 Open Access Article
Open Access ArticleRecent advances in peptide-based nanomaterials for targeting hypoxia
Jun
Wang†
 *,
Jing
Liu†
and
Zhongxing
Yang
*,
Jing
Liu†
and
Zhongxing
Yang
School of Pharmacy, Jining Medical University, Rizhao, 276800, China
First published on 3rd September 2021
Abstract
Hypoxia is a prominent feature of many severe diseases such as malignant tumors, ischemic strokes, and rheumatoid arthritis. The lack of oxygen has a paramount impact on angiogenesis, invasion, metastasis, and chemotherapy resistance. The potential of hypoxia as a therapeutic target has been increasingly recognized over the last decade. In order to treat these disease states, peptides have been extensively investigated due to their advantages in safety, target specificity, and tumor penetrability. Peptides can overcome difficulties such as low drug/energy delivery efficiency, hypoxia-induced drug resistance, and tumor nonspecificity. There are three main strategies for targeting hypoxia through peptide-based nanomaterials: (i) using peptide ligands to target cellular environments unique to hypoxic conditions, such as cell surface receptors that are upregulated in cells under hypoxic conditions, (ii) utilizing peptide linkers sensitive to the hypoxic microenvironment that can be cleaved to release therapeutic or diagnostic payloads, and (iii) a combination of the above where targeting peptides will localize the system to a hypoxic environment for it to be selectively cleaved to release its payload, forming a dual-targeting system. This review focuses on recent developments in the design and construction of novel peptide-based hypoxia-targeting nanomaterials, followed by their mechanisms and potential applications in diagnosis and treatment of hypoxic diseases. In addition, we address challenges and prospects of how peptide-based hypoxia-targeting nanomaterials can achieve a wider range of clinical applications.
1. Introduction
Hypoxia is one of the most prominent features of solid tumors.1 The uncontrolled growth and proliferation of tumors consume both nutrition and oxygen, resulting in both an unmet demand for oxygen and compromised delivery of oxygen to other parts of the body. The proliferation of tumor cells stimulates the generation of blood vessels; however, these vessels are abnormal in terms of both their macro- and micro-structure, leading to challenges in regulating the delivery of oxygen.2 The tumor hypoxic microenvironment directly induces healthy cell apoptosis or necrosis and promotes tumor cell progression by upregulating related pathways including growth-factor signalling, tissue infiltration, metastasis, and genomic instability.3 Hypoxia is related to the degree of malignancy, invasion, and prognosis of a tumor, as well as resistance to radiotherapy and chemotherapy, with higher degrees of hypoxia in tumors decreasing therapeutic response.4,5 For some anticancer drugs, oxygen increases the cytotoxicity of the cell damage they cause. Therefore, hypoxia has become the main target of cancer treatment in tumor progression as well as a significant challenge to overcome in the development of new therapies that do not result in resistance to treatment.6Peptides are short oligomers formed by amino acid units connected via amide bonds.7 They are important components of proteins and the causal agent of biological structure and function. Owing to their good compatibility with tissues, cells, and other biological components, peptides are incredibly biocompatible and biodegradable, increasing their advantages for biomedical applications.8 The ability to change the amino acid side chains allows for precise tuning of the secondary and tertiary structures of peptides. This modification can lead to increased cell penetration, increased payload retention, or self-assembling functionality. These secondary structures, which include α-helices and β-sheets, can also cause interactions between peptide chains.9 The interactions of secondary structures can lead to peptides forming nanostructures, such as nanofibers and micelles, which allows for increased cell penetration and large surface area to enable conjugation of the drug and imaging agent. In addition, the formation of these peptides can be triggered under certain conditions, allowing for flexibility and control. Peptide-based materials have been developed as unique and promising tools for the treatment of diseases. They have a variety of activities, including drug delivery, sensing, cell targeting, deep penetration of tissues, and immune responses to enhance anti-tumor treatment effects.10–12 By combining several functionalities such as responsive cleavage sites, cellular targeting, endocytosis transporters, and therapeutic activities, peptides can also be used as components of functionalized composite materials.13–15
There are three main ways through which these peptides have been used in the treatment of hypoxia-related diseases. The first is through utilizing a targeting ligand for therapy, usually involving targeting a specific cell, tissue, or microenvironment for the peptides' intended effect, with a majority of these strategies being where the peptide itself is the targeting ligand.16,17 These strategies usually involve a cytotoxic small molecular peptide that has a method of selecting the target over non-target cells, and will either induce cytotoxic effects or provide protection from harmful external stimuli, such as hypoxia. The second method involves utilization of hypoxic sensitive linkers that can allow hypoxia triggered delivery of therapeutic or imaging payloads. The pathological environment of hypoxia increases reductive stress, leading to overexpression of different bio-reductases including nitroreductase (NTR), azoreductase (AZR), and quinone reductase.18,19 Under hypoxic conditions, the reducible functional groups, including nitro, azo, and quinone, located on small molecules present in the cellular environment can accept electrons and be reduced.20 With the reduction of these hypoxia-responsive moieties, the physicochemical properties and functions of the nanosystem can be changed, such as particle size, fluorescence, and hydrophilicity. Utilizing this information, significant improvements in nanotechnology have occurred by incorporating hypoxia-sensitive moieties, such as nitroimidazole (NI),21 nitrobenzyl alcohol (NA),22,23 and azobenzene (Azo)24 derivatives to achieve the response to bio-reductase in hypoxic tissues.25 Peptides can be conjugated to either imaging agents or therapeutics through the use of stimuli-responsive elements that will allow release of payloads in a specific environment into the targeted tissue. The third possible method where peptides are used involves a combination of the above strategies.26 This involves a targeting motif of some type, allowing fewer non-target effects to occur, improving the effective dose, and enabling more complete imaging of hard to image areas. Peptides are ideal in these three strategies as they can be highly specific, making them ideal targeting agents, while also being biodegradable, allowing for clearance or recycling from the body.
In this review, we will focus on novel peptide-based functional nanomaterials containing hypoxia-responsive functional groups as well as hypoxia-targeting nanomaterials. We will explore how these groups can be further utilized with peptides in order to increase biocompatibility, lower the toxicity, and improve the effectiveness of existing imaging and cytotoxic drugs. In addition, we will discuss the various ways in which these peptides can act alone to produce their intended effects. Using multiple models of peptides including small molecular peptides, self-assembling peptides, polypeptides, peptide–polymer conjugates, and peptides functionalized to either liposomes or polysaccharides, we hope to illustrate the ways through which these systems can overcome common difficulties inherent to hypoxic diseases. Here, a series of peptide-based hypoxia-targeting nanomaterials and their targeting strategies are listed in Table 1.
