Visible-light-driven cuprous oxide nanomotors with surface-heterojunction-induced propulsion†
Wenjuan
Liu
 *ab,
Xiao
Chen
a,
Xiaoyong
Ding
a,
Qiang
Long
a,
Xiaolong
Lu
*c,
Qiang
Wang
*ab,
Xiao
Chen
a,
Xiaoyong
Ding
a,
Qiang
Long
a,
Xiaolong
Lu
*c,
Qiang
Wang
 *d and
Zhongwei
Gu
ab
*d and
Zhongwei
Gu
ab
aCollege of Materials Science and Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: liuwenjuan@njtech.edu.cn
bJiangsu Collaborative Innovation Center for Advanced Inorganic Functional Composites, Nanjing Tech University, Nanjing 211816, China
cState Key Laboratory of Mechanics and Control of Mechanical Structures, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China. E-mail: long_8446110@nuaa.edu.cn
dSchool of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: wangqiang@njtech.edu.cn
First published on 15th January 2021
Abstract
The controllable synthesis and customized design of micro/nanomotors represents a highly desired paradigm in the field of intelligent nanovehicles. Exploiting asymmetrical structures and geometry-dependent propulsion are the two main strategies for achieving light-driven micro/nanomotors. However, inherent crystal-structure differences in a single colloidal motor have rarely been explored. Here, we propose the first surface-heterojunction-induced propulsion methodology for cuprous oxide (Cu2O) nanomotors, by tailoring the crystal morphology of a Cu2O crystalloid from a sphere into a truncated octahedron and preserving the controllable-index crystal facets of {100} and {111} in a single colloid. Due to the high crystallinity and distinct activity of the exposed crystal facets, a surface heterojunction between the {100} and {111} facets is formed to enhance electron–hole separation, as confirmed by density functional theory (DFT) calculations, thus endowing the truncated octahedral Cu2O nanomotors with autonomous and vigorous movement in biocompatible fuels under visible light. These Cu2O nanomotors can reach a propulsion speed in water of over two times faster than that of polycrystalline spherical motors with low crystallinity. The efficient Cu2O nanomotors offer a promising guideline not only for the synthesis of novel light-driven motors with desired structures, but also for potential applications in biocompatible environments.
New conceptsUnlike traditional asymmetrical structure- or geometry-dependent propulsion, we report the first surface heterojunction-induced propulsion methodology for Cu2O nanomotors by tailoring the crystal morphology of a Cu2O crystalloid. By changing the morphology from a sphere into a truncated octahedron and preserving the controllable-index crystal facets of {100} and {111} in a single colloid, this novel Cu2O nanomotor can propel well in pure water and achieve a speed that is approximately three times faster than that of the reported BiOI-Au Janus micromotor. Since a surface heterojunction is formed between the {100} and {111} facets, as confirmed by DFT calculations, the photogenerated charge separation/transport efficiency was enhanced, thereby accelerating the motion of Cu2O nanomotors. The Cu2O nanomotors with a surface heterojunction via co-exposed {111} and {100} facets could reach a speed of twice that of polycrystalline spherical motors with low crystallinity. Furthermore, the good motility behavior of the photoactive Cu2O nanomotors could extend to different biocompatible environments. This work is the first experimental example to demonstrate the surface heterojunction-induced propulsion of micro/nanomotors, and will offer a promising guideline not only for the synthesis of novel light-driven motors with desirable structures, but also for potential applications in biocompatible environments. |
Introduction
The controllable synthesis of micro/nano-structured colloids is of great significance for constructing artificial intelligent micro/nanomotors on demand. Over the past decades, numerous colloids with specially designed structures (e.g., microtubes,1 Janus microspheres,2,3 dumbbell-shaped colloids,4 nanotrees,5 brush-shaped colloids,6 microbowls7 and pocket-like cells8,9) have been successfully implemented in micro/nanomotors and have enabled the conversion of energy10–17 into autonomous motion in various applications.18–21 However, inherent crystal-structure differences in a single micro/nanomotor have rarely been explored. Understanding such differences could help to overcome future challenges in the synthesis of micro/nanomachines and lead to more promising application prospects. Although there is already some preliminary research on polymorphic anatase/rutile TiO2 micromotors with inherent phase asymmetry,22 harmful ultraviolet light illumination is required. In contrast, visible-light-driven Cu2O (with 2.1 eV band gap) micro/nanomotors have recently attracted scientific attention,23–27 but most of this research has focused on constructing asymmetrical structures,23 forming heterostructures with other conductors/semiconductors24–26 or introducing defects.27 Visible-light-driven Cu2O micro/nanomotors are still in their infancy, and great efforts must be devoted to the inherent crystal-structure design for understanding the underlying photocatalytic mechanism and opening new avenues for the synthesis of novel light-powered intelligent micro/nanomachines.Nowadays, there is an enormous interest in controlling the crystal facets of Cu2O due to their fascinating shape-dependent physicochemical properties.28,29 Generally, growth of the crystal facets of Cu2O nanoparticles can induce crystal-structure differences. Cu2O colloids featuring high-activity {110} and {111} facets exhibit distinct and remarkable optical and electrical properties.30,31 In addition, Cu2O with excellent crystallinity can effectively inhibit the recombination of photo-generated charges, thereby improving the photocatalytic activity.32,33 However, all currently reported Cu2O micromotors are approximately spherical in shape before an effective surface modification. As a result, they exhibit low crystallinity and poor crystal facet exposure, hence resulting in poor photoexcited electron/hole pair separation and weak light-driven propulsion. According to the Gibbs-Wulff's theorem, crystal facets possessing high surface energies decrease gradually or even disappear during crystal growth under equilibrium conditions. Indeed, the final structures of crystals and the exposed facets are a result of the synergistic interaction of thermodynamics and kinetics.34,35 Therefore, tailoring crystal morphology and preserving controllable-index facets in a single colloid is a feasible way to construct and to propel micro/nanomotors.
