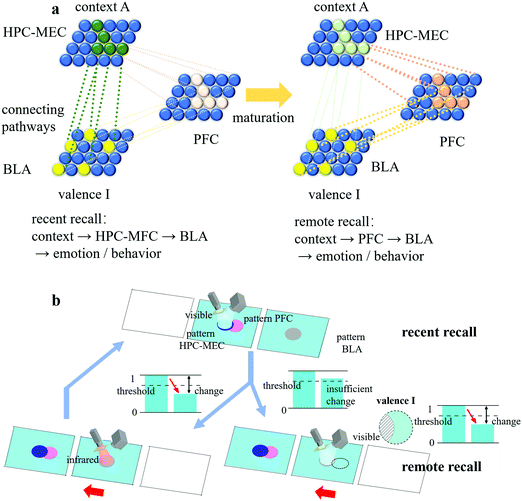
Memory consolidation
In biology, memory consolidation consists of cellular consolidation and system consolidation. Cellular consolidation is a fast process that increases the synaptic weights between engrams, while system consolidation is a slow time-dependent process that reorganizes the brain circuit. Long-term memory is attributed to the transformation of the memory path, from the engram complex distributed over the hippocampal cortex (HPC), medial entorhinal cortex (MEC) and basolateral amygdala (BLA) to the engram complex distributed over the prefrontal cortex (PFC) and basolateral amygdala (BLA).26 As shown in Fig. 6(a), during memory consolidation, the complex of HPC-MEC-BLA gradually weakened and the complex of PFC-BLA continued to strengthen, leading to the transition of the brain circuit. This process required inputs from the HPC-MEC engram ensemble to mature the PFC engram ensemble. Over time, the HPC-MEC engram ensemble eventually became silent, so the route of “context → HPC-MEC → BLA → emotion/behaviour” for recent recall was switched to that of “context → PFC → BLA → emotion/behaviour” for remote recall.To mimic the same function of memory consolidation, two identical artificial engram devices were prepared, one for the HPC-MEC-BLA complex and the other for the PFC ensemble, as shown in Fig. 6(b). The HPC-MEC pattern and PFC pattern had the same pattern shape and position relative to their own device. Each time the valence information of pattern BLA was successfully retrieved when pattern HPC-MEC was illuminated by visible light, pattern PFC on the other device was treated with infrared irradiation. Over time, the topological change in the PFC pattern was strengthened due to repetitive cycles, corresponding to the maturation of the PFC engram ensemble. After a certain time, due to the natural restoring effect of the HPC-MEC-BLA device, the BLA response was no longer able to be activated by illuminating the HPC-MEC pattern with visible light. In contrast, when pattern PFC was illuminated by visible light, the valence information I in the virtual pattern BLA in the right-hand side device (the black dotted outline in Fig. 6(b), which had the same pattern shape and position relative to its own device compared with that in the left-hand side device) can be successfully retrieved. Thus, from this point on, a memory consolidation was established in the PFC, a place in the brain mediating higher-order behaviours, such as intellectual or executive functions, and only the recall of the PFC pattern could activate the BLA response.
Integration and scalability
A brain integrates about 14 billion neurons in the cerebral cortex alone and the cell bodies of neurons are typically tens of micrometres in diameter. In order to emulate the high integration of a brain, a potential solution is to reduce the pixel size or increase the number of pixels per unit area. Since the minimal stimulable area is defined as a pixel, the lower limit of the pixel size is mainly determined by the activated area of a stimulus, which is the beam size of either an infrared or UV laser beam. This means it is potentially feasible to achieve a pixel with the size similar to that of a biological engram cell. The pursuit of high integration also effectively reduces the power consumption, which is an important feature of brain operations, since the energy consumption of the UV irradiation and infrared heating is directly proportional to the size of a pixel. Apart from the beam size, the minimal step length of the translational stage, the thermal diffusion coefficient of UVCR, and the incident wavelength and grating period also have effects on the lower limit of the pixel size, therefore an improvement on device integration and scalability needs to consider all these factors.Conclusions
We have demonstrated an artificial engram device based on a nanoimprinted SPC-347 UVCR. The variation in the relative diffraction efficiency based on the asymmetric reversible topological change of the nanoimprinted grating enables the device to meet all the requirements for artificial engrams, including synaptic plasticity, long memory storage time, asymmetric memorizing–forgetting behaviour and measurable changes and responses. Furthermore, we demonstrate the concept of realizing memory formation, memory manipulation and implantation, and memory consolidation using our artificial engram device in comparison with its biological counterpart. This device is flexible, and nanoimprint fabrication is compatible with up-scaling production. The device has the potential to be composed of pixels with an ultrahigh density and thus low energy consumption since the footprint of a pixel is defined by the size of the infrared and UV beams, which can be narrowed down to an extremely small value. We believe this work will initiate the development of materials such as phase change materials, photoswitch materials and hydrogels in the field of artificial engrams, and it will be interesting to see how this sort of device works synergistically with artificial synaptic devices to promote the realization of high-efficiency brain-like computing in the future.Author contributions
Conceptualization, X. L. and S. Z.; investigation, X. L. and Y. Z.; formal analysis, X. L., P. Z. and F. W.; writing draft, X. L. and S. Z.; review editing, X.L. and S.Z.; project administration, X. L., P. Z. and S. Z.; funding acquisition, S. Z. and Q. O.; resources, S. Z., R. L., X. W. and Q.O.; visualization, X. L., D. Z. and P. Z.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (61705042, 11975081 and 51677031).References
- J. S. Tang, F. Yuan, X. K. Shen, Z. R. Wang, M. Y. Rao, Y. Y. He, Y. H. Sun, X. Y. Li, W. B. Zhang, Y. J. Li, B. Gao, H. Qian, G. Q. Bi, S. Song, J. J. Yang and H. Q. Wu, Adv. Mater., 2019, 31, 1902761 CrossRef CAS PubMed.