| Hypoxia-targeting strategies | Hypoxia-responsive motif | Peptide agent | Structures of nanomaterial/loaded agents | Results under hypoxic conditions | Ref. |
|---|---|---|---|---|---|
| Hypoxia-responsive motif | NI | Poly-glutamic acid | Nanoparticles/DOX | Showed faster release | 32 |
| Poly-aspartic acid | Micelles/(Dox + Ce6) | Enhanced chemotherapy and PDT efficacy | 34 | ||
| Poly-aspartic acid | Vesicles/insulin | Enhanced glucose responsive insulin delivery | 35 | ||
| Poly-glutamide | Nanoparticles/siRNA | Silencing hypoxia-correlated pro-tumorigenic gene and significant suppression of tumor growth | 36 | ||
| NA | Poly-lysine | Micelles/DOX | Enhanced tumor penetration and improved anti-tumor efficacy | 37 | |
| Poly-glutamate | Nanoparticles/DOX | Enhanced DOX release and superior tumor cell-killing ability | 38 | ||
| Azo | Poly-aspartic acid | Micelles/cytochrome C | Showed great killing effect on HepG2 liver cancer cells | 39 | |
| Poly-aspartic acid | Micelles/Ce6 | Enhanced antitumor PDT efficacy | 42 | ||
| NP | Poly-lysine&AVPI-NP-C12 | Nanofibers | Released a pro-apoptotic AVPI peptide | 52 | |
| Cy7 | Surfactin | Nanoparticles/gambogic acid | Enhanced tumor localization, excellent biodistribution, and superior therapeutic efficacy | 54 | |
| Hypoxia-responsive motif and peptide ligands | Azo | iRGD | Polymersomes/gemcitabine | Enhanced ability to target, penetrate and deliver drugs | 68 and 86 |
| Azo | iRGD | Nanoparticles/DOX | Significantly diminished tumor growth | 69 | |
| Azo | TAT peptide | Nanoparticles/Ce6+TPZ | Enhanced PDT and bioreductive chemotherapy efficiency | 71 | |
| Azo | CRGDK | Nanoparticles/IR-780 + PFOB | Alleviated hypoxia and improved PDT efficiency | 72 | |
| Peptide ligands | (D)-Phe-(D)-Phe-(D)-Lys-OH | Nanofibers/DOX | Sensitized tumors to DOX administration and expedited conventional chemotherapy | 59 | |
| CCGNKRTRGC | Nanoparticles/radiolabeled with 131I | Improved local hypoxia and significantly inhibited tumor growth and metastasis | 73 | ||
| iVR1 peptide | Nanoparticles/salidroside + apatinib | Showed stronger anti-tumor effect | 74 | ||
| cRGD | Liposomes/Ce6 + TPZ + ICG | Enhanced the tumor therapeutic effect by the combination of PTT, PDT and chemotherapy | 87 | ||
| iRGD | Nanoparticles/TPZ + ICG | Effectively inhibited primary tumor growth and metastasis | 88 | ||
| GGGGDRVYIHPF | Liposomes/BDP-NO2 | Could be used for real-time imaging of hypoxia levels of myocardial ischemia | 93 | ||
| Cyclopeptide RA-V | Liposomes/RX-0047+ anti-DR5 | Enhance the chemotherapy efficacy and achieved therapeutic self-monitoring | 94 | ||
| YPHIDSLGHWRR | Hydrogel | Enhanced cell survival, proliferation, and migration and improved cardiac repair | 95 | ||
| TAT peptide | Nanoparticles/siRNAs | Effectively inhibited tumor growth and angiogenesis | 105 | ||
| RGD | Nanoparticles/HIF-1α-AA | Promoted angiogenesis and improved the recovery of nerve function | 107 |
2. Polypeptides
Polypeptides are a kind of biomedical polymer with α-amino acid structural units.27 Compared with traditional synthetic polymers, polypeptides allow for control of polarity and charge and also show stability against hydrolysis, are easily synthesized, and are biodegradable by enzymes in vivo. These protein-mimicking polymers inherit many properties of proteins, such as biocompatibility, versatility, bioactivity, and hierarchical assembly properties.28–30 In addition, the combination of polypeptides and synthetic polymers can enhance solubility, processability, and antifouling properties, which greatly broadens the application of biological materials.31 Introducing a hypoxia-responsive moiety (such as NI, Azo, or NA) into the side chain of the polypeptide can enhance the micellization, encapsulation of hydrophobic drugs, and hypoxia responsiveness due to its hydrophobic effect, resulting in selective drug release at the hypoxic regions.Many polypeptides containing hypoxia-responsive NI motifs have been reported in drug delivery and tumor therapy.32–34 For example, Yu et al. developed novel hypoxia and H2O2 dual-sensitive polymersome-based vesicles (d-GRPs) for enhanced glucose-responsive insulin delivery. The vesicles were self-assembled by a diblock copolymer consisting of PEG and NI-modified polyserine linked by thioether (PEG-poly(Ser-S-NI)), which could encapsulate recombinant human insulin and glucose oxidase. In the process of the enzymatic conversion of glucose to gluconic acid, a local hypoxic environment was generated quickly, which promoted the biological reduction of NI to hydrophilic 2-aminoimidazoles. Meanwhile, the thioether moiety in the polymer eliminated the excess H2O2 and promoted the disassembly of vesicles, releasing the encapsulated insulin. The glucose-responsive polymersomes could be further integrated with a painless microneedle-array patch platform for insulin delivery (Fig. 1).35 Shi and co-workers developed a hypoxia-responsive nanoparticle consisting of NI-modified polypeptide methoxy poly(ethylene glycol)-block-poly(L-glutamide-graft-2-nitroimidazole) (mPEG-b-(PLG-g-NI)) and a cationic lipid-like compound, used to deliver siRNA to silence cell division cycle 20 gene (CDC20) expression in breast cancer cells. The delivery nanosystem showed prolonged blood circulation, high tumor accumulation, efficient CDC20 silencing, and high potent antitumor efficacy.36
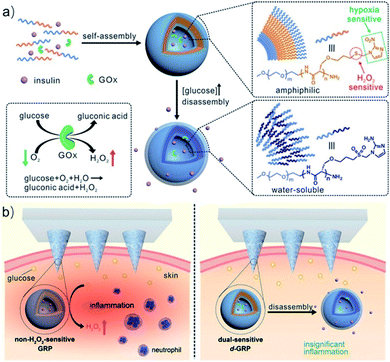 | ||
| Fig. 1 Schematic of the glucose-responsive insulin delivery system using hypoxia and H2O2 dual-sensitive polymersome-based vesicle (d-GRP) loaded microneedle-array patches. (a) Formation and mechanism of d-GRPs consisting of PEG-poly(Ser-S-NI). (b) Schematic of local inflammation induced by a non-H2O2-sensitive GRP-loaded microneedle-array patch, and schematic of a d-GRP-loaded microneedle-array patch for in vivo insulin delivery triggered by a hyperglycemic state for potential prevention of the long-term side effect associated with inflammation. Reproduced with permission from ref. 35, copyright © 2017, American Chemical Society. | ||
Hypoxia-responsive polypeptides based on nitrobenzyl alcohol derivatives (NADs) are commonly found in the literature. One example of these, investigated by Park and co-workers, involved preparing hypoxia-responsive polymeric micelles formed by an amphiphilic block copolymer, poly(ethylene glycol)-poly(ε-(4-nitro)benzyloxycarbonyl-L-lysine) (PEG-b-PLys-g-NBCF).22 The copolymer can self-assemble into micelles and encapsulate doxorubicin (DOX) under aqueous conditions. Under hypoxic conditions, the DOX-loaded micelles tend to be highly unstable and easily degraded by a 1,6-elimination reaction, resulting in rapid intracellular release of DOX. Similarly, the Shi group developed a complex micelle containing an NA motif which was prepared by co-assembly of poly(ε-caprolactone)-block-poly(ethylene glycol) (PCL-b-PEG) and poly(ε-caprolactone)-block-poly(L-lysine)-graft-4-nitrobenzyl chloroformate (PCL-b-PLL-g-NBCF). The micelle design includes a PCL core and a hypoxia-responsive shell (NBCF-modified PLL and PEG). This core–shell structure can inhibit rapid removal by the immune system in the process of blood circulation. Once these micelles have reached the tumor site, the NBCF-modified PLL can degrade under the hypoxic microenvironment, leading to the increase of positively charged PLL on the surface of the micelles, enabling the penetration of the micelles into the tumor. In addition, in vitro and in vivo experiments show that these DOX-loaded micelles have better penetration ability and inhibitory effects on tumor tissues.37 In another example, Zhang et al. synthesized a hypoxia-responsive polymer, mPEG-PLG-NC, by conjugating hydrophobic 4-nitrobenzyl (3-azidopropyl) carbamate (AP-NC) to the side chains of methoxy poly(ethylene glycol)-b-poly(γ-propargyl-L-glutamate) (mPEG-PPLG) copolymers (Fig. 2). The polymer self-assembled into nanoparticles in aqueous solution and could load DOX with a high encapsulation efficiency of 97.8% due to the strong π–π interaction between the p-nitrobenzyl group and DOX. The DOX-loaded nanoparticles (PPGN@DOX) showed hypoxia-responsive drug release behavior in vitro. PPGN@DOX can be effectively internalized by 4T1 cells and release DOX into the cell nucleus in a hypoxic environment. In addition, since PPGN@DOX provided a longer circulation time and improved the biodistribution of DOX, there were increased antitumor outcomes and reduced side effects in vivo.38 A further study with an NA modified hypoxia-sensitive polypeptide was carried out by Sun et al. who prepared polymeric micelles that were self-assembled by an amphiphilic polymer methoxy PEG-block-poly(diethylenetriamine-graft-4-nitrobenzyl chloroformate)-L-glutamate [mPEG-b-P(Deta-NBCF)LG] for the targeted delivery of metalloprotein cytochrome c (CC) to cancer cells. These CC-loaded micelles are effectively taken up by cancer cells and greatly enhance the cytotoxicity of CC to cancer cells under hypoxic conditions.39
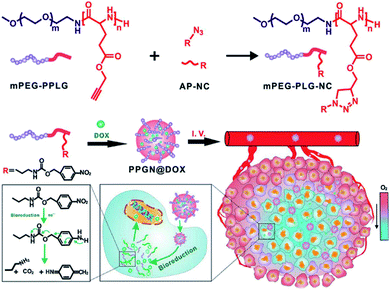 | ||
| Fig. 2 Schematic of the synthesis of mPEG-PLG-NC and preparation of PPGN@DOX for hypoxia-responsive drug delivery in vivo. Reproduced with permission from ref. 38, copyright © 2020 American Chemical Society. | ||
Most nanocarriers consist of a steric PEG protective layer to maintain the stability of the system for protein adsorption.40 However, the PEG layer usually hinders the cellular uptake of nanocarriers, resulting in the loss of photodynamic therapy (PDT) efficacy.41 Designing a sheddable PEG (dePEGylation) is an effective means of balancing system stability and cellular uptake of nanocarriers. For this purpose, Li et al. reported hypoxia and singlet oxygen dually responsive multifunctional micelles to enhance antitumor PDT. The micelles were obtained by self-assembly of the amphiphilic methoxy poly(ethylene glycol)-azobenzene-poly(aspartic acid) copolymer conjugated with imidazole (IM) side chains (mPEG-Azo-PAsp-IM). Azo and IM are hypoxic and singlet oxygen-responsive (SR), respectively. Chlorin e6 (Ce6) could be efficiently loaded by the micelles as a model photosensitizer. The promotion of cellular uptake was achieved by triggering the collapse of Azo to cause the shedding of PEG. The oxidation of imidazole by the singlet oxygen decomposed the micelles and led to release of Ce6. In addition, singlet oxygen-mediated cargo release consumes oxygen and increases the degree of hypoxia, which in turn further improves cellular uptake of micelles (Fig. 3).42
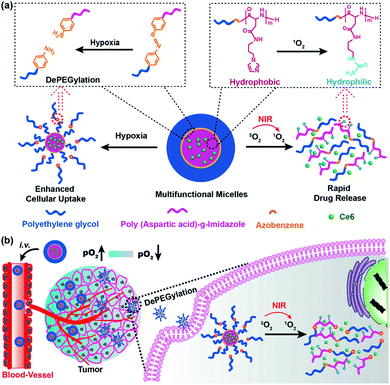 | ||
| Fig. 3 Illustration of the interactively hypoxia- and singlet oxygen-sensitive tailor-made micelles for improved photodynamic antitumor therapy; (a) the hypoxia-responsive Azo linker was used to connect the hydrophilic PEG block and hydrophobic polypeptide block, producing a self-assembling amphiphilic copolymer; Ce6 was the model photosensitizer; the Azo and imidazole moieties are hypoxia- and SR moieties, respectively; (b) upon micelles reaching the tumor, the hypoxia-sensitivity would enable the DePEGylation and enhanced cellular uptake of micelles, while the singlet oxygen-responsiveness could lead to micelle disassembly and rapid Ce6 release. Reproduced with permission from ref. 42, copyright © 2018, American Chemical Society. | ||
Hypoxia-responsive amphiphilic block copolymers formed by polypeptides could assemble into nanocarriers with different morphologies, which were used for the controlled release of drugs under hypoxic conditions. Due to the passive targeting effect, these nanocarriers showed high tumor accumulation, better penetration ability, and improved inhibition efficacy on tumor tissues. Therefore, these polypeptide-based hypoxia-targeting nanosystems present a promising and advanced strategy for precision cancer therapy.