Herein, a highly photoactive Cu2O nanomotor was constructed by customizing its crystalline structure into a truncated octahedron with co-exposed {100} and {111} facets in a single colloid motor. This work is the first experimental example to demonstrate the surface-heterojunction-induced propulsion of visible-light-driven micro/nanomotors. Controlling the crystal facets of a catalyst colloid is significant to improve its photocatalytic activity, due to the different atomic coordination and electronic structures of various exposed crystal facets. Notably, polyhedral Cu2O architectures with well-defined facets (including low-index facets of {100}, {111} and {110} and high-index facets of {211}, {311}, {332} and {544}) display enhanced photoelectrochemical/photocatalytic properties and effective separation of photogenerated electron–hole pairs.28,32,36 Due to the strong interaction with different metallic ions, poly(vinylpyrrolidone) PVP is employed here to reduce the surface energy and expose different facets during crystal growth. The crystalline structures of Cu2O are tunable by varying the dose of PVP. Truncated octahedral Cu2O nanomotors were obtained and showed stable performance and invariant structures under high temperature conditions. The propulsion performance of truncated octahedral Cu2O nanomotors was later evaluated in different biocompatible fuels (pure water, glucose solution and tannic acid at low concentration) and under three light sources (ambient, blue and green light). Furthermore, a “surface heterojunction” concept is proposed on the basis of density functional theory (DFT) calculations and a relevant propulsion mechanism is established to explain the distinct propulsion performance of truncated octahedral Cu2O with co-exposed {100} and {111} facets. These novel truncated octahedral Cu2O nanomotors are useful for fundamental structural studies and potential applications as visible-light driven motors.
Results and discussion
During a low temperature liquid phase reduction process, with CuSO4 as the copper salt precursor and PVP as the capping agent, Cu2O nanoparticles were fabricated on a large scale after reducing the copper–citrate complex solution with glucose in an alkaline environment at a specific temperature (Fig. S1, ESI†), according to the following reaction:37| Cu(citrate) (aq) + C5H11O5–CHO (aq) → Cu2O (s) + C5H11O5COOH (aq) | (1) |
Due to the preferential adsorption of organic additives on certain crystallographic surfaces, PVP is the key variable to effectively freeze the Cu2O low-index facets and build different crystal structures in this system. With the increase of PVP dosage, the final crystalline Cu2O particles vary from truncated cube to truncated octahedron. When the PVP dosage is about 3.5 g, most of the particles are approximately spherical in shape with rough surfaces (Fig. S2, ESI†). Up to a PVP dosage of 1.5 g, regular truncated octahedral Cu2O nanoparticles were obtained, with an average diameter of 864 nm (Fig. S3, ESI†). Meanwhile, the spherical Cu2O particles have a larger average diameter of 1.46 μm (Fig. S3, ESI†), and do not exhibit any obvious agglomeration.