- S. Choi, J. Yang and G. Wang, Adv. Mater., 2020, 32, 2004659 CrossRef CAS PubMed.
- Q. F. Xia and J. J. Yang, Nat. Mater., 2019, 18, 309–323 CrossRef CAS PubMed.
- Z. Y. Lv, Y. Wang, J. G. Chen, J. J. Wang, Y. Zhou and S. T. Han, Chem. Rev., 2020, 120, 3941–4006 CrossRef CAS PubMed.
- Y. Tuchman, T. N. Mangoma, P. Gkoupidenis, Y. van de Burgt, R. A. John, N. Mathews, S. E. Shaheen, R. Daly, G. G. Malliaras and A. Salleo, MRS Bull., 2020, 45, 619–630 CrossRef.
- L. F. Abbott and W. G. Regehr, Nature, 2004, 431, 796–803 CrossRef CAS PubMed.
- H. L. Park, Y. Lee, N. Kim, D. G. Seo, G. T. Go and T. W. Lee, Adv. Mater., 2020, 32, 1903558 CrossRef CAS PubMed.
- E. Goi, Q. Zhang, X. Chen, H. Luan and M. Gu, PhotoniX, 2020, 1, 3 CrossRef.
- D. O. Hebb, The Organization of Behavior: A Neuropsychological Theory, Wiley, New York, 1949 Search PubMed.
- S. L. Dai, Y. W. Zhao, Y. Wang, J. Y. Zhang, L. Fang, S. Jin, Y. L. Shao and J. Huang, Adv. Funct. Mater., 2019, 29, 1903700 CrossRef CAS.
- G. Cao, P. Meng, J. Chen, H. Liu, R. Bian, C. Zhu, F. Liu and Z. Liu, Adv. Funct. Mater., 2020, 31, 2005443 CrossRef.
- Y. C. Shen, N. C. Harris, S. Skirlo, M. Prabhu, T. Baehr-Jones, M. Hochberg, X. Sun, S. J. Zhao, H. Larochelle, D. Englund and M. Soljacic, Nat. Photonics, 2017, 11, 441 CrossRef CAS.
- C. Rios, N. Youngblood, Z. G. Cheng, M. Le Gallo, W. H. P. Pernice, C. D. Wright, A. Sebastian and H. Bhaskaran, Sci. Adv., 2019, 5, eaau5759 CrossRef CAS PubMed.
- J. Feldmann, N. Youngblood, C. D. Wright, H. Bhaskaran and W. H. P. Pernice, Nature, 2019, 569, 208–214 CrossRef CAS PubMed.
- Z. G. Cheng, C. Rios, W. H. P. Pernice, C. D. Wright and H. Bhaskaran, Sci. Adv., 2017, 3, e1700160 CrossRef PubMed.
- R. Semon, Die Mneme als erhaltendes Prinzip im Wechsel des organischen Geschehens, Wilhelm Engelmann, Leipzig, 1904 Search PubMed.
- D. L. Schacter, J. E. Eich and E. Tulving, J. Verb. Learn. Verb. Behav., 1978, 17, 743 Search PubMed.
- S. Tonegawa, M. Pignatelli, D. S. Roy and T. J. Ryan, Curr. Opin. Neurobiol., 2015, 35, 101–109 CrossRef CAS PubMed.