3. Self-assembled peptides
Self-assembled peptides can spontaneously construct a series of well-defined supramolecular nanostructures, such as nanotubes, nanofibers, vesicles, and hydrogels.43 Non-covalent interactions including hydrogen bonding, van der Waals interaction, electrostatic interaction, and hydrophobic effects maintain the self-assembled structure in a stable low-energy state.44 The self-assembly of peptides can be effectively regulated by inherent or external stimuli such as pH, temperature, ionic strength, metal ion complexation, and enzymatic reactions.45–47 Based on the characteristics of the individual self-assembled peptide, these nanostructures can have diverse applications in biomedicine, including drug delivery, tissue engineering, and regenerative medicine.48–50The introduction of hypoxia-responsive motifs or targeting peptide ligands into the self-assembled peptide nanostructure can be used for the diagnosis and treatment of hypoxic diseases. An example is a study performed by Ikeda et al. who explored pro-apoptotic peptide amphiphiles that could form self-assembled nanostructures.51 The peptide amphiphile AVPI-NP-C12 was composed of a pro-apoptotic AVPI tetrapeptide connected with a hydrophobic dodecyl chain through a nitrophenyl (NP) linker. In the presence of poly-L-lysine, AVPI-NP-C12 formed self-assembled nanostructures comprising entanglements of nanofibers. Mechanistically, the proposed theory for why this occurs is due to the electrostatic interaction between cations and the carboxylate anion of AVPI-NP-C12. Upon the addition of sodium dithionite, a reducing agent used to mimic the hypoxic environment, the NP motif can be reduced, leading to the decomposition of AVPI-NP-C12 and the release of a pro-apoptotic peptide. Apoptotic peptides can enhance the activity of anticancer drugs by inhibiting apoptosis protein inhibitors;52 therefore, the nanostructures of pro-apoptotic self-assembling peptides have potential applications in nanomedicine for encapsulation and delivery of drugs, and sensing of hypoxic environments.
The charges of peptides can greatly affect the performance of the assembled nanostructures in nanomedicine. A negative charge can protect the nanostructures from being cleared before reaching the tumor tissue. However, due to the negative potential of the cytomembrane, the nanostructures are difficult to be engulfed by target cells.53 Based on this concept, Wang et al. explored a selective-release “mosaic-type” nanoparticle, GA-Cy7-NP, for targeting hypoxic cancer cells. These nanoparticles were prepared by the self-assembly of surfactin, in addition to a single conjugate of heptamethine carbocyanine dye (Cy7) and gambogic acid (GA) in an aqueous solution. In this system, surfactin is used as a carrier platform, with Cy7 and GA being used as a hypoxia target group and antitumor drug, respectively. The Cy7 group is embedded in the surface of negatively charged nanoparticles formed by a surfactant, which enables these nanoparticles to selectively release the drug conjugates into hypoxic cancer cells without internalization of particles. GA-Cy7-NP also showed long-term circulation characteristics and high cell uptake in tumor cells. Compared with the prototype drug, it has stronger antitumor activity in cell proliferation, tumor growth, and angiogenesis.54 Another example of using self-assembled peptides as drug delivery systems is the work of Zhu and co-workers who prepared an angiogenesis vessel-targeting nanoparticle (AVT-NP) containing photosensitizer 5-(4-carboxyphenyl)-10,15,20-trisphenylchlorin (TPC), angiogenesis vessel-targeting cyclopeptide (peptide sequence: CGNSNPKSC), and bio-reductive drug TPZ. Upon exposure to irradiation, the photosensitizer can generate large amounts of reactive oxygen species (ROS), which will reduce TPZ to form cytotoxic free radicals. In vivo experiments demonstrated that AVT-NP can specifically accumulate around tumor cells through the targeting of angiogenesis vessels. The system also exhibited enhanced antitumor efficiency with the chemo–photo synergistic effect.55
The intracellular self-assembly of nanomaterials shows a high retention rate and low cytotoxicity, which has great application potential in long-term tumor biological imaging.14 Based on the strategies of intracellular transglutaminase (TG2) mediated catalytic polymerization and in situ self-assembly of polymerized elastin-like polypeptides (ELPs), Li and co-workers reported a peptide-based probe for imaging hypoxic neuroblastoma cells. In this system, the polymeric monomer peptides are composed of N- and C-terminal amino acid polymeric active sites (e.g., Q and K) and elastin repeating units (XGVGP or GYGXP). To optimize TG2-catalyzed polymerization into ELPs, many parameters, namely peptide sequences, heat sensitivity, and the upper critical solution temperature (UCST), are studied to fine-tune the system for the maximum effect. In cells that overexpress TG2 (such as HeLa), intramolecular polymerization and self-assembly can enhance retention efficiency and intracellular accumulation. Due to the up-regulation of TG2 expression under hypoxia, FITC-labelled peptide probes can selectively image hypoxic neuroblastoma cells for diagnostic work (Fig. 4).56
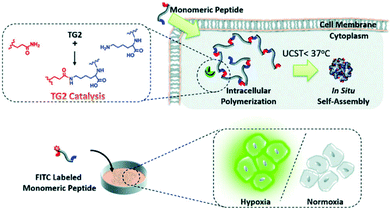 | ||
| Fig. 4 Schematic illustration of specific transglutaminase 2 (TG2)-catalyzed intracellular polymerization, temperature induced in situ self-assembly and hypoxic neuroblastoma cell imaging. Reproduced with permission from ref. 56, copyright © 2020, Royal Society of Chemistry. | ||
Self-assembling peptides can be designed to form tunable supramolecular nanostructures triggered by targeted cellular environments.57 These pericellular nanostructures provide effective strategies to change the interaction mechanism of enzymes with cells and promote or reduce their cellular uptakes.58 Recently, Li et al. developed a self-assembling carbonic anhydrase (CA) inhibitor based on self-assembling peptides, which specifically interacted with the overexpressed CA on hypoxic cancer cell membranes. This complex boosted the inhibition and selectivity of the CA inhibitor through the strategy of hypoxia-triggered self-assembly. The self-assembling CA IX inhibitor N-pepABS was synthesized by conjugating a commercially available CA inhibitor, 4-(2-aminoethyl) benzenesulfonamide (ABS), with a self-assembled motif [2-naphthaleneacetic acid-(D)-Phe-(D)-Phe-(D)-Lys-OH (N-pep)]. N-pepABS can target CA IX and self-assemble into nanofibers, which enables CA IX inhibitors to concentrate on the membrane of hypoxic cancer cells, resulting in interruption of hypoxic cancer cells. These CA-triggered nanofibers promote cellular uptake through CA IX-mediated endocytosis. In the process of internalization, nanofibers may transform into larger nanofiber bundles under low pH conditions. The bundles will in turn cause damage to the intracellular acid vesicles and blockage of protective autophagy. These results provide evidence that the cell milieu-triggered tunable nanostructure produces highly selective toxicities to hypoxic cancer cells. Moreover, the antimetastatic and antiangiogenic functions of N-pepABS were evaluated in murine 4T1 breast cancer cells. N-pepABS efficiently decreased tumor volume and reduced the number of lung metastases. Upon the detection of endothelial marker CD31, the intact tumor vessels turned dissociative and were muted after treatment with N-pepABS. In addition, the combination therapy of N-pepABS and doxorubicin (Dox) showed that N-pepABS treatment can effectively make tumors sensitive to Dox administration and significantly enhance anti-tumor efficacy (Fig. 5).59
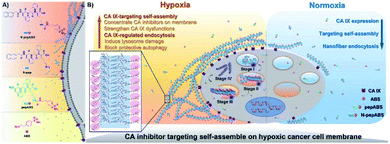 | ||
| Fig. 5 Molecular design of self-assembled CA IX inhibitors (A) and their hypoxic cancer cell-targeted self-assembly (B). Adapted with permission from ref. 59, copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. | ||
Based on the inherent biocompatibility and biodegradability, the bioinspired nanostructures originating from self-assembled peptides extraordinarily benefit tumor therapy. The versatility of tunable nanostructures responding to the cell milieu impressively provides strategic therapy for hypoxic tumors. In addition, self-assembly in vivo may be a potential nanotechnology in biomimetic structure fabrication in vivo in the future. Using peptide-based monomers for intracellular catalytic polymerization and assembly may provide a new strategy for hypoxic cancer therapy.