As reported, an ideal truncated octahedron structure of a Cu2O colloid should consist of eight hexagonal {111} facets and six square {100} facets.37,38 Accordingly, the separation of photogenerated electron–hole pairs could be promoted through the different facets of polyhedral Cu2O.39 The possible photocatalytic cycles are demonstrated in the ESI.† Electron migration is facilitated by the different surface energies of the valence and conduction bands (VB and CB) between facets, thus, electrons and holes can accumulate on different facets. Namely, exposed facets can effectively hinder charge recombination. Therefore, as for the low-index facets ({100}, {111} and {110}) of an anisotropic faceted Cu2O crystal (such as a truncated octahedral structure), the {111} facet possesses more positive valence band energy, prompting the transfer of photogenerated holes to the {100} facet and {110} facet.32,38 The enhanced photogenerated charge separation/transport efficiency results in the improved photocatalytic performance of truncated octahedral Cu2O motors and good nanomotor motility (Fig. 1a). This experimentally demonstrates that truncated octahedral Cu2O nanomotors with high crystallinity and co-exposed {111} and {100} facets exhibit enhanced propulsion speed that is twice more than that of spherical motors. This may be ascribed to the better charge separation between different active crystal facets (Fig. 1b and c). It should be noted that, even without any rigorous surface modification, Cu2O nanomotors with truncated octahedral structure could perform self-propulsion at a speed of ∼5.3 μm s−1 in pure water, under weak ambient light triggering. This is approximately three times faster than the speed of visible-light-driven fuel-free BiOI-Au Janus micromotors.40 In addition, most reported single-component Cu2O micro/nanomotors merely display weak Brownian motion, even in efficient chemical fuels and under intensive visible-light irradiation, because of their low photocatalytic efficiency.23,25 Furthermore, these previously reported Cu2O colloids are all almost spherical, which greatly suppresses the exposure of photoactive facets. It is also confirmed in our system that, regardless of the fuel used, the Brownian motion of spheroidal Cu2O micromotors is observed (Video S1, ESI†). Conversely, truncated octahedral Cu2O nanomotors exhibit vigorous self-propulsion in pure water as expected. Moreover, the speed of such motors reaches ∼10.8 μm s−1 in 0.01 v% H2O2 solution, which is almost twice the speed of the spherical type (Fig. 1d). The band structure and simple cubic structure of the Cu2O crystal is illustrated in Fig. 1e; the unit cell contains four Cu atoms in a linear coordination and two O atoms at the tetrahedral sites. Charge recombination is inevitable in parallel with the charge transfer process. As shown in Fig. S4 (ESI†), truncated octahedral Cu2O exhibits a relatively lower energy band gap (∼1.64 eV) than the spherical particles (∼1.74 eV). This demonstrates that Cu2O nanomotors with a truncated octahedral structure have higher efficiency in terms of light utilization, expressing better motion performance under the same conditions. To further confirm the conjecture of the surface heterojunction structure enhancing the separation rate of photogenerated charges and the self-propulsion of motors, the motion behaviors of cubic and octahedral Cu2O micro/nanomotors were explored. Cubic and octahedral Cu2O particles with {100} and {111} facets, respectively, were fabricated by controlling the dosage of PVP 0.1 g and 1.95 g. The corresponding SEM and EDX images are provided in Fig. S5a–f (ESI†). Additionally, these motors also present self-propulsion behaviors in pure water and 0.01% H2O2 under ambient light exposure. The velocity of the cubic and octahedral motors can reach approximately 6.8 μm s−1 and 7.7 μm s−1, respectively (Fig. S5g, ESI†). However, due to the absence of a surface heterojunction structure, the speed of such motors is lower than the truncated octahedral type.
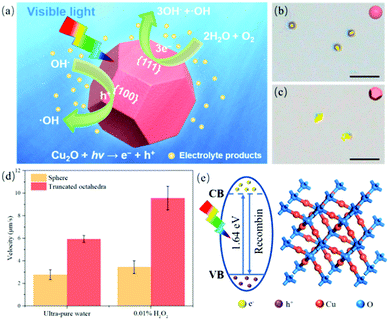 | ||
| Fig. 1 (a) Schematic of the propulsion mechanism of a truncated octahedral Cu2O nanomotor in pure water under visible light irradiation. (b and c) Travel trajectories (taken from Video S1, ESI†) of spherical and truncated octahedral Cu2O motors over 3 seconds, respectively. Scale bar: 5 μm. (d) Velocity of truncated octahedral and spheroidal Cu2O motors in ultra-pure water and 0.01 v% H2O2 solution under ambient light exposure. (e) Schematic of the band gap structure of truncated octahedral Cu2O nanomotors. | ||
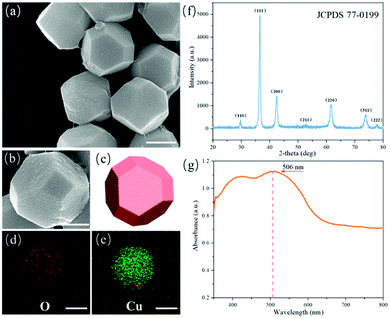
The detailed morphology and crystalline structure of the truncated octahedral Cu2O nanomotors are disclosed in Fig. 2. Well-defined crystalline facets can be found in Fig. 2a and b, and a three-dimensional model (Fig. 2c) further illustrates the special truncated octahedral structure with 6 tetragonal and 8 hexagonal planes. The elemental composition of these Cu2O nanomotors was confirmed, as shown in Fig. 2d and e. Meanwhile, the XRD pattern in Fig. 2f demonstrates that the typical diffraction peaks nearly match JCDPS card no. 77-0199 of a pure cubic Cu2O phase. Furthermore, Fig. 2g reveals that the Cu2O nanomotors have an absorption characteristic peak at about 506 nm, which is the wavelength of blue-green visible light.