- M. M. Poo, M. Pignatelli, T. J. Ryan, S. Tonegawa, T. Bonhoeffer, K. C. Martin, A. Rudenko, L. H. Tsai, R. W. Tsien, G. Fishell, C. Mullins, J. T. Goncalves, M. Shtrahman, S. T. Johnston, F. H. Gage, Y. Dan, J. Long, G. Buzsaki and C. Stevens, BMC Biol., 2016, 14, 40 CrossRef PubMed.
- S. Tonegawa, X. Liu, S. Ramirez and R. Redondo, Neuron, 2015, 87, 918–931 CrossRef CAS PubMed.
- X. Liu, S. Ramirez, P. T. Pang, C. B. Puryear, A. Govindarajan, K. Deisseroth and S. Tonegawa, Nature, 2012, 484, 381–415 CrossRef CAS PubMed.
- S. Ramirez, X. Liu, P. A. Lin, J. Suh, M. Pignatelli, R. L. Redondo, T. J. Ryan and S. Tonegawa, Science, 2013, 341, 387–391 CrossRef CAS PubMed.
- K. Z. Tanaka, H. He, A. Tomar, K. Niisato, A. J. Y. Huang and T. J. McHugh, Science, 2018, 361, 392–397 CrossRef CAS PubMed.
- S. A. Josselyn and S. Tonegawa, Science, 2020, 367, 39 CrossRef PubMed.
- T. J. Ryan, D. S. Roy, M. Pignatelli, A. Arons and S. Tonegawa, Science, 2015, 348, 1007–1013 CrossRef CAS PubMed.
- T. Kitamura, S. K. Ogawa, D. S. Roy, T. Okuyama, M. D. Morrissey, L. M. Smith, R. L. Redondo and S. Tonegawa, Science, 2017, 356, 73 CrossRef CAS PubMed.
- D. S. Roy, S. Muralidhar, L. M. Smith and S. Tonegawa, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 9972–9979 CrossRef PubMed.
- C. Zhang, H. Subbaraman, Q. Li, Z. Pan, J. G. Ok, T. Ling, C.-J. Chung, X. Zhang, X. Lin, R. T. Chen and L. J. Guo, J. Mater. Chem. C, 2016, 4, 5133–5153 RSC.
- L. J. Guo, Adv. Mater., 2007, 19, 495–513 CrossRef CAS.
- A. Habermehl, P. Brenner, R. Huber, A. Mertens, F. Winkler, L. Hahn, M. Guttmann, C. Eschenbaum and U. Lemmer, Adv. Eng. Mater., 2019, 21, 1900110 CrossRef.
- M. Xu, X. Mai, J. Lin, W. Zhang, Y. Li, Y. He, H. Tong, X. Hou, P. Zhou and X. Miao, Adv. Funct. Mater., 2020, 30, 2003419 CrossRef CAS.
- S. Raoux, W. Welnic and D. Ielmini, Chem. Rev., 2010, 110, 240–267 CrossRef CAS PubMed.
- W. Zhang, R. Mazzarello, M. Wuttig and E. Ma, Nat. Rev. Mater., 2019, 4, 150–168 CrossRef CAS.
- X. J. Zhu, D. Li, X. G. Liang and W. D. Lu, Nat. Mater., 2019, 18, 141 CrossRef CAS PubMed.
- A. Goulet-Hanssens, F. Eisenreich and S. Hecht, Adv. Mater., 2020, 32, 1905966 CrossRef CAS PubMed.
- C. Yu, H. Guo, K. Cui, X. Li, Y. N. Ye, T. Kurokawa and J. P. Gong, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18962–18968 CrossRef CAS PubMed.
- H. Zhang, H. Zeng, A. Priimagi and O. Ikkala, Nat. Commun., 2019, 10, 3267 CrossRef PubMed.
- D. I. Bower, An Introduction to Polymer Physics, Cambridge University Press, London, 1981 Search PubMed.
- L. H. Sperling, Introduction to Physical Polymer Science, 4th edn, John Wiley & Sons, New Jersey, 2005 Search PubMed.
- K. Teo, A. Hassan and S. Gan, Polymers, 2018, 10, 1374 CrossRef PubMed.
- H. L. Roediger, Y. Dudai and S. M. Fitzpatrick, Science of Memory: Concepts, Oxford University Press, London, 2007 Search PubMed.
- G. Vetere, L. M. Tran, S. Moberg, P. E. Steadman, L. Restivo, F. G. Morrison, K. J. Ressler, S. A. Josselyn and P. W. Frankland, Nat. Neurosci., 2019, 22, 933 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nh00064k |
| This journal is © The Royal Society of Chemistry 2021 |