4. Peptide–polymer conjugates
Peptide–polymer conjugates are a kind of soft material formed by the covalent linkage of peptides and polymers. In these structures, polymer conjugation imparts processability to the peptides under different conditions (i.e., solvent, temperature, and pressure), while the peptide imparts precision (e.g., sequence control and low dispersity) to the peptide–polymer conjugates.60 Due to their biocompatibility, biodegradability and mechanical strength, many polymers such as polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic acid glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL) combined with PEG have been widely used in peptide–polymer conjugate studies.61,62 In addition, when tumor-targeting peptides are conjugated with these polymers, the affinity for the receptor, cell internalization, and tissue penetration ability can be enhanced.63–65 Based on these characteristics, materials composed of peptide–polymer conjugates have a wide range of applications in biomedical sciences, such as drug delivery, tumor therapy, gene delivery, and antibacterial coatings.66,67Recently there have been increasing studies on tumor hypoxia therapy utilizing peptide–polymer conjugates. One such design is centered on hypoxia-reactive motifs. An example of such a system is the work by Mallik and co-workers who developed tissue-penetrating, hypoxia-responsive polymersomes that could deliver the anticancer drug gemcitabine to solid tumors. The polymersomes are composed of a hypoxia-responsive Azo incorporated polylactic acid–polyethylene glycol (PLA–PEG) polymer and a tissue penetrating peptide iRGD (peptide sequence: CRGDKGPDC) functionalized PLA–PEG polymer. Drug-encapsulated polymersomes greatly reduced the viability of pancreatic cancer cells68 and triple-negative breast cancer cells69 in spherical cultures and successfully released the encapsulated contents in vitro and in vivo under hypoxic conditions. More research in this area by Ma et al. developed a novel AZR responsive nanoprobe (Micelle@Mito-rHP@TATp, MCM@TATp) for specific imaging of mitophagy in living cells under hypoxia by encapsulating mitochondria-targeted rhodamine spirolactam derivatives (Mito-rHP). The micelle MCM was formed by self-assembling of a hypoxia-responsive amphiphilic polymer, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-Azo-N-[maleimide(polyethylene glycol)2000] (DSPE-Azo-PEG-Mal), in aqueous solution and simultaneously encapsulating a rhodamine spirolactam derivative (Mito-rHP). Then MCM was functionalized by a cell-penetrating peptide (TATp, RKKRRQRRRC), which ensured that the nanoprobe could be transported into the cytoplasm in a receptor-independent manner, thus avoiding the restriction of the nanoprobe in the endosome or lysosome. Under hypoxic conditions, the nanoprobe was disrupted by the highly expressed AZR. The encapsulated probe Mito-rHP was then released and able to target mitochondria. Because Mito-rHP is also pH-sensitive, it is converted into a protonated form during mitophagy, thus stimulating a fluorescent signal to switch from “off” to “on”.70 Another study with the Azo motif was carried out by Jiang and co-workers who developed a hypoxia-responsive drug delivery system for combined PDT and bioreductive chemotherapy. There, sensitive amphiphilic polymer monomethoxy PEG-azobenzene-PLGA (PEG-Azo-PLGA) was first synthesized and conjugated with TAT peptide (peptide sequence: YGRKKRRQRRRC-NH2) and 2,3-dimethylmaleic anhydride (DA) successively to obtain the polymer DATAT–PEG–PLGA. After that nanoparticles TAT + AzoNPs were prepared by PEG-Azo-PLGA and DATAT-PEG-PLGA. Due to the TAT peptide attached on the surface, TAT + AzoNPs can keep the cargoes C6e and TPZ and accumulate within tumor cells. Under laser irradiation, and with sufficient oxygen supply, TAT + AzoNPs achieved effective PDT on tumor cells proximal to vessels. Oxygen consumption during PDT further generated a hypoxic microenvironment, which could trigger the release of TPZ by breakage of the azobenzene bond, and accelerated the activation of TPZ, thereby improving the efficacy of combined treatment in tumor cells distal to the vessel (Fig. 6).71
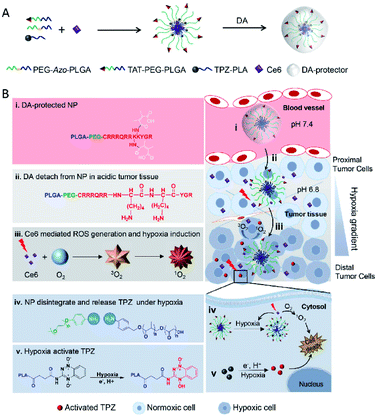 | ||
| Fig. 6 Schematic illustration of tumoral pH activatable and hypoxia responsive NPs (TAT + AzoNPs) for enhancing PDT-induced hypoxia activated chemotherapy. (A) Fabrication of tumoral pH activatable and hypoxia responsive NPs. (B) Proposed mechanism of TAT + AzoNPs. TAT + AzoNPs followed multiple steps to meet ideal anticancer therapy. Reproduced with permission from ref. 71, copyright © 2020, Elsevier Ltd. | ||
Recently, Liu et al. reported reactive oxygen species (ROS) sensitive arylboronic ester-based nanocarriers modified with red blood cell membrane and iRGD peptide. These nanocarriers can be utilized for co-encapsulation of Ce6 and TPZ to achieve tumor specific release and synergistic PDT.65 Similarly, Zhao et al. developed deep penetrating and oxygen self-sufficient PDT nanoparticles based on peptide–polymer conjugates, which were used to balance the distribution of ROS in tumors. These nanoparticles (CNP/IP) are prepared by the co-assembly of peptide (peptide sequence: CRGDK)–PEG–PCL and PEG–PCL and simultaneously encapsulating a photosensitizer, IR780, and an artificial blood substitute, perfluorooctyl bromide (PFOB), with higher oxygen storage capacity. Modification of CRGDK peptide on the nanoparticles greatly promoted the accumulation and penetration of the encapsulated IR780 and PFOB into the interior of the tumor. In hypoxic regions, PFOB released oxygen, effectively alleviating hypoxia and enhancing PDT efficacy.72
In another study, Song et al. developed a multifunctional platform composed of 131I-labeled dendrimers modified with a LyP-1 (peptide sequence: CCGNKRTRGC (C2–C10)) peptide for targeted antitumor and antimetastasis therapy.73 The nanosystem was prepared by generation 5 (G5) poly(amidoamine) dendrimers conjugated with PEG-LyP-1 and 3-(4′-hydroxyphenyl)propionic acid-OSu (HPAO), and then the remaining dendrimers were acetylated and radiolabeled with 131I. The 131I-labeled LyP-1-modified dendrimers had promising biocompatibility and could be utilized as a diagnostic probe for single-photon emission computed tomography (SPECT) imaging. Meanwhile, the LyP-1 peptide could recognize tumor cells in hypoxic regions and relieve hypoxia, in addition to being used as a carrier for anti-tumor and anti-metastatic therapy. The nanotheranostic system showed specific targeting in vitro and in tumor sites and could significantly inhibit tumor growth and metastasis in vivo, further providing evidence of its possible usefulness in therapy (Fig. 7).
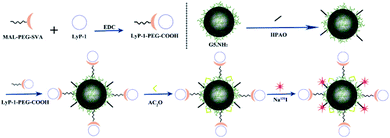 | ||
| Fig. 7 Schematic diagram of the formation of G5.NHAc-HPAO-131I-(PEG-LyP-1). Reproduced with permission from ref. 73. Copyright © 2020, American Chemical Society. | ||
A hypoxic microenvironment in solid tumors usually significantly reduces the chemosensitivity of cancer cells. Recent work by Zhang et al. developed a novel combined treatment strategy that can combat the drug resistance of gastric cancer in vivo and in vitro.74 Salidroside (Sal) and apatite (Apa) were co-loaded by PLGA nanoparticles (NPs) to enhance the chemotherapy effect of apatinib on gastric cancer. To improve the drug delivery efficacy, the tumor recognizable peptide iVR1 was further modified on the NPs-Apa/Sal. iVR1 peptide can specifically target the vascular endothelial growth factor receptor 1 (VEGFR1) and inhibit angiogenesis and progression of certain cancers by selectively antagonizing VEGFR1.75 The peptide-modified iVR1-NPs-Apa/Sal displayed excellent capability of tumor targeting drug delivery and showed an effective inhibitory effect on cell growth, invasion, and migration, as well as tumor progression in vivo. A mechanistic study showed that the enhancement of the chemotherapeutic effect of Apa was likely due to Sal inducing cell apoptosis and reprograming the hypoxic tumor micro-environment.
