Open Access Article
Open Access ArticleDesigning a mechanically driven spin-crossover molecular switch via organic embedding†
Sumanta
Bhandary
 *a,
Jan M.
Tomczak
*a,
Jan M.
Tomczak
 b and
Angelo
Valli
b and
Angelo
Valli
 c
c
aSchool of Physics, Trinity College Dublin, The University of Dublin, Dublin 2, Ireland. E-mail: sumanta.bhandary@tcd.ie; Tel: +353-1-896 8455
bInstitute of Solid State Physics, Vienna University of Technology, 1040 Vienna, Austria
cInstitute for Theoretical Physics, Vienna University of Technology, 1040 Vienna, Austria
First published on 26th July 2021
Abstract
Among spin-crossover complexes, Fe–porphyrin (FeP) stands out for molecular spintronic applications: an intricate, yet favourable balance between ligand fields, charge transfer, and the Coulomb interaction makes FeP highly manipulable, while its planar structure facilitates device integration. Here, we theoretically design a mechanical spin-switch device in which external strain triggers the intrinsic magneto-structural coupling of FeP through a purely organic embedding. Exploiting the chemical compatibility and stretchability of graphene nanoribbon electrodes, we overcome common reliability and reproducibility issues of conventional inorganic setups. The competition between the Coulomb interaction and distortion-induced changes in ligand fields requires methodologies beyond the state-of-the-art: combining density functional theory with many-body techniques, we demonstrate experimentally feasible tensile strain to trigger a low-spin (S = 1) to high-spin (S = 2) crossover. Concomitantly, the current through the device toggles by over an order of magnitude, adding a fully planar mechanical current-switch unit to the panoply of molecular spintronics.
The idea of utilising the electrons' spin for information-processing in molecular-scale devices is the foundation for the emerging field of molecular spintronics.1–4 Elementary molecular devices, including spin-switches,5–7 spin transistors,8–11 or spin-valves,12 exploit reversible control over magnetic states via spin-crossover, magnetic coupling, Kondo resonance, or magnetic anisotropy mechanisms. Among these, the molecular spin-crossover (SCO) is the most robust and versatile mechanism: it can be triggered upon contact with surfaces,13–15 through external stimuli, e.g., voltage13,16 or strain,17 and, importantly, it can operate reliably also at ambient conditions.18
In standard experimental setups, a functional molecule is contacted between metallic electrodes (typically Au). However, the resulting properties strongly depend on the local atomic configuration of the junction, which is extremely difficult to control. Moreover, high atomic mobility and strong metal-molecule coupling often destroy intrinsic properties of the embedded molecule,16,19 rendering its functionality unexploitable. To date, ensuring reliability and reproducibility remains a key challenge for device fabrication. A promising route to overcome the above issues is to integrate the magnetic molecule into a more compatible organic environment. In fact, carbon-based architectures have recently been demonstrated to yield highly mechanically stable and atomically precise molecular junctions,20–24 while at the same time preserving the intrinsic magnetic properties of the contacted molecule. Therefore it is timely to conceptualize functional molecular devices with organic embedding.
Here, we explore the theoretical design of a mechanically-driven SCO device, that consists of a magnetic iron–porphyrin (FeP) molecule covalently bonded to graphene nanoribbon (GNR) electrodes. We identify a strategy to reversibly manipulate the molecular spin state by applying uniaxial strain. Exploiting the stretchability of the GNRs, we can directly engage the Fe–N coupling that controls the competition between ligand-fields and the Coulomb interaction, at the heart of the SCO mechanism.