Structural stability is of significance for efficient photocatalysts, critically determining their functions and applications. For example, TiO2 can convert from metastable anatase or non-crystalline to the stable rutile phase after calcination at a specific temperature. Based on this temperature-induced phase transition, a novel anatase/rutile TiO2 micromotor with excellent motility was successfully accomplished, just by controlling the calcination temperature.22 Generally, colloids with poor stability might exhibit reduced activity or even undergo a phase transition when they are exposed to changes in temperature. As a typical metastable phase, the structural characteristics (including morphology, phase component and exposed crystal facets) of a Cu2O crystal may change under high temperature and high pressure. In particular, in an aerobic environment with high temperature, additional redox reactions of Cu2O could occur, generating by-products of copper (Cu) and cupric oxide (CuO).41,42 In order to evaluate structural stability, the truncated octahedral Cu2O nanomotors were subjected to different heat treatments by changing the sintering temperature and operating time under an argon atmosphere. As shown in Fig. S6 (ESI†), truncated octahedral Cu2O nanomotors exhibit a favorable structural stability in high-temperature systems under an argon atmosphere. Unlike the previously reported phase transformations of Cu2O films and nanoparticles after annealing in air,41,42 the sintering temperature and heating duration time under an argon atmosphere have no effect on the morphology or crystal structure of the Cu2O nanomotors (Fig. S6a–c, ESI†). Meanwhile, the structural stability is also supported by the light-induced motion behaviors of the nanomotors after different heat treatment regimes. No noticeable propulsion speed change is found for the truncated octahedral Cu2O nanomotors in pure water (Fig. S6d and e, ESI†).
Attributed to selective adsorption of the {111} planes of Cu2O with PVP, ideal Cu2O nanomotors with truncated octahedral structures should be enclosed by eight hexagonal {111} facets and six square {100} facets.37,38 To further substantiate crystal structure differences in the Cu2O motors, the microstructures were examined using TEM characterization. Remarkably, sharp edges and regular shapes can be clearly observed in the TEM images of the truncated octahedral Cu2O nanomotors (Fig. 3a and b). Comparatively, spherical Cu2O micromotors exhibit irregular shapes with rough edges (Fig. S7a and b, ESI†). Fig. 3c and d illustrate the SAED patterns of truncated octahedral Cu2O nanomotors with the electron beam parallel to the [001] and [01—1] direction, respectively. The corresponding high-resolution TEM images reveal resolved lattice fringes of (110) (0.30 nm), (200) (0.21 nm) and (111) (0.24 nm) lattice planes of Cu2O (Fig. 3e and f), confirming the single crystal structure of the truncated octahedral Cu2O nanomotors and the co-existence of the {100} and {111} facets. However, the ring-like SAED patterns and high-resolution TEM images in Fig. S7c–f (ESI†) indicate that the spherical Cu2O micromotors are polycrystalline with low crystallinity, resulting in a poor crystal structure and inferior photocatalytic activity.
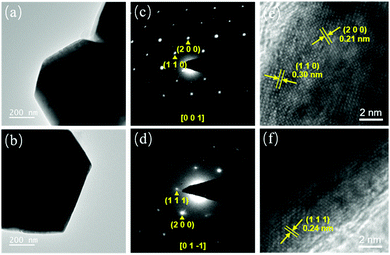 | ||
| Fig. 3 Detailed morphology and crystal structure of truncated octahedral Cu2O nanomotors. TEM images (a and b), SAED patterns (c and d), and high-resolution TEM images (e and f). | ||
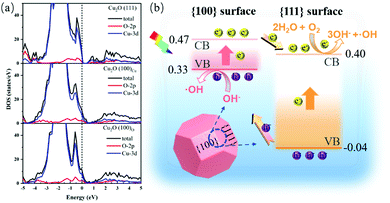
The electron transfer properties of different crystal facets were further supported by DFT calculations. As can be seen in Fig. 4a, the Fermi levels of the Cu2O{100}Cu and Cu2O{100}O facets enter their valence band, while the Fermi level of the Cu2O{111} facet is located at the top of the valence band of the Cu2O{111} facet. Because the {100} and {111} facets contact each other in Cu2O nanomotors, their Fermi levels should be equal. Therefore, the {100} and {111} surfaces can form a surface heterojunction, as illustrated in Fig. 4b, which is beneficial to the transfer and separation of photogenerated electrons and holes. In addition, the work functions of the Cu2O{111} plane, the Cu2O{100}Cu plane terminated with copper and the Cu2O{100}O plane terminated with oxygen in Table S2 and Fig. S8 (ESI†) show that the work functions of the Cu2O{100}Cu (6.64 eV) and Cu2O{100}O (6.45 eV) facets are larger than that of the Cu2O{111} (4.98 eV) facet. So the Cu2O{111} facet is more likely to photogenerate electrons than Cu2O{100}Cu/O. Simultaneously, the electrons of the Cu2O{100}Cu/O facet will migrate to the Cu2O{111} facet in surface-heterojunction Cu2O nanomotors. Therefore, the photogenerated electrons and holes migrate to the Cu2O{111} and Cu2O{100}Cu/O facets during the photocatalytic process, respectively. Meanwhile, the corresponding oxidation reactions occur on Cu2O{100}Cu/O surfaces, while reduction reactions occur on Cu2O{111} surfaces, which is in accordance with a previous report.33 Other calculation information can be found in Tables S1, S3 and Fig. S9 (ESI†).