In this section, nanocarriers formed by polymers modified with peptide ligands greatly facilitated the accumulation and penetration of the encapsulated contents into both the tumor periphery and hypoxic tumor inner regions. Such a strategy may have great potential in inhibiting tumor growth and metastasis, and could also have possible application in alleviating the hypoxia-induced chemotherapy resistance.
5. Peptide functionalized liposomes
Liposomes are phospholipid vesicles with a lipid bilayer structure and an inner aqueous compartment formed by self-assembly of amphiphilic molecules in water.76 These structures can not only encapsulate the hydrophilic compound in their core, but also encapsulate the hydrophobic compound in the bilayer.77 Due to their biocompatibility, self-assembly capacity, and the ability to direct passive targeting by enhancing the permeability and retention in the tumor area, liposomes are promising systems for drug and gene delivery, ophthalmology, vaccines, imaging, and cosmetics.78–80 Liposomes modified with a targeting peptide increase the targeting specificity and transfection efficiency in cells, and make nanocomplexes have better biocompatibility.81,82 These peptide-liposomal systems targeting hypoxia for drug delivery,83 gene delivery,84 and cancer therapy85 have been explored. Kulkarni et al. prepared liposomes composed of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), a hypoxia-responsive PEGylated lipid palmitoyl oleoylphosphatidylethanolamine (POPE)-Azo-PEG, and an iRGD peptide coupled lipid DSPE-PEG-iRGD. The iRGD peptide on the surface allowed liposomes to penetrate deeper and deliver the anticancer drug to the hypoxic cores. Under hypoxic conditions, the Azo moieties of the hypoxia-responsive lipids were reduced, which destroyed the stability of the lipid membrane and released the encapsulated drug from liposomes, resulting in increased cytotoxicity of the cultured pancreatic cancer cell.86Liposome-based multiple responsive and multiple functional nanoplatforms for hypoxic tumor therapy have been reported. One such report by Dai et al. involved the preparation of a liposome via self-assembly of an amphiphilic molecule (mPEG-Ce6-C18), lecithin, and DSPE-PEG-cRGD (peptide sequence: RGD-D-FK). The liposome can encapsulate indocyanine green (ICG) and hypoxia-activated prodrug tirapazamine (TPZ), followed by chelation with GdIII to form a multifunctional theranostic liposome, ICG/TPZ@Ce6-GdIII. These multifunctional liposomes can be used not only as a multimodal imaging contrast agent but also as a PTT (photothermal therapy)–PDT–chemotherapy cascade activated antitumor agent. cRGD targeting and photothermally activated PDT can effectively minimize side effects on normal cells. Meanwhile, TPZ can enhance the therapeutic effect allowing this novel nanosystem to achieve the selective combination treatment of tumors (Fig. 8).87 A similar synergetic mechanism was utilized by Wang et al. to achieve hypoxia-activated chemotherapy combined with PDT against metastatic breast cancer by simultaneously delivering ICG and TPZ to solid tumors through iRGD-modified liposomes.88 In another study, You and co-workers proposed a dual endoplasmic reticulum (ER) targeting strategy to achieve PDT–PTT–immunotherapy. The nanosystem was composed of modified ER-targeting padaxin (FAL) peptides, ICG conjugated hollow gold nanospheres (FAL-ICG-HAuNs), and oxygen carrying HB liposomes (FAL-HB-lipo) for reversing hypoxia. Under NIR light irradiation, the ER-targeting nanosystem can induce strong ER stress and calreticulin (CRT) exposure on the cell surface. CRT exposure is a signal that stimulates the maturation of naive dendritic cells and induces an enhanced immune response, including CD8+ T cell proliferation and cytotoxic cytokine secretion. ER-targeting PDT–PTT promotes immunotherapy associated with immunogenic cell death through direct ROS-based ER stress and exhibits enhanced antitumor efficacy.89
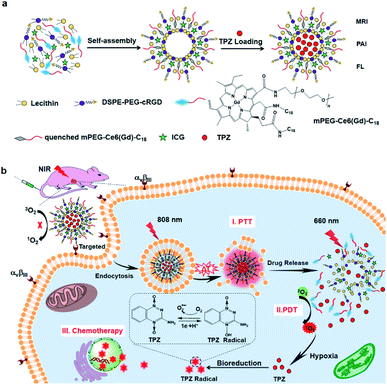 | ||
| Fig. 8 Synthesis of ITC-GdIII TLs and NIR-triggered cascade-activated combination therapy. (a) Synthesis of ITC-GdIII TLs by self-assembly; (b) cascade-activated combination therapy for improved lung tumor therapeutic efficacy triggered by NIR light. Reproduced with permission from ref. 87. Copyright © 2019, American Chemical Society. | ||
Utilizing the overexpression of several reductases under hypoxic conditions, many reductase fluorescent probes have been developed and successfully used for imaging the hypoxia status of tumor cells.90–92 Recently, Fan et al. developed a liposome-based nanoprobe functionalized with a peptide (GGGGDRVYIHPF) to target cardiac cells and encapsulate them with nitrobenzene substituted BODIPY (BDP-NO2) for myocardial hypoxia imaging. Due to the peptide (GGGGDRVYIHPF), the system can specifically target the angiotensin II type 1 (AT1) receptor overexpressed on ischemic heart cells. The nanoprobe is taken up by the cardiac cells and releases BDP-NO2. Under hypoxic conditions, the nitro group of BDP-NO2 is reduced to BDP-NH2 by NTR, resulting in a fluorescence enhancement of the nanoprobe. Furthermore, the nanoprobe can be used for real-time imaging of hypoxia levels in a mouse model of myocardial ischemia.93 Yao et al. used real-time therapeutic monitoring to explore a combination therapy liposome nanosystem for high-efficiency therapy against hypoxic tumors and real-time imaging during apoptosis. In this study, a pH-sensitive liposome co-encapsulated a chemotherapeutic drug cyclopeptide RA-V (deoxybouvardin) and antisense oligonucleotides (RX-0047), as well as a caspase-8 probe. The obtained liposomes could selectively enter the lysosome of colon cancer cells through receptor-mediated endocytosis, and the lysosomal acidic microenvironment stimulated the liposomes to release the payloads. The released RA-V could induce apoptosis of cancer cells through the mitochondrial pathway, while the antisense oligonucleotides could inhibit the expression of HIF-1α to alleviate tumor hypoxia. Moreover, the liposome can also be used for therapeutic self-monitoring through the fluorescence of the caspase-8 probe (Fig. 9).94
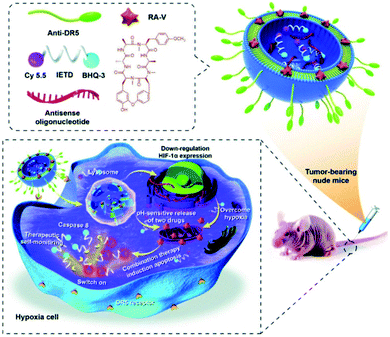 | ||
| Fig. 9 Schematic illustration of co-delivery of cyclopeptide RA-V and antisense oligonucleotide with therapeutic self-monitoring in colon cancer. Reproduced with permission from ref. 94. Copyright 2020, Royal Society of Chemistry. | ||
For a brief summary, nanosystems originating from peptide functionalized liposomes for targeting hypoxia have attracted more and more attention for the synergistic treatment of cancer. The introduction of hypoxia-responsive or other responsive motifs into the liposome structure will provide a new strategy for the design and development of multi-response, multifunctional therapeutic nanoplatforms.