1 The proposed device
In Fig. 1, we present the device set-up where an FeP molecule is chemically anchored to semi-infinite zigzag-edge graphene nano-ribbon (zGNR) electrodes. The dangling bonds of the edge C atoms of the zGNRs are passivated with H atoms for an enhanced stability and, at the same time, preserving the sp2 planar structure.25 The FeP molecule is connected to the electrodes by replacing two H atoms of the pyrrole moiety and two H atoms on the middle of the armchair facet of the GNR with direct C–C bonds, creating a side-sharing hexagon–pentagon interface.We exploit the magneto-structural relationship of the encapsulated FeP molecule26–29 as the basis for the device functionality. FeP has been demonstrated to realize a spin-crossover (SCO) under various conditions, e.g., through surface adsorption,29–32 in molecular assembly,33 or in single-molecule junctions by approaching an STM tip,34,35 all of which create internal strains in the embedded molecule. However, depending on the surface topology and surface–molecule interactions, outer ligand groups, metal atom coordination and oxidation state, and the nature of external stimuli, the type of magnetic transition, i.e., low-spin (LS) to high-spin (HS) or vice versa, as well as the amount of required strain, vary substantially, often diminishing the propensity for a SCO.23,36
In fact, the spin-state of the central Fe atom is the result of a delicate interplay of structural distortions in the porphyrin molecule and the Coulomb interaction. We manipulate this balance by mechanical strain, as indicated in Fig. 1, with the objective of inducing a spin-crossover. To effectively include the feedback of a SCO on the atomic structure, we relax the device by taking into account the Coulomb interaction within a static mean-field approximation (DFT + U). With the obtained structures we then go beyond this approximation and include dynamical many-body effects within the Fe-3d multiplet in order to obtain a better description of the electronic and transport properties across the SCO. For technical details, see the ESI.†
Upon atomic relaxation, the free-standing device undergoes a slight out-of-plane buckling due to an internal stress, caused by the mismatch in C–C bond-length at the interface between the hexagon of the zGNR and the C4N pentagon of the porphyrin ring. Also, the coordination of the central Fe atom becomes asymmetrical and consequently, the D4h symmetry of the FeN4 core (present in a free molecule) is lifted, as the Fe–N bonds along (perpendicular to) the zGNR coordination direction are elongated (shortened) to [Fe–N]x = 2.03 Å ([Fe–N]y = 1.99 Å).
The uniaxial strain applied to the device is quantified by the relative change in the unit-cell size along the x-direction. The covalent nature of the molecule-zGNR bonds and the high stretchability of zGNRs37 allow a sizeable expansion of the device. A 1% strain is sufficient to release the internal stress caused by the bonding between FeP and the zGNR electrodes and restore planarity, which is retained for higher strains. In Fig. 2(a) we show how the molecule-zGNR contacts and the molecule itself change due to the applied strain, by comparing structures without and with (5%) strain. Strain stretches the C–C bonds at the contacts as well as the longitudinal Fe–N bonds of FeP, which are identified as the softest in the system, by far. As expected, the changes in the Fe–N bond-length is direction-dependent and the [Fe–N]x distances expand while the [Fe–N]y distances shrink, see Fig. 2(b) and (c), further enhancing the asymmetry of the active molecule. Between 2% and 2.5% strain, the evolution of interatomic distances is discontinuous as the FeN4 core suddenly expands in both x- and y-direction. This phenomenon is, as we shall see, the structural signature of the system switching from a LS (S = 1) to a HS (S = 2) ground-state.
2 Spin state switching
We now take the perspective of the central Fe-ion, as it carries the magnetic degrees of freedom responsible for the device functionality. More specifically, we quantify how the above structural changes modify the Fe-ligand coupling, by considering the dynamical hybridization function38–40 | (1) |
 denote the Fe-3d orbitals, εbn the energies of the molecular orbitals of the ligands, which include both the porphyrin ring and the zGNR leads, and
denote the Fe-3d orbitals, εbn the energies of the molecular orbitals of the ligands, which include both the porphyrin ring and the zGNR leads, and  their coupling. The diagonal component
their coupling. The diagonal component  with
with  is the strongest due to the large axial overlap between dx2−y2 and N-2p orbitals. The second strongest contribution comes from the dyz orbital, whereas the dxz one is suppressed by the elongation of the [Fe–N]x distance and together with all other diagonal and off-diagonal components, are already insignificant for the description of the SCO (see Fig. S1 and the corresponding discussion in the ESI†). In the following we focus on the dx2−y2 component of Δ(ω), which is displayed in Fig. 2(d) and denoted as
is the strongest due to the large axial overlap between dx2−y2 and N-2p orbitals. The second strongest contribution comes from the dyz orbital, whereas the dxz one is suppressed by the elongation of the [Fe–N]x distance and together with all other diagonal and off-diagonal components, are already insignificant for the description of the SCO (see Fig. S1 and the corresponding discussion in the ESI†). In the following we focus on the dx2−y2 component of Δ(ω), which is displayed in Fig. 2(d) and denoted as  . At 0% strain,
. At 0% strain,  is characterized by a broad resonance around ω ∼ −2.0 eV. As a function of strain, it shifts towards higher binding energy and its height decreases, a hallmark of the weakening metal–ligand coupling. A sudden drop between 2% and 2.5% strain reflects again the structural discontinuity connected with the SCO.