Furthermore, the surface-heterojunction-induced propulsion behaviors of the truncated octahedral Cu2O nanomotors were evaluated in different environments. Whether in pure water or other fuels, the truncated octahedral nanomotors exhibit good motility under visible light sources (ambient, blue and green light) (Fig. 5). Expectedly, the motion becomes more intense with increasing H2O2 concentration under just weak ambient light, with the velocity reaching ∼9.1 μm s−1 in 0.001 v% H2O2 solution and ∼10.8 μm s−1 in 0.01 v% H2O2 solution (Fig. 5a). The trajectories of the motors in pure water and 0.01 v% H2O2 (taken from Videos S2 and S3, ESI†) under visible-light irradiation also demonstrate the intensive movement (Fig. 5b and Fig. S10, ESI†). Notably, because Cu2O is readily oxidized to CuO in the presence of a high concentration of H2O2, such nanomotors stop moving immediately when the H2O2 concentration rises to 0.1 v%. In addition, according to the existence of the 506 nm absorption peak, the fast movement of truncated octahedral Cu2O nanomotors indicates an enhanced light response under blue and green light stimulation. In 0.0001 v% H2O2 solution, the motor speed reaches a maximum of ∼11.0 μm s−1 under green light, which is much faster than corresponding nanomotors under ambient light. In addition, these nanomotors display a similar diffusiophoretic propulsion behavior in biofuels (glucose and tannic acid) under the same light sources, with speeds of up to ∼9.2 μm s−1 in 30 mM glucose solution and ∼9.8 μm s−1 in 0.2 mM tannic acid solution (Fig. S11a, ESI†). The corresponding effective diffusion (Deff) coefficient of the Cu2O nanomotors is shown in Fig. S11b (ESI†), obtained from the following cacluations:43,44
| D = kBT/(6πηR) | (2) |
| τR = kBT/(8πηR3) | (3) |
| Deff = D + ν2τR/4 | (4) |
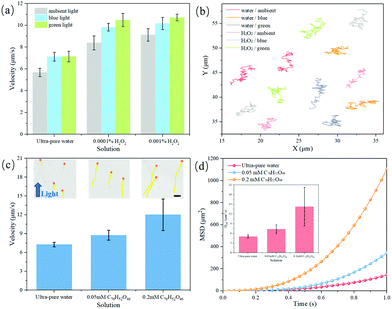 | ||
| Fig. 5 Propulsion behavior of truncated octahedral Cu2O nanomotors. (a) Velocity of truncated octahedral Cu2O nanomotors under different light sources (ambient light, 4 W, blue light, 103 W, green light, 103 W) in ultra-pure water and low-concentration H2O2 solution. (b) Trajectory of truncated octahedral Cu2O nanomotors under different light sources for 3 s in ultra-pure water and 0.01 v% H2O2 solution (taken from Video S2 and S3, ESI†). (c) Velocity of truncated octahedral Cu2O nanomotors under 1.8 W cm−2 blue light point source in different solutions; the insets are the trajectories of negative phototaxis behavior of Cu2O nanomotors for 3 s (taken from Video S4, ESI†). Scale bar: 10 μm. (d) The corresponding MSD and effective diffusion coefficient of Cu2O nanomotors in ultra-pure water and tannic acid solution under 1.8 W cm−2 blue light point source. | ||
Conclusions
In summary, we have proposed a novel surface-heterojunction-induced propulsion methodology for Cu2O nanomotors, which exhibit autonomous and continuous movement in various biocompatible environments under visible light irradiation (ambient, blue and green light). The excellent photocatalytic propulsion of such nanomotors is based on their unique truncated octahedral structures. Compared to a spheroid, the polyhedral Cu2O crystal with better crystallinity and more exposed controllable-index facets exhibits more vigorous movement under visible light due to the faster and better separation efficiency of photogenerated electron–hole pairs, because of the formation of a surface heterojunction between the {100} and {111} facets. In addition, the light-driven motility of the truncated octahedral nanomotors is demonstrated to be more effective not only in chemical fuels such as H2O2, but also in biofuels such as pure water, glucose and tannic acid. Additionally, the motors exhibit effective negative phototactic behavior when exposed to a blue light point source. Significantly, this is the first time that the propulsion performance of visible-light-driven micro/nanomotors has been enhanced just by tailoring the crystal structure within a single colloid. This research might pave the way for the synthesis of micro/nanomotors with high photoactivity.Experimental section
Chemicals
Anhydrous copper(II) sulfate (CuSO4; 99%) was purchased from Shanghai Xinbao Fine Chemical Factory, while poly(vinylpyrrolidone) (PVP-K30; 95%) was obtained from Beijing Solarbio Science & Technology Co., Ltd. Anhydrous sodium carbonate (NaCO3; 99.8%) was supplied by Shanghai Lingfeng Chemical Regent Co., Ltd. Sodium citrate (C6H5Na3O7·2H2O; 99%) was acquired from Shanghai Jiuyi Chemical Regents Co., Ltd. D-(+)-Glucose (C6H12O6·H2O) and hydrogen peroxide (H2O2; 30%) (Cat.7722-84-1) were purchased from Sinopharm Chemical Regents Co., Ltd. Tannic acid (C76H52O46) was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Sterile ultra-pure water was purchased from Beijing Leagene biotech Co., Ltd. All chemicals were used as received without further purification. Distilled water (18.3 MΩ) was used for all solution preparations.Cu2O nanomotor synthesis