6. Peptide functionalized polysaccharides
Polysaccharides are a kind of natural macromolecular polymer composed of long chains of monosaccharide units linked by glycosidic bonds.95 As natural biomaterials, polysaccharides are generally regarded as water-soluble, safe, non-toxic, biodegradable, and nonimmunogenic, making them widely applicable in biomedicine.96,97 In polysaccharides, hydrophilic functional groups such as the hydroxy group and carboxy group are easy to be chemically and biochemically modified and can also cause bioadhesion with biological tissues (mainly epithelium and mucosa) through non-covalent interactions. These nanocarriers made of bioadhesive polysaccharides can prolong the residence time in the mucosa, thus increasing the bioavailability of drugs. In addition, the surface of polysaccharide nanocarriers could be modified with ligands to actively target drug molecules.98 In recent years, polysaccharide-based nanosystems for hypoxic tumor therapy have been developed,99–102 among which peptide-functionalized polysaccharide nanomaterials have been reported, although research in this area is not as deep. An example is the work of Shu et al. who developed a RoY (peptide sequence: YPHIDSLGHWRR) peptide modified chitosan chloride hydrogel (CSCl-RoY). Using the targeting properties of RoY peptide, CSCl-RoY hydrogel can regulate the expression level of 78 kDa glucose-regulated protein (GRP78) receptor on the membrane of human umbilical vein endothelial cells, then activate protein kinase B (Akt) and extracellular signal-regulated kinase (ERK1/2) signaling pathways related to cell survival/proliferation, thereby enhancing cell survival, proliferation, migration and tube formation under hypoxic conditions. The study also demonstrated that the introduction of Roy peptide can not only induce angiogenesis at the infarct region but also improve cardiac repair.103The CD73 molecule (ecto-5′-nucleotidase) is one of the molecules affected by hypoxia, as well as HIF-1α, which plays a key role in the growth and spread of cancer.104 Ghalamfarsa et al. developed a delivery system for silencing CD73 and HIF-1α genes using superparamagnetic iron oxide (SPION) nanocarriers loaded with siRNA for cancer therapy (Fig. 10). In this nanosystem, siRNA-loaded SPIONs were encapsulated by thiolated chitosan (TC) and trimethyl chitosan (TMC) to improve their stability and functionality. The produced TMC-TC-SPIONs were conjugated with TAT peptide (C (Npys) GRKKRRQRRR) to increase the cellular uptake and form nanoparticles TAT-TMC-TCSPIONs. These nanoparticles could significantly reduce the expression of HIF-1α and CD73 in cancer cells, resulting in a significant reduction in the migration and proliferation of cancer cells. In addition, in vivo evaluation showed that these nanoparticles could effectively inhibit tumor growth and angiogenesis (Fig. 10).105
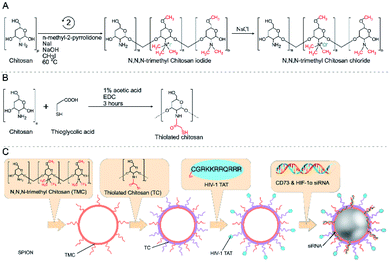 | ||
| Fig. 10 Reaction scheme for the synthesis of trimethyl chitosan and thiolated chitosan (A and B). Production of siRNA-loaded TAT-TMC–TC-SPION NPs (C). Reproduced with permission from ref. 105. Copyright 2020, © 2020 Elsevier B.V. | ||
A hyperbranched cationic amylopectin derivative conjugated with 3-(dimethylamino)-1-propylamine (DMAPA-Amyp) has good blood compatibility and low cytotoxicity, allowing it to be used as an effective gene carrier.106 In a recent study by Deng et al., an RGD (peptide sequence: KYGRGDC)-modified targeted gene nanocarrier, RGD-DMAPA-Amyp, for ischemic stroke treatment was synthesized. The targeting strategy was involved in the selection of RGD peptides, binding them to the nanocarriers and delivering them to the vascular endothelial cells in the peri-infarct region. The HIF-1α mutant form (HIF-1α-AA) was loaded with RGD-DMAPA-Amyp in the treatment of cerebral ischemia. The results demonstrated that this nanocomplex had good biocompatibility and could significantly improve the recovery of nerve function and the formation of new blood vessels in vivo.107
Because many polysaccharides have a high potential for binding to oligonucleotides, nanocarriers formed by peptide functionalized polysaccharides have been used as an effective targeted gene vector into hypoxic cells. These strategies can enhance the efficiency of gene transfection and the therapeutic effect of cancer or ischemic diseases.
7. Conclusion and future perspectives
In summary, utilizing the properties of the peptide system in diagnosis and treatment provides an opportunity to overcome the hurdles hypoxic diseases pose. As outlined, peptides have several advantages, including targeting specificity, tumor penetrability, and high drug delivery efficiency. In this review, we summarized the latest advances in the design and construction of different peptide-based hypoxia-targeting nanomaterials, including polypeptides, self-assembling peptides, and peptide–polymer conjugates, in addition to peptide functionalized liposomes and polysaccharides. The utilization of both peptide ligands and hypoxia-sensitive linkers, either together or apart, is the main strategy for targeting hypoxia. Through this strategy, peptide-based nanomaterials can be made to accumulate in hypoxic regions, target hypoxic cells and successfully deliver the payloads across biological barriers. In addition, these peptide-based nanomaterials can prolong blood circulation, improve permeability into angiogenic vessels and tumor tissues, and promote tumor cell uptake and therapeutic efficacy. Moreover, through intramolecular and intermolecular forces, peptides and their derivatives are easy to self-assemble into various nano-functional materials with different structures. These materials can take advantage of the unique characteristics of the hypoxic microenvironment or cancer cells, thereby improving the specificity of cancer cells. The literature provides strong evidence that the application of these peptide-based nanomaterials could improve the overall treatment efficiency of hypoxic diseases.However, these studies are still in the preliminary stage. There are still several challenges preventing peptide-based nanomaterials from clinical application of hypoxic diseases. Peptides are easily degraded by enzymes and can be taken up by mononuclear phagocytes. Renal filtration also shortens the circulation time of peptides. Therefore, it is necessary to consider the system design and material modification of these peptide-based nanomaterials. More peptide ligands with new sequences need to be explored for specifically and efficiently targeting the hypoxic regions of different hypoxia diseases. Currently, many nanomaterials for medical applications are passively targeting tumor tissues through the enhanced permeability and retention (EPR) effect. Although the EPR effect can increase the enrichment of nanocarriers at the tumor site, poor cellular uptake and internalization limit the efficacy of chemotherapy. Therefore, it is necessary to combine peptides with various nanomaterials to construct a positive targeted multistage or multifunctional nanocomposite system to enhance cellular uptake and internalization, thereby enhancing the synergistic therapeutic effect of various therapies (PDT, photothermal therapy, thermodynamic therapy, chemotherapy, radiotherapy, etc.) under hypoxic conditions. Critically, different hypoxia-related diseases such as tumors, bone tissue inflammation, cardiovascular diseases, and ischemic strokes all have specific hypoxic microenvironments, which requires rationally designed microenvironment-induced peptide-based materials for the treatment of these diseases according to the properties of peptides. In addition, the change in microenvironment in vivo could cause significant changes in the physicochemical properties of nanomaterials. Therefore, the biocompatibility, biodistribution, and in vivo targetability of these peptide-based nanomaterials should be evaluated in detail before clinical trials. We anticipate and look forward to the development of peptide-based hypoxia-targeting nanomaterials, as we believe they will make an important contribution to the progress in the diagnosis and treatment of hypoxia-related diseases in the near future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Teachers' Research of Jining Medical University (No. JYFC2018KJ068), the National Innovation Training Program for College Students (No. 201710443006), the PhD Scientific Research Start-up Fund of Jining Medical University, the Project of Shandong Province Higher Educational Science and Technology Program (No. J18KA093), and the Project of study abroad for excellent Young and middle-aged teachers sponsored by Jining Medical College. We thank Prof. Toshio Masuda and Dr Maocai Yan for assistance with English editing.Notes and references
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2018, 140, 14851–14859 CrossRef CAS PubMed.
- L. Zhao, C. Fu, L. Tan, T. Li, H. Zhong and X. Meng, Nanoscale, 2020, 12, 2855–2874 RSC.
- G. L. Semenza, N. Engl. J. Med., 2011, 365, 537–547 CrossRef CAS PubMed.
- J. M. Brown and W. R. Wilson, Nat. Rev. Cancer, 2004, 4, 437–447 CrossRef CAS PubMed.
- H. Zhou, F. Qin and C. Chen, Adv. Healthcare Mater., 2021, 10, 2001277 CrossRef CAS PubMed.
- W. R. Wilson and M. P. Hay, Nat. Rev. Cancer, 2011, 11, 393–410 CrossRef CAS PubMed.
- A. Dehsorkhi, V. Castelletto and I. W. Hamley, J. Pept. Sci., 2014, 20, 453–467 CrossRef CAS PubMed.
- S. Wong, M. S. Shim and Y. J. Kwon, J. Mater. Chem. B, 2014, 2, 595–615 RSC.
- S. Zhang, Interface Focus, 2017, 7, 20170028 CrossRef PubMed.
- H. Sun, Y. Dong, J. Feijen and Z. Zhong, J. Controlled Release, 2018, 290, 11–27 CrossRef CAS PubMed.
- J. Zhao, Q. Li, X. Hao, X. Ren, J. Guo, Y. Feng and C. Shi, J. Mater. Chem. B, 2017, 5, 8035–8051 RSC.
- J. S. Rudra, T. Sun, K. C. Bird, M. D. Daniels, J. Z. Gasiorowski, A. S. Chong and J. H. Collier, ACS Nano, 2012, 6, 1557–1564 CrossRef CAS PubMed.
- L. Zhang, Y. Huang, A. R. Lindstrom, T. Y. Lin, K. S. Lam and Y. Li, Theranostics, 2019, 9, 7807–7825 CrossRef CAS PubMed.
- D. Zhang, G. B. Qi, Y. X. Zhao, S. L. Qiao, C. Yang and H. Wang, Adv. Mater., 2015, 27, 6125–6130 CrossRef CAS PubMed.
- A. N. Shirazi, D. Oh, R. K. Tiwari, B. Sullivan, A. Gupta, G. D. Bothun and K. Parang, Mol. Pharmaceutics, 2013, 10, 4717–4727 CrossRef PubMed.
- S. Dissanayake, W. A. Denny, S. Gamage and V. Sarojini, J. Controlled Release, 2017, 250, 62–76 CrossRef CAS PubMed.
- Y. Gilad, E. Noy, H. Senderowitz, A. Albeck, M. A. Firer and G. Gellerman, Bioorg. Med. Chem., 2016, 24, 294–303 CrossRef CAS PubMed.