is characterized by a broad resonance around ω ∼ −2.0 eV. As a function of strain, it shifts towards higher binding energy and its height decreases, a hallmark of the weakening metal–ligand coupling. A sudden drop between 2% and 2.5% strain reflects again the structural discontinuity connected with the SCO.
In order to reveal the connection between structural and magnetic changes under strain, we now take into account many-body effects in the Fe-3d multiplet embedded into its surrounding via the hybridization function, eqn (1). The latter is restricted to the dominant dx2−y2 and dyz channels which are treated as bath degrees of freedom. We then solve the ensuing realistic Anderson impurity model that incorporates the full Coulomb tensor41 with an exact diagonalization (ED) technique (for details, see the ESI†).
In Fig. 3 we show the evolution of the electronic properties under strain. In Fig. 3(a), the spin-transition energy ΔE = ELS − EHS, i.e., the energy difference between the lowest-lying many-body states in the LS and HS sectors, displays a discontinuity as a function of mechanical strain. We identify the system to undergo a transition from a LS to a HS state (sign change in ΔE) at the same critical strain (2–2.5%) for which DFT + U accounted for the structural transition. The corresponding electronic configurations are schematically shown in Fig. 3(b) in the basis of the Fe-3d and the two ligand orbitals. The LS and HS states differ for the occupation of the dxy and dx2−y2 orbitals, and the alignment of spins on different orbitals follows the Hund's rule. The above scenario is confirmed by the discontinuous evolution with strain of the total spin S projected onto the Fe-3d, computed from the operator 〈S2〉 = S(S + 1). We find SLS ∼ 1.2 (strain ≤2%) and SHS ∼ 1.9 (strain >2%). Note that the Fe atom is in a paramagnetic state, i.e., 〈Sz〉 = 0. The spin configuration displayed in Fig. 3(b) therefore corresponds to a snapshot in time, as the local moments fluctuate freely—subject only to the constraint of the spin-state S. Concomitant with the spin crossover, the orbital occupation  shows a charge transfer from the dxy to the dx2−y2 orbital. The slight deviation from a pure SLS = 1 and SHS = 2 can be mainly attributed to the projection on to the Fe-3d orbitals, as explained previously.42 The expectation values of the observables are obtained as a quantum mechanical and thermal average from many-body calculations at T = 300 K, indicating that the SCO survives up to room temperature. The mechanism underlying the SCO is represented schematically by the level diagram in Fig. 3(e), obtained through a static Hartree-like approximation of the many-body simulation (see ESI† for details). The resulting diagram can be understood in terms of the competition between the Coulomb interaction and the ligand field: both in the gas phase and in the device at 0% strain, the anti-bonding orbital with predominant dx2−y2 character lies far above the Fermi level, unoccupied, due to the strong hybridization to the ligands. As the Fe–N bonds soften along the strain direction, the splitting between bonding and anti-bonding orbitals is strongly reduced. At a critical strain, it becomes energetically favorable to promote an electron from the doubly-occupied dxy to the anti-bonding dx2−y2 orbital, in order to evade the intra-orbital Coulomb repulsion. At the same time, the Hund's exchange couplings within the Fe-3d multiplet suppress inter-orbital
shows a charge transfer from the dxy to the dx2−y2 orbital. The slight deviation from a pure SLS = 1 and SHS = 2 can be mainly attributed to the projection on to the Fe-3d orbitals, as explained previously.42 The expectation values of the observables are obtained as a quantum mechanical and thermal average from many-body calculations at T = 300 K, indicating that the SCO survives up to room temperature. The mechanism underlying the SCO is represented schematically by the level diagram in Fig. 3(e), obtained through a static Hartree-like approximation of the many-body simulation (see ESI† for details). The resulting diagram can be understood in terms of the competition between the Coulomb interaction and the ligand field: both in the gas phase and in the device at 0% strain, the anti-bonding orbital with predominant dx2−y2 character lies far above the Fermi level, unoccupied, due to the strong hybridization to the ligands. As the Fe–N bonds soften along the strain direction, the splitting between bonding and anti-bonding orbitals is strongly reduced. At a critical strain, it becomes energetically favorable to promote an electron from the doubly-occupied dxy to the anti-bonding dx2−y2 orbital, in order to evade the intra-orbital Coulomb repulsion. At the same time, the Hund's exchange couplings within the Fe-3d multiplet suppress inter-orbital  double occupations with opposite spins
double occupations with opposite spins  thus stabilizing a HS configuration.41
thus stabilizing a HS configuration.41
One can estimate the Coulomb contribution to the spin-transition energy between the electronic configurations in Fig. 3(b). Within a density–density approximation of the Coulomb tensor (see ESI† for an extended discussion) ΔCoulomb = 2J1 + 3J3. The system gains energy in the HS state associated with two exchange interaction parameters: J1, that couples both the dxy and dx2−y2 to the dxz and dyz orbitals, and J3, that couples the dxy and dx2−y2 orbitals. We find ΔCoulomb ≈ 2.83 eV to be of the same order of magnitude as the ligand field between the dxz and dx2−y2 orbitals. The competition between these two energy scales, which in the full calculation is represented by ΔE in Fig. 3(a), is the root cause of the SCO in FeP.
3 Electron transport
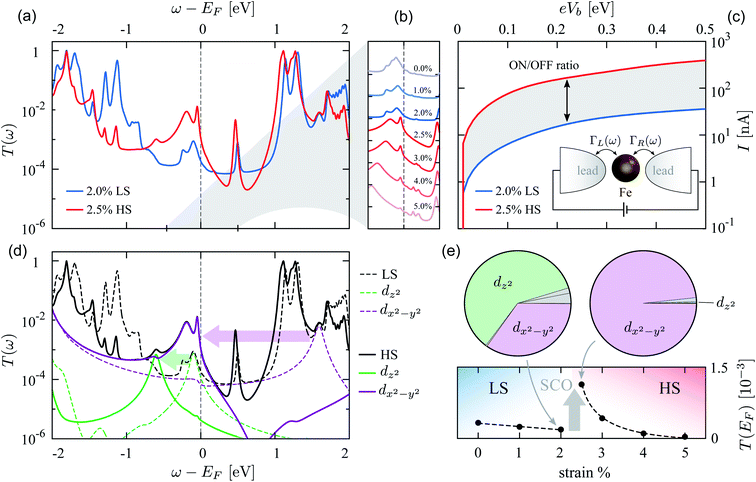
The change of the spin state across the SCO has practical implications, as it drastically changes the charge transport properties through the device. In order to demonstrate that, we map the device onto an effective quantum-dot model. We calculate the Landauer transmission function| T(ω)=Tr[ΓL(ω)G†(ω)ΓR(ω)G(ω)], | (2) |
The simulated transmission function T(ω) in the LS and HS state is shown in Fig. 4(a) for strains across the SCO, while Fig. 4(b) shows its behavior at low-energy for all strain values. At the Fermi level we identify a significant enhancement of the conductance from about 2 × 10−4G0 at 2% strain to values >10−3G0 at 2.5% strain, where G0 = e2/h is the conductance quantum. This jump is mirrored by an increase in the electric current (per spin) evaluated as
 | (3) |
4 Conclusions
In summary, we have demonstrated the potential of a molecular spin-switch device, where a mechanically driven spin-crossover results in a bias-independent enhancement of the current through the device by one order of magnitude. This is revealed by realistic simulations that include many-body effects without breaking the spin symmetry of the Fe core. The key advantage of the proposed design is the organic anchoring of the active FeP molecule. The strong covalent bonding to the zGNR electrodes assures a high reliability and reproducibility. The relatively smaller contact-resistance is also expected to enable the proposed device to identify small conductance differences in electrical measurements. Recent technical breakthroughs, such as dash-line lithography20 or on-surface-synthesis22,49 make it possible to anchor molecules with graphene-nano structures with high atomic-precision. These graphene–molecule–graphene junctions have already shown remarkable functionalities, including magnetism,21,23,24,49 or rectifying behaviour,50 and can be engineered to work in reversible mechanical break-junction setups.51 These early successes strongly suggest that our proposed device, which couples magnetic and structural degrees of freedom in a fully planar geometry, is experimentally realizable, adding a new building block for organic spintronic devices.Author contributions
SB designed the project. SB and AV carried out the numerical simulations. All authors discussed the results and contributed to writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Science Foundation Ireland [19/EPSRC/3605], the Engineering and Physical Sciences Research Council [EP/S030263/1], the European Research Council through Consolidator Grant no. 617196 ‘CORRELMAT’ (SB), and the Austrian Science Fund (FWF) through project ‘LinReTraCe’ P 30213 (JMT, AV) and project P 31631 (AV).Notes and references
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed.
- A. R. Rocha, V. M. García-suárez, S. W. Bailey, C. J. Lambert, J. Ferrer and S. Sanvito, Nat. Mater., 2005, 4, 335 CrossRef CAS PubMed.
- S. Sanvito, Chem. Soc. Rev., 2011, 40, 3336–3355 RSC.
- S. Schmaus, A. Bagrets, Y. Nahas, T. K. Yamada, A. Bork, M. Bowen, E. Beaurepaire, F. Evers and W. Wulfhekel, Nat. Nanotechnol., 2011, 6, 185–189 CrossRef CAS PubMed.
- M. Ormaza, P. Abufager, B. Verlhac, N. Bachellier, M. L. Bocquet, N. Lorente and L. Limot, Nat. Commun., 2017, 8, 1974 CrossRef CAS PubMed.
- K. Bairagi, O. Iasco, A. Bellec, A. Kartsev, D. Li, J. Lagoute, C. Chacon, Y. Girard, S. Rousset, F. Miserque, Y. J. Dappe, A. Smogunov, C. Barreteau, M.-L. Boillot, T. Mallah and V. Repain, Nat. Commun., 2016, 7, 12212 CrossRef CAS PubMed.
- H. Wan, B. Zhou, X. Chen, C. Q. Sun and G. Zhou, J. Phys. Chem. C, 2012, 116, 2570–2574 CrossRef CAS.
- E. A. Osorio, K. Moth-Poulsen, H. S. J. van der Zant, J. Paaske, P. Hedegård, K. Flensberg, J. Bendix and T. Bjørnholm, Nano Lett., 2010, 10, 105–110 CrossRef CAS PubMed.
- W. Liang, M. P. Shores, M. Bockrath, J. R. Long and H. Park, Nature, 2002, 417, 725 CrossRef CAS PubMed.
- R. Gaudenzi, E. Burzurí, D. Reta, I. d. P. R. Moreira, S. T. Bromley, C. Rovira, J. Veciana and H. S. J. van der Zant, Nano Lett., 2016, 16, 2066–2071 CrossRef CAS PubMed.
- A. S. Zyazin, J. W. G. van den Berg, E. A. Osorio, H. S. J. van der Zant, N. P. Konstantinidis, M. Leijnse, M. R. Wegewijs, F. May, W. Hofstetter, C. Danieli and A. Cornia, Nano Lett., 2010, 10, 3307–3311 CrossRef CAS PubMed.
- M. Urdampilleta, S. Klyatskaya, J.-P. Cleuziou, M. Ruben and W. Wernsdorfer, Nat. Mater., 2011, 10, 502 CrossRef CAS PubMed.
- T. G. Gopakumar, F. Matino, H. Naggert, A. Bannwarth, F. Tuczek and R. Berndt, Angew. Chem., Int. Ed., 2012, 51, 6262–6266 CrossRef CAS PubMed.
- M. Bernien, H. Naggert, L. M. Arruda, L. Kipgen, F. Nickel, J. Miguel, C. F. Hermanns, A. Krüger, D. Krüger, E. Schierle, E. Weschke, F. Tuczek and W. Kuch, ACS Nano, 2015, 9, 8960–8966 CrossRef CAS PubMed.
- B. Warner, J. C. Oberg, T. G. Gill, F. El Hallak, C. F. Hirjibehedin, M. Serri, S. Heutz, M.-A. Arrio, P. Sainctavit, M. Mannini, G. Poneti, R. Sessoli and P. Rosa, J. Phys. Chem. Lett., 2013, 4, 1546–1552 CrossRef CAS PubMed.
- T. Miyamachi, M. Gruber, V. Davesne, M. Bowen, S. Boukari, L. Joly, F. Scheurer, G. Rogez, T. K. Yamada, P. Ohresser, E. Beaurepaire and W. Wulfhekel, Nat. Commun., 2012, 3, 938 CrossRef PubMed.
- R. Frisenda, G. D. Harzmann, J. A. Celis Gil, J. M. Thijssen, M. Mayor and H. S. J. van der Zant, Nano Lett., 2016, 16, 4733–4737 CrossRef CAS PubMed.
- F. Prins, M. Monrabal-Capilla, E. A. Osorio, E. Coronado and H. S. J. van der Zant, Adv. Mater., 2011, 23, 1545–1549 CrossRef CAS PubMed.
- C. Jia and X. Guo, Chem. Soc. Rev., 2013, 42, 5642–5660 RSC.
- Y. Cao, S. Dong, S. Liu, L. He, L. Gan, X. Yu, M. L. Steigerwald, X. Wu, Z. Liu and X. Guo, Angew. Chem., Int. Ed., 2012, 51, 12228–12232 CrossRef CAS PubMed.
- C. Jia, B. Ma, N. Xin and X. Guo, Acc. Chem. Res., 2015, 48, 2565–2575 CrossRef CAS PubMed.
- Y. He, M. Garnica, F. Bischoff, J. Ducke, M.-L. Bocquet, M. Batzill, W. Auwärter and J. V. Barth, Nat. Chem., 2017, 9, 33–38 CrossRef CAS PubMed.
- J. Li, N. Friedrich, N. Merino, D. G. de Oteyza, D. Peña, D. Jacob and J. I. Pascual, Nano Lett., 2019, 19, 3288–3294 CrossRef CAS PubMed.
- Y. Li, C. Yang and X. Guo, Acc. Chem. Res., 2020, 53, 159–169 CrossRef CAS PubMed.
- S. Bhandary, O. Eriksson, B. Sanyal and M. I. Katsnelson, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 165405 CrossRef.
- W. R. Scheidt and C. A. Reed, Chem. Rev., 1981, 81, 543–555 CrossRef CAS.
- S. Bhandary, M. Schüler, P. Thunström, I. di Marco, B. Brena, O. Eriksson, T. Wehling and B. Sanyal, Phys. Rev. B, 2016, 93, 155158 CrossRef.
- S. Bhandary, S. Ghosh, H. Herper, H. Wende, O. Eriksson and B. Sanyal, Phys. Rev. Lett., 2011, 107, 257202 CrossRef PubMed.
- S. Bhandary, B. Brena, P. M. Panchmatia, I. Brumboiu, M. Bernien, C. Weis, B. Krumme, C. Etz, W. Kuch, H. Wende, O. Eriksson and B. Sanyal, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 024401 CrossRef.
- B. Liu, H. Fu, J. Guan, B. Shao, S. Meng, J. Guo and W. Wang, ACS Nano, 2017, 11, 11402–11408 CrossRef CAS PubMed.
- W. Wang, R. Pang, G. Kuang, X. Shi, X. Shang, P. N. Liu and N. Lin, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 045440 CrossRef.