The synthesis of Cu2O nanomotors was based on previous reports.37 18 mL water, 108.5 mg CuSO4 and 1.5 g PVP were firstly added into a glass beaker. After continuously stirring until the mixed powders were dissolved completely (about 15–20 min), 1 mL of 1.2 M NaCO3 and 0.74 M sodium citrate mixture solution were dropped slowly to provide a slightly alkaline pH. The solution changed to deep blue without any apparent precipitation. 10 min later, this was followed by the slow addition of 1 mL of 1.4 M glucose solution. Afterwards, the obtained clear solution was placed in a 70 °C water bath for 2 h and then left to cool down to room temperature. The brick-red precipitate was collected and washed by centrifugation with sterile ultra-pure water five times, and dried eventually at 60 °C in a vacuum to obtain the pure Cu2O nanomotors. Cu2O colloids with other structures were obtained by varying the dosage of PVP.Heat treatment
The heat treatment experiments were carried out in a tube furnace (KTL1600 Dia80*1000) with temperature control under an argon atmosphere, to determine the effect of sintering temperature on the structure of Cu2O nanomotors. 150 mg of the Cu2O nanomotors were placed in five quartz crucibles, each containing one-fifth of the amount. Then, the samples were transferred to a furnace with a heating rate of 10 °C min−1 from room temperature to 100 °C, 150 °C, 200 °C, 250 °C and 300 °C, respectively. After calcination had continued for 30 min, the samples were naturally cooled to room temperature in the furnace. The whole process was completed in an argon atmosphere. The effect of sintering time on the structure of the Cu2O nanomotors was also investigated. The process was similar to the method described above, and calcination was kept at 200 °C for 1 h, 2 h or 3 h.Characterization
The morphologies and elemental distributions of the Cu2O nanomotors were attained on a field emission scanning electron microscope (SEM) (JSM-7600F), equipped with an Oxford X-MAX energy dispersive X-ray spectrometer (EDX). X-ray diffraction (XRD) patterns were recorded by an X-ray diffractometer (Bruker D8 Advance) with a Cu Kα X-ray source at a scanning rate of 10° min−1. The detailed transmission electron microscopy (TEM) and selected area electron diffraction (SAED) characterization were performed using a Jeol Transmission Electron Microscope (JEM-3200FS). The UV-vis absorption spectrum was obtained on a Shimadzu 3101 spectrophotometer and BaSO4 was used as the reflectance sample.Motion observation
A 2 μL aqueous suspension of the Cu2O nanomotors was dropped onto a glass substrate, followed by the addition of 2 μL of different concentrations of H2O2 solution (0.02 v%, 0.002 v% and 0.0002 v%). The visible light source was generated by 103W Mercury lamp sockets and a dichroic mirror. Barrier filters (U-FBW and U-FGW) were used to generate blue light and green light. The ambient light was generated from a 4 W LED light equipped in the microscope. The optical images and videos of the nanomotors under visible light irradiation were obtained using an inverted optical microscope (CKX53, Olympus Instrument Inc., Tokyo, Japan) coupled with a 40× microscope objective and a DP74 digital camera (Olympus Instrument Inc., Tokyo, Japan). In addition, the motion behaviors of the nanomotors in ultra-pure water, glucose solution (10 mM and 60 mM) and tannic acid (0.1 mM and 0.4 mM) were also investigated. Meanwhile, the blue light was generated by a point light source (1.8 W cm−2, 460 nm, Shenzhen Lamplic Science Co. Ltd) for the controllable motion of the nanomotors. All the motion videos of the nanomotors were analyzed using Video Spot Tracker V08.01 software.Computational methods
All the computations were performed with the Vienna Ab initio simulation package (VASP), which is based on density functional theory.45,46 The exchange–correlation interaction uses the general gradient approximation (GGA) formulated by Perdew–Burke–Ernzerhof (PBE).46 All electron interactions were described with projector augmented wave (PAW) pseudo potentials. An 11 × 11 × 1 k-point mesh was used for the interaction of the Brillouin-zone. The cutoff energy for the plane wave basis set was restricted to 400 eV, and a vacuum region of at least 15 Å was used in building the slab models. The two bottom layers of the Cu2O slab were fixed to their bulk lattice positions, whereas the three top layers were allowed to fully relax during the calculation, and the convergence threshold was set as 10−6 eV in energy and 0.01 eV Å−1 in force.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51975278), the Natural Science Foundation of Jiangsu Province (BK20181292), the Nanjing Tech University Supported Program, the PAPD-A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Research Fund of State Key Laboratory of Mechanics and Control of Mechanical Structures (Nanjing University of Aeronautics and Astronautics) (Grant No. MCMS-I-0318Y01) and the Open fund project of innovation, entrepreneurship and practice of Jiangsu Province (No. 201910291026Z). All authors declare no conflicts of interest. The authors acknowledge Suqian Advanced Materials Industry Technology Innovation Center of Nanjing Tech University for support.References
- B. Xu, B. Zhang, L. Wang, G. Huang and Y. Mei, Adv. Funct. Mater., 2018, 28, 1705872 CrossRef.
- A. M. Pourrahimi and M. Pumera, Nanoscale, 2018, 10, 16398–16415 RSC.
- T. Maric, M. Z. M. Nasir, R. D. Webster and M. Pumera, Adv. Funct. Mater., 2020, 30, 1908614 CrossRef CAS.
- Y. Wu, T. Si, C. Gao, M. Yang and Q. He, J. Am. Chem. Soc., 2018, 140, 11902–11905 CrossRef CAS.
- B. Dai, J. Wang, Z. Xiong, X. Zhan, W. Dai, C. Li, S. Feng and J. Tang, Nat. Nanotechnol., 2016, 11, 1087–1092 CrossRef CAS.
- Y. Ying, A. M. Pourrahimi, C. L. Manzanares-Palenzuela, F. Novotny, Z. Sofer and M. Pumera, Small, 2020, 16, 1902944 CrossRef CAS.
- S. Tang, F. Zhang, J. Zhao, W. Talaat, F. Soto, E. Karshalev, C. Chen, Z. Hu, X. Lu, J. Li, Z. Lin, H. Dong, X. Zhang, A. Nourhani and J. Wang, Adv. Funct. Mater., 2019, 29, 1809003 CrossRef.
- Z. Wu, T. Li, J. Li, W. Gao, T. Xu, C. Christianson, W. Gao, M. Galarnyk, Q. He, L. Zhang and J. Wang, ACS Nano, 2014, 8, 12041–12048 CrossRef CAS.
- C. Gao, Z. Lin, D. Wang, Z. Wu, H. Xie and Q. He, ACS Appl. Mater. Interfaces, 2019, 11, 23392–23400 CrossRef CAS.
- E. Karshalev, B. Esteban-Fernández de Ávila and J. Wang, J. Am. Chem. Soc., 2018, 140, 3810–3832 CrossRef CAS.
- J. Wang, Z. Xiong, J. Zheng, X. Zhan and J. Tang, Acc. Chem. Res., 2018, 51, 1957–1965 CrossRef CAS.
- L. Bouffier, V. Ravaine, N. Sojic and A. Kuhn, Curr. Opin. Colloid Interface Sci., 2016, 21, 57–64 CrossRef CAS.
- Z. Lin, X. Fan, M. Sun, C. Gao, Q. He and H. Xie, ACS Nano, 2018, 12, 2539–2545 CrossRef CAS.
- Z. Li, L. Bai, C. Zhou, X. Yan, L. Mair, A. Zhang, L. Zhang and W. Wang, Part. Part. Syst. Charact., 2017, 34, 1600277 CrossRef.
- J. G. S. Moo, C. C. Mayorga-Martinez, H. Wang, W. Z. Teo, B. H. Tan, T. D. Luong, S. R. Gonzalez-Avila, C. Ohl and M. Pumera, Adv. Funct. Mater., 2018, 28, 1702618 CrossRef.
- M. Xuan, Z. Wu, J. Shao, L. Dai, T. Si and Q. He, J. Am. Chem. Soc., 2016, 138, 6492–6497 CrossRef CAS.
- W. Liu, X. Chen, X. Lu, J. Wang, Y. Zhang and Z. Gu, Adv. Funct. Mater., 2020, 30, 2003195 CrossRef CAS.
- J. Parmar, D. Vilela, K. Villa, J. Wang and S. Sánchez, J. Am. Chem. Soc., 2018, 140, 9317–9331 CrossRef CAS.
- C. Chen, E. Karshalev, J. Guan and J. Wang, Small, 2018, 14, 1704252 CrossRef.
- G. Hwang, A. J. Paula, E. E. Hunter, Y. Liu, A. Babeer, B. Karabucak, K. Stebe, V. Kumar, E. Steager and H. Koo, Sci. Rob., 2019, 4, eaaw2388 CrossRef.
- J. Wang, R. Dong, H. Wu, Y. Cai and B. Ren, Nano-Micro Lett., 2020, 12, 1–19 CrossRef.
- J. Zhang, F. Mou, Z. Wu, S. Tang, H. Xie, M. You, X. Liang, L. Xu and J. Guan, ACS Appl. Mater. Interfaces, 2019, 11, 16639–16646 CrossRef CAS.
- D. Zhou, Y. C. Li, P. Xu, N. S. McCool, L. Li, W. Wang and T. E. Mallouk, Nanoscale, 2017, 9, 75–78 RSC.
- É. O'Neel-Judy, D. Nicholls, J. Castañeda and J. G. Gibbs, Small, 2018, 14, 1801860 CrossRef.
- Q. Wang, R. Dong, C. Wang, S. Xu, D. Chen, Y. Liang, B. Ren, W. Gao and Y. Cai, ACS Appl. Mater. Interfaces, 2019, 11, 6201–6207 CrossRef CAS.
- J. Wang, H. Wu, X. Liu, Q. Liang, Z. Bi, Z. Wang, Y. Cai and R. Dong, Adv. Intell. Syst., 2020, 2, 1900159 CrossRef.
- Q. Wang, R. Dong, Q. Yang, J. Wang, S. Xu and Y. Cai, Nanoscale Horiz., 2020, 5, 325–330 RSC.
- S. Sun, X. Zhang, Q. Yang, S. Liang, X. Zhang and Z. Yang, Prog. Mater. Sci., 2018, 96, 111–173 CrossRef CAS.
- Y. Shang and L. Guo, Adv. Sci., 2015, 2, 1500140 CrossRef.
- W. Huang, L. Lyu, Y. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261–1267 CrossRef CAS.
- H. Zhu, Y. Li and X. Jiang, J. Alloys Compd., 2019, 772, 447–459 CrossRef CAS.
- C. Y. Toe, J. Scott, R. Amal and Y. H. Ng, J. Photochem. Photobiol., C, 2019, 40, 191–211 CrossRef CAS.
- Z. Zheng, B. Huang, Z. Wang, M. Guo, X. Qin, X. Zhang, P. Wang and Y. Dai, J. Phys. Chem. C, 2009, 113, 14448–14453 CrossRef CAS.
- Z. Quan, C. Li, X. Zhang, J. Yang, P. Yang, C. Zhang and J. Lin, Cryst. Growth Des., 2008, 8, 2384–2392 CrossRef CAS.
- A. S. Barnard, Acc. Chem. Res., 2012, 45, 1688–1697 CrossRef CAS.
- H. Li, J. Tian, F. Xiao, R. Huang, S. Gao, F. Cui, S. Wang and X. Duan, J. Hazard. Mater., 2020, 385, 121518 CrossRef CAS.
- Y. Sui, W. Fu, H. Yang, Y. Zeng, Y. Zhang, Q. Zhao, Y. Li, X. Zhou, Y. Leng, M. Li and G. Zou, Cryst. Growth Des., 2010, 10, 99–108 CrossRef CAS.
- S. Lee, C. Liang and L. W. Martin, ACS Nano, 2011, 5, 3736–3743 CrossRef CAS.
- L. Zhang, J. Shi, M. Liu, D. Jing and L. Guo, Chem. Commun., 2014, 50, 192–194 RSC.
- R. Dong, Y. Hu, Y. Wu, W. Gao, B. Ren, Q. Wang and Y. Cai, J. Am. Chem. Soc., 2017, 139, 1722–1725 CrossRef CAS.
- M. Balık, V. Bulut and I. Y. Erdogan, Int. J. Hydrogen Energy, 2019, 44, 18744–18755 CrossRef.
- Y. Zhu, J. Ma, J. Su, L. Zhou, M. Jiang and X. Zhu, Nanotechnology, 2019, 30, 335711 CrossRef CAS.
- J. R. Howse, R. A. L. Jones, A. J. Ryan, T. Gough, R. Vafabakhsh and R. Golestanian, Phys. Rev. Lett., 2007, 99, 048102 CrossRef.
- A. M. Pourrahimi, K. Villa, C. L. M. Palenzuela, Y. Ying, Z. Sofer and M. Pumera, Adv. Funct. Mater., 2019, 29, 1808678 CrossRef.
- E. Sanville, S. D. Kenny, R. Smith and G. Henkelman, J. Comput. Chem., 2007, 28, 899–908 CrossRef CAS.
- X. Liu, L. Fu, N. Liu, T. Gao, Y. Zhang, L. Liao and Z. Liu, J. Phys. Chem. C, 2011, 115, 11976–11982 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nh00663g |
| This journal is © The Royal Society of Chemistry 2021 |