- K. Xu, F. Wang, X. Pan, R. Liu, J. Ma, F. Kong and B. Tang, Chem. Commun., 2013, 49, 2554–2556 RSC.
- S. Xu, Q. Wang, Q. Zhang, L. Zhang, L. Zuo, J. D. Jiang and H. Y. Hu, Chem. Commun., 2017, 53, 11177–11180 RSC.
- Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4563–4601 RSC.
- T. Thambi, V. G. Deepagan, H. Y. Yoon, H. S. Han, S. H. Kim, S. Son, D. G. Jo, C. H. Ahn, Y. D. Suh, K. Kim, I. C. Kwon, D. S. Lee and J. H. Park, Biomaterials, 2014, 35, 1735–1743 CrossRef CAS PubMed.
- T. Thambi, S. Son, D. S. Lee and J. H. Park, Acta Biomater., 2016, 29, 261–270 CrossRef CAS PubMed.
- J. Zheng, Y. Shen, Z. Xu, Z. Yuan, Y. He, C. Wei, M. Er, J. Yin and H. Chen, Biosens. Bioelectron., 2018, 119, 141–148 CrossRef CAS PubMed.
- Y. Zhou, M. Maiti, A. Sharma, M. Won, L. Yu, L. X. Miao, J. Shin, A. Podder, K. N. Bobba, J. Han, S. Bhuniya and J. S. Kim, J. Controlled Release, 2018, 288, 14–22 CrossRef CAS PubMed.
- A. Shah, M. S. Malik, G. S. Khan, E. Nosheen, F. J. Iftikhar, F. A. Khan, S. S. Shukla, M. S. Akhter, H.-B. Kraatz and T. M. Aminabhavi, Chem. Eng. J., 2018, 353, 559–583 CrossRef CAS.
- T. R. Pearce, K. Shroff and E. Kokkoli, Adv. Mater., 2012, 24, 3803–3822 CrossRef CAS PubMed.
- Y. Liu, D. Li, J. X. Ding and X. S. Chen, Chin. Chem. Lett., 2020, 31, 3001–3014 CrossRef CAS.
- Y. Ou, Z. H. Tang, L. Sun, H. Y. Yu, J. Li, M. H. Zhao and H. Xu, Asian J. Pharm. Sci., 2018, 13, 191–196 CrossRef PubMed.
- H. Yu, Z. Tang, D. Zhang, W. Song, Y. Zhang, Y. Yang, Z. Ahmad and X. Chen, J. Controlled Release, 2015, 205, 89–97 CrossRef CAS PubMed.
- C. Deng, J. Wu, R. Cheng, F. Meng, H.-A. Klok and Z. Zhong, Prog. Polym. Sci., 2014, 39, 330–364 CrossRef CAS.
- L. A. Canalle, D. W. Lowik and J. C. van Hest, Chem. Soc. Rev., 2010, 39, 329–353 RSC.
- Z. Ahmad, S. X. Lv, Z. H. Tang, A. Shah and X. S. Chen, J. Biomater. Sci., Polym. Ed., 2016, 27, 40–54 CrossRef CAS PubMed.
- W. Yin, M. Qiang, W. D. Ke, Y. Han, J. F. Mukerabigwi and Z. S. Ge, Biomaterials, 2018, 181, 360–371 CrossRef CAS PubMed.
- J. Deng, F. Liu, L. N. Wang, Y. An, M. Gao, Z. Wang and Y. J. Zhao, Biomater. Sci., 2019, 7, 429–441 RSC.
- J. C. Yu, C. G. Qian, Y. Q. Zhang, Z. Cui, Y. Zhu, Q. D. Shen, F. S. Ligler, J. B. Buse and Z. Gu, Nano Lett., 2017, 17, 733–739 CrossRef CAS PubMed.
- Y. Li, J. Ding, X. Xu, R. Shi, P. E. Saw, J. Wang, S. Chung, W. Li, B. M. Aljaeid, R. J. Lee, W. Tao, L. Teng, O. C. Farokhzad and J. Shi, Nano Lett., 2020, 20, 4857–4863 CrossRef CAS PubMed.
- J. Zhen, S. Tian, Q. Liu, C. Zheng, Z. Zhang, Y. Ding, Y. An, Y. Liu and L. Shi, Biomater. Sci., 2019, 7, 2986–2995 RSC.
- P. Zhang, H. L. Yang, W. Shen, W. G. Liu, L. Chen and C. S. Xiao, ACS Biomater. Sci. Eng., 2020, 6, 2167–2174 CrossRef CAS PubMed.
- X. S. Sun, M.-S. Jang, Y. Fu, J. H. Lee, D. S. Lee, Y. Li and H. Y. Yang, Mater. Sci. Eng., C, 2020, 114, 111069 CrossRef CAS PubMed.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed.
- E. Blanco, H. Shen and M. Ferrari, Nat. Biotechnol., 2015, 33, 941–951 CrossRef CAS PubMed.
- J. J. Li, X. Meng, J. Deng, D. Lu, X. Zhang, Y. R. Chen, J. D. Zhu, A. P. Fan, D. Ding, D. L. Kong, Z. Wang and Y. J. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 17117–17128 CrossRef CAS PubMed.
- L. Zhao, Q. Zou and X. Yan, Bull. Chem. Soc. Jpn., 2019, 92, 70–79 CrossRef CAS.
- S. Lee, T. H. T. Trinh, M. Yoo, J. Shin, H. Lee, J. Kim, E. Hwang, Y.-b. Lim and C. Ryou, Int. J. Mol. Sci., 2019, 20, 5850 CrossRef CAS PubMed.
- Z. Feng, H. Wang, X. Chen and B. Xu, J. Am. Chem. Soc., 2017, 139, 15377–15384 CrossRef CAS PubMed.
- J. Wang, F. Shao, W. Li, J. Yan, K. Liu, P. Tao, T. Masuda and A. Zhang, Chem. –Asian J., 2017, 12, 497–502 CrossRef CAS PubMed.
- S. Yang, D. Xu and H. Dong, J. Mater. Chem. B, 2018, 6, 7179–7184 RSC.
- J. Boekhoven and S. I. Stupp, Adv. Mater., 2014, 26, 1642–1659 CrossRef CAS PubMed.
- S. H. Kim and J. R. Parquette, Nanoscale, 2012, 4, 6940–6947 RSC.
- N. Habibi, N. Kamaly, A. Memic and H. Shafiee, Nano Today, 2016, 11, 41–60 CrossRef CAS PubMed.
- M. Ikeda, M. Kawakami and Y. Kitade, Chem. Lett., 2015, 44, 1137–1139 CrossRef CAS.
- C. R. Arnt, M. V. Chiorean, M. P. Heldebrant, G. J. Gores and S. H. Kaufmann, J. Biol. Chem., 2002, 277, 44236–44243 CrossRef CAS PubMed.
- M. J. Ernsting, M. Murakami, A. Roy and S. D. Li, J. Controlled Release, 2013, 172, 782–794 CrossRef CAS PubMed.
- W. W. Wang, X. Y. Li, Z. H. Wang, J. F. Zhang, X. Dong, Y. Z. Wu, C. Fang, A. W. Zhou and Y. L. Wu, Nanoscale, 2019, 11, 2211–2222 RSC.
- D. Guo, S. Xu, N. Wang, H. Jiang, Y. Huang, X. Jin, B. Xue, C. Zhang and X. Zhu, Biomaterials, 2017, 144, 188–198 CrossRef CAS PubMed.
- B. Peng, X. Zhao, M. S. Yang and L. L. Li, J. Mater. Chem. B, 2019, 7, 5626–5632 RSC.
- H. Wang, Z. Feng and B. Xu, Chem. Soc. Rev., 2017, 46, 2421–2436 RSC.
- T. B. Potocky, A. K. Menon and S. H. Gellman, J. Am. Chem. Soc., 2005, 127, 3686–3687 CrossRef CAS PubMed.
- J. Li, K. Shi, Z. F. Sabet, W. Fu, H. Zhou, S. Xu, T. Liu, M. You, M. Cao, M. Xu, X. Cui, B. Hu, Y. Liu, C. Chen, Z. F. Sabet and C. Chen, Sci. Adv., 2019, 5, eaax0937 CrossRef CAS PubMed.
- P. A. Taylor and A. Jayaraman, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 257–276 CrossRef CAS PubMed.
- L. Milane, Z. Duan and M. Amiji, Mol. Pharmaceutics, 2010, 8, 185–203 CrossRef PubMed.
- H. S. Abyaneh, A. H. Soleimani, M. R. Vakili, R. Soudy, K. Kaur, F. Cuda, A. Tavassoli and A. Lavasanifar, Pharmaceutics, 2018, 10, 196 CrossRef CAS PubMed.
- A. Vasconcelos, E. Vega, Y. Perez, M. J. Gomara, M. L. Garcia and I. Haro, Int. J. Nanomed., 2015, 10, 609–631 Search PubMed.
- T. Teesalu, K. N. Sugahara and E. Ruoslahti, Front. Radiat. Oncol., 2013, 3, 216 Search PubMed.
- H. Liu, W. Jiang, Q. Wang, L. Hang, Y. Wang and Y. Wang, Biomater. Sci., 2019, 7, 3706–3716 RSC.