- S. Bhandary, O. Eriksson and B. Sanyal, Sci. Rep., 2013, 3, 3405 CrossRef PubMed.
- D. Rolf, C. Lotze, C. Czekelius, B. W. Heinrich and K. J. Franke, J. Phys. Chem. Lett., 2018, 9, 6563–6567 CrossRef CAS PubMed.
- B. W. Heinrich, C. Ehlert, N. Hatter, L. Braun, C. Lotze, P. Saalfrank and K. J. Franke, ACS Nano, 2018, 12, 3172–3177 CrossRef CAS PubMed.
- G. Kuang, Q. Zhang, T. Lin, R. Pang, X. Shi, H. Xu and N. Lin, ACS Nano, 2017, 11, 6295–6300 CrossRef CAS PubMed.
- H. C. Herper, M. Bernien, S. Bhandary, C. F. Hermanns, A. Krüger, J. Miguel, C. Weis, C. Schmitz-Antoniak, B. Krumme, D. Bovenschen, C. Tieg, B. Sanyal, E. Weschke, C. Czekelius, W. Kuch, H. Wende and O. Eriksson, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 174425 CrossRef.
- C. Liu, B. Yao, T. Dong, H. Ma, S. Zhang, J. Wang, J. Xu, Y. Shi, K. Chen, L. Gao and L. Yu, npj 2D Mater. Appl., 2019, 3, 23 CrossRef.
- R. Bulla, T. A. Costi and T. Pruschke, Rev. Mod. Phys., 2008, 80, 395–450 CrossRef CAS.
- M. Schüler, S. Barthel, T. Wehling, M. Karolak, A. Valli and G. Sangiovanni, Eur. Phys. J.: Spec. Top., 2017, 226, 2615–2640 Search PubMed.
- G. Gandus, A. Valli, D. Passerone and R. Stadler, J. Chem. Phys., 2020, 153, 194103 CrossRef CAS PubMed.
- A. Valli, M. P. Bahlke, A. Kowalski, M. Karolak, C. Herrmann and G. Sangiovanni, Phys. Rev. Res., 2020, 2, 033432 CrossRef CAS.
- J. Steinbauer, S. Biermann and S. Bhandary, Phys. Rev. B, 2019, 100, 245418 CrossRef CAS.
- Y. Meir and N. S. Wingreen, Phys. Rev. Lett., 1992, 68, 2512 CrossRef PubMed.
- D. Jacob, J. Phys.: Condens. Matter, 2015, 27, 245606 CrossRef PubMed.
- A. Droghetti and I. Rungger, Phys. Rev. B, 2017, 95, 085131 CrossRef.
- A. Valli, A. Amaricci, V. Brosco and M. Capone, Nano Lett., 2018, 18, 2158 CrossRef CAS PubMed.
- A. Valli, A. Amaricci, V. Brosco and M. Capone, Phys. Rev. B, 2019, 100, 075118 CrossRef CAS.
- J. L. Zhang, J. Q. Zhong, J. D. Lin, W. P. Hu, K. Wu, G. Q. Xu, A. T. S. Wee and W. Chen, Chem. Soc. Rev., 2015, 44, 2998–3022 RSC.
- J. Li, N. Merino-Díez, E. Carbonell-Sanromà, M. Vilas-Varela, D. G. de Oteyza, D. Peña, M. Corso and J. I. Pascual, Sci. Adv., 2018, 4, eaaq0582 CrossRef PubMed.
- H. Wan, Y. Xu and G. Zhou, J. Chem. Phys., 2012, 136, 184704 CrossRef PubMed.
- S. Caneva, P. Gehring, V. M. García-Suárez, A. García-Fuente, D. Stefani, I. J. Olavarria-Contreras, J. Ferrer, C. Dekker and H. S. J. van der Zant, Nat. Nanotechnol., 2018, 13, 1126–1131 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details for ab initio, many-body, and transport calculations; embedding of the Fe-3d multiplet; benchmark against quantum Monte Carlo. See DOI: 10.1039/d1na00407g |
| This journal is © The Royal Society of Chemistry 2021 |