- N. Dube, J. W. Seo, H. Dong, J. Y. Shu, R. Lund, L. M. Mahakian, K. W. Ferrara and T. Xu, Biomacromolecules, 2014, 15, 2963–2970 CrossRef CAS PubMed.
- H. Sun, Y. Hong, Y. Xi, Y. Zou, J. Gao and J. Du, Biomacromolecules, 2018, 19, 1701–1720 CrossRef CAS PubMed.
- P. Kulkarni, M. K. Haldar, F. Karandish, M. Confeld, R. Hossain, P. Borowicz, K. Gange, L. Xia, K. Sarkar and S. Mallik, Chemistry, 2018, 24, 12490–12494 CrossRef CAS PubMed.
- B. Mamnoon, J. Loganathan, M. I. Confeld, N. De Fonseka, L. Feng, J. Froberg, Y. Choi, D. M. Tuvin, V. Sathish and S. Mallik, ACS Appl. Bio Mater., 2021, 4, 1450–1460 CrossRef CAS PubMed.
- D. Ma, C. Huang, J. Zheng, W. Zhou, J. Tang, W. Chen, J. Li and R. Yang, Anal. Chem., 2019, 91, 1360–1367 CrossRef CAS PubMed.
- K. M. Ihsanullah, B. N. Kumar, Y. Zhao, H. Muhammad, Y. Liu, L. Wang, H. Liu and W. Jiang, Biomaterials, 2020, 245, 119982 CrossRef CAS PubMed.
- C. Y. Zhao, Y. J. Tong, X. L. Li, L. H. Shao, L. Chen, J. Q. Lu, X. W. Deng, X. Wang and Y. Wu, Small, 2018, 14, 1703045 CrossRef PubMed.
- N. Song, L. Zhao, X. Xu, M. Zhu, C. Liu, N. Sun, J. Yang, X. Shi and J. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 12395–12406 CrossRef CAS PubMed.
- Z. Zhang, W. Yang, F. Ma, Q. Ma, B. Zhang, Y. Zhang, Y. Liu, H. Liu and Y. Hua, Drug Delivery, 2020, 27, 691–702 CrossRef CAS PubMed.
- V. Cicatiello, I. Apicella, L. Tudisco, V. Tarallo, L. Formisano, A. Sandomenico, Y. Kim, A. Orlandi, J. Ambati, M. Ruvo, R. Bianco and S. D. Falco, Oncotarget, 2015, 6, 10563–10576 CrossRef PubMed.
- R. Cheng, L. Liu, Y. Xiang, Y. Lu, L. Deng, H. Zhang, H. A. Santos and W. Cui, Biomaterials, 2020, 232, 119706 CrossRef CAS PubMed.
- A. Gonzalez Gomez and Z. Hosseinidoust, ACS Infect. Dis., 2020, 6, 896–908 CrossRef CAS PubMed.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi and K. Nejati-Koshki, Nanoscale Res. Lett., 2013, 8, 102 CrossRef PubMed.
- K. S. Ahmed, S. A. Hussein, A. H. Ali, S. A. Korma, Q. Lipeng and C. Jinghua, J. Drug Targeting, 2019, 27, 742–761 CrossRef CAS PubMed.
- S. Zununi Vahed, R. Salehi, S. Davaran and S. Sharifi, Mater. Sci. Eng., C, 2017, 71, 1327–1341 CrossRef CAS PubMed.
- C. Yu-Wai-Man, A. D. Tagalakis, M. D. Manunta, S. L. Hart and P. T. Khaw, Sci. Rep., 2016, 6, 21881 CrossRef CAS PubMed.
- A. D. Tagalakis, L. He, L. Saraiva, K. T. Gustafsson and S. L. Hart, Biomaterials, 2011, 32, 6302–6315 CrossRef CAS PubMed.
- A. A. Kale and V. P. Torchilin, J. Liposome Res., 2007, 17, 197–203 CrossRef CAS PubMed.
- Y. T. Ko, W. C. Hartner, A. Kale and V. P. Torchilin, Gene Ther., 2009, 16, 52–59 CrossRef CAS PubMed.
- Y. Wang, M. Fu, J. Liu, Y. Yang, Y. Yu, J. Li, W. Pan, L. Fan, G. Li, X. Li and X. Wang, Int. J. Nanomed., 2019, 14, 4071–4090 CrossRef CAS PubMed.
- P. Kulkarni, M. K. Haldar, P. Katti, C. Dawes, S. You, Y. Choi and S. Mallik, Bioconjugate Chem., 2016, 27, 1830–1838 CrossRef CAS PubMed.
- Y. Dai, B. Wang, Z. Sun, J. Cheng, H. Zhao, K. Wu, P. Sun, Q. Shen, M. Li and Q. Fan, ACS Appl. Mater. Interfaces, 2019, 11, 39410–39423 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, J. Li, Z. H. Peng, Y. Sheinin, J. Zhou and D. Oupicky, ACS Nano, 2017, 11, 2227–2238 CrossRef CAS PubMed.
- W. Li, J. Yang, L. Luo, M. Jiang, B. Qin, H. Yin, C. Zhu, X. Yuan, J. Zhang, Z. Luo, Y. Du, Q. Li, Y. Lou, Y. Qiu and J. You, Nat. Commun., 2019, 10, 3349 CrossRef PubMed.
- R. B. P. Elmes, Chem. Commun., 2016, 52, 8935–8956 RSC.
- Q. Yang, S. Wang, D. Li, J. Yuan, J. Xu and S. Shao, Anal. Chim. Acta, 2020, 1103, 202–211 CrossRef CAS PubMed.
- C. Zhu, Z. Zou, C. Huang, J. Zheng, N. Liu, J. Li and R. Yang, Chem. Commun., 2019, 55, 3235–3238 RSC.
- Y. Fan, M. Lu, X. A. Yu, M. He, Y. Zhang, X. N. Ma, J. Kou, B. Y. Yu and J. Tian, Anal. Chem., 2019, 91, 6585–6592 CrossRef CAS PubMed.
- Y. Yao, L. Feng, Z. Wang, H. Chen and N. Tan, Biomater. Sci., 2020, 8, 256–265 RSC.
- J. H. Xie, M. L. Jin, G. A. Morris, X. Q. Zha, H. Q. Chen, Y. Yi, J. E. Li, Z. J. Wang, J. Gao, S. P. Nie, P. Shang and M. Y. Xie, Crit. Rev. Food Sci. Nutr., 2016, 56(1), S60–S84 CrossRef CAS PubMed.
- M. Swierczewska, H. S. Han, K. Kim, J. H. Park and S. Lee, Adv. Drug Delivery Rev., 2016, 99, 70–84 CrossRef CAS PubMed.
- M. Li, F. Lin, Y. Lin and W. Peng, Biochem. Biophys. Res. Commun., 2015, 466, 748–754 CrossRef CAS PubMed.
- B. Laha, S. Maiti, K. K. Sen and S. Jana, in Green Synthesis, Characterization and Applications of Nanoparticles, Elsevier, 2019, pp. 347–368 Search PubMed.
- S. W. Shin, W. Jung, C. Choi, S. Y. Kim, A. Son, H. Kim, N. Lee and H. C. Park, Mar. Drugs, 2018, 16, 510 CrossRef CAS PubMed.
- S. Uthaman, Y. Kim, J. Y. Lee, S. Pillarisetti, K. M. Huh and I. K. Park, ACS Appl. Mater. Interfaces, 2020, 12, 28004–28013 CrossRef CAS PubMed.
- W. Park, B. C. Bae and K. Na, Biomaterials, 2016, 77, 227–234 CrossRef CAS PubMed.
- C. Zhang, Q. Li, C. Wu, J. Wang, M. Su and J. Deng, Nanotechnology, 2021, 32, 095107 CrossRef CAS PubMed.
- Y. Shu, T. Hao, F. Yao, Y. Qian, Y. Wang, B. Yang, J. Li and C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6505–6517 CrossRef CAS PubMed.
- G. Ghalamfarsa, M. H. Kazemi, S. Raoofi Mohseni, A. Masjedi, M. Hojjat-Farsangi, G. Azizi, M. Yousefi and F. Jadidi-Niaragh, Expert Opin. Ther. Targets, 2019, 23, 127–142 CrossRef CAS PubMed.
- F. Hajizadeh, S. Moghadaszadeh Ardebili, M. Baghi Moornani, A. Masjedi, F. Atyabi, M. Kiani, A. Namdar, V. Karpisheh, S. Izadi, B. Baradaran, G. Azizi, G. Ghalamfarsa, G. Sabz, M. Yousefi and F. Jadidi-Niaragh, Eur. J. Pharmacol., 2020, 882, 173235 CrossRef CAS PubMed.
- Y. Zhou, B. Yang, X. Ren, Z. Liu, Z. Deng, L. Chen, Y. Deng, L. M. Zhang and L. Yang, Biomaterials, 2012, 33, 4731–4740 CrossRef CAS PubMed.
- L. Deng, F. Zhang, Y. Wu, J. Luo, X. Mao, L. Long, M. Gou, L. Yang and D. Y. B. Deng, ACS Biomater. Sci. Eng., 2019, 5, 6254–6264 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |
