Open Access Article
Open Access ArticleSurface modification for improving the photoredox activity of CsPbBr3 nanocrystals†
Syed
Akhil
,
V. G. Vasavi
Dutt
and
Nimai
Mishra
 *
*
Department of Chemistry, SRM University-AP, Amaravati, Neerukonda, Guntur (Dt), Andhra Pradesh 522240, India. E-mail: nimai.m@srmap.edu.in
First published on 26th February 2021
Abstract
In recent years inorganic lead halide perovskite nanocrystals (PNCs) have been used in photocatalytic reactions. The surface chemistry of the PNCs can play an important role in the excited state interactions and efficient charge transfer with redox molecules. In this work, we explore the impact of CsPbBr3 nanocrystal surface modification on the excited state interactions with the electron acceptor benzoquinone (BQ) for three different ligand environments: as oleic acid/oleylamine (OA/OAm), oleic acid (OA)/trioctylphosphine (TOP), and oleic acid (OA)/oleylamine (OAm)/trioctylphosphine (TOP) ligands. Our finding concludes that amine-free PNCs (OA/TOP capped) exhibit the best excited state interactions with benzoquinone compared to the conventional oleylamine ligand environment. The photoinduced electron transfer (PET) rate constants were measured from PL-lifetime decay measurement. The amine-free PNCs show the highest PET which is 9 times higher than that of conventional ligand capped PNCs. These results highlight the impact of surface chemistry on the excited-state interactions of CsPbBr3 NCs and in photocatalytic applications. More importantly, this work concludes that amine-free PNCs maintain a redox-active surface with a high photoinduced electron transfer rate which makes them an ideal candidate for photocatalytic applications.
Introduction
Inorganic cesium lead halide perovskite nanocrystals (CsPbX3, X = I, Br, Cl) have been of great interest to researchers because of their very high photoluminescence quantum yield (∼up to 90%), broad absorbance, and narrow emission with wide spectral tunability.1–7 These materials are at the forefront of research due to their low-temperature facile synthesis, tolerance to surface defects, and low exciton binding energies.1,8–10 Due to these excellent optoelectronic properties1,11–14 and facile synthesis PNCs received great attention as active materials in several optoelectronic devices such as light-emitting diodes,15,16 solar cells,17 photodetectors,18 scintillators,19 and photocatalysts.20–22 The carrier diffusion in cesium lead halide perovskite PNCs plays an important role in the performance of optoelectronic devices.23 Carrier diffusion is affected by several factors such as the energy levels, crystallinity, exciton binding energy, and surface chemistry of PNCs.24–26 Typically, in optoelectronic devices upon photoexcitation, the exciton dissociates and the electron/hole is transferred from the conduction/valence band to the electron/hole acceptors, respectively.27Hence examining the factors that impact the charge transport is significant for improving the performance of inorganic halide PNCs and broadening their applications. While most of these PNCs are used in photovoltaic28 and optoelectronic devices,29–32 only limited research has been done towards their use as photocatalysts.33–35 The reason behind the limited use of PNCs in photocatalytic studies is their instability in the polar medium and the presence of high energy barrier surfactants on the surfaces of the nanocrystals. The use of surfactant molecules in PNC synthesis is unavoidable but it limits the access of the active surface sites to electron/hole acceptors.
The use of PNCs as photocatalysts was first realized by Park et al. when they reported their pioneering work using methylammonium lead iodide for hydrogen evolution from a saturated HI solution.36 Soon after the initial work, many research groups reported photocatalytic applications which include CO2 reduction,37,38 derivatives of thiophene by polymerization,33 oxidation of benzyl alcohol,39 activation of C(sp3)–H,40 and alkylation of aldehydes.41 Composite structures (such as CsPbBr3 NCs with graphene oxide and with porous g-C3N4) have been utilized to enhance the charge transport efficiency for further improvement in photocatalytic conversion.36,37 Thus the use of secondary conducting materials as co-catalysts has been proved as a successful strategy. Nevertheless, direct modification of PNCs may be the most viable and straightforward option to accelerate charge transport which therefore improves the photocatalytic performance efficiency.42
To design an efficient photo-catalyst the competition between radiative charge recombination and charge transfer remains the main focus. In metal chalcogenide semiconductor nanocrystals, efficient charge separation was achieved through morphological and band engineering modifications via core/shell heterostructures.43,44 Band engineering with core/shell structures for efficient charge separation in perovskite nanocrystals is not yet well established. Hence, researchers are working on alternative techniques to improve interfacial charge separation. Modification in surface chemistry could be a viable option to improve charge separation and electron transfer.45 Surface capping groups are necessary to improve their colloidal stability and maintain their morphology but their presence may reduce or even block interfacial carrier transfer. To overcome this problem, it is desirable to find out suitable surface capping ligands that can facilitate electron transfer while preserving the morphology and maintaining the stability of the perovskite NCs. Therefore, there is an urgent need for alternative surface capping groups that may facilitate faster charge transfer from PNCs to the acceptor.
Herein, we sought to investigate the effects of a new surface capping shell on the redox activity of PNCs. CsPbBr3 PNCs with three different surface capping groups were synthesized and utilized as photoinduced electron donors. To understand surface capping shell-dependent charge transfer we performed photoinduced electron transfer (PET) experiments with a standard electron acceptor, benzoquinone (BQ). The schematic reaction mechanism is shown in Fig. 1. Previous studies have shown that a rapid PET occurs at the CsPbBr3/BQ interface.46 The PET process relies on the reduction potential of NCs and the permeability of the surface capping shell. The PET process has been probed with the help of steady-state and time-resolved PL of CsPbBr3 PNCs with different concentrations of BQ molecules. The steady-state PL demonstrated an improved photoinduced electron transfer (PET) when completely amine-free CsPbBr3 PNCs are used as photoactive materials. This could be attributed to the absence of the strongly binding oleylamine which facilitates the fast charge transfer process. This observation was further confirmed by the time-resolved photoluminescence spectra, in which the amine-free CsPbBr3 NCs have the longest lifetime, therefore, this indicates longer existence of the exciton which can be easily dissociated and transferred to the electron acceptor. Thus, we envisioned that amine-free CsPbBr3 PNCs could be an ideal candidate for a photocatalyst, and it will open a new avenue in PNC photocatalytic-based organic synthesis.
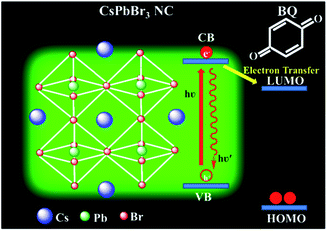 | ||
| Fig. 1 Schematic energy level diagram of CsPbBr3 NCs and the possible electron transfer that occurs from NCs to benzoquinone (BQ) molecule. | ||
Results and discussion
Three different surface chemistry-based CsPbBr3 perovskites were synthesized according to the reported hot injection method with a slight modification.47,48 Briefly, amine free-CsPbBr3 perovskite nanocrystals were prepared by a modified three-precursor approach in which the Cs, Pb, and Br precursors are used independently. Amine free-CsPbBr3 PNCs were synthesized in the presence of oleic acid (OA) and trioctylphosphine (TOP) as surface capping ligands. In this process, oleylamine is replaced with TOP.In contrast, amine-CsPbBr3 PNCs were synthesized by the conventional two precursor approach where oleylamine (OAm) and oleic acid (OA) were used as surface capping ligands.48 The amine + TOP capped CsPbBr3 PNCs were synthesized by the above-mentioned conventional synthesis in the presence of TOP as the surface capping ligand in addition to OAm and OA. The detailed synthesis can be found in the Experimental section in the ESI.†
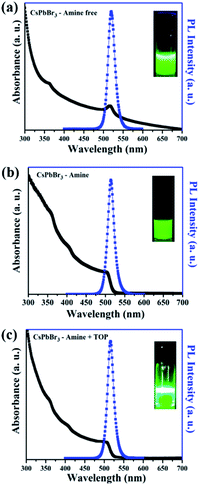
The detailed optical characterization of the three different surface ligand capped CsPbBr3 PNCs can be found in Fig. 2. Fig. 2(a)–(c) show the narrow excitonic-emission spectra (blue color) and broad absorbance spectra (black line) of amine-free, amine, and amine + TOP based CsPbBr3 PNCs and in the inset, the digital photograph of the colloidal solution taken under UV irradiation (with excitation wavelength of 365 nm) is provided, which shows good dispersibility in toluene and is highly emissive in nature. The calculated photoluminescence quantum yield (PLQY) values of the three samples are 69.2% (amine-free), 79.4% (amine), and 81.1% (amine + TOP). The absorbance and emission spectra with the 1st excitonic peak at 516 nm and the emission maximum at 518 nm are presented in Fig. 2(a) for amine-free CsPbBr3 PNCs. The full width at half maximum (FWHM) of 23.4 nm indicates moderately monodisperse particles. The CsPbBr3-amine PNCs and CsPbBr3-amine + TOP PNCs exhibit absorption peaks at 510 nm and 511 nm and PL emission peaks at 513 nm (FWHM = 18.3 nm) and 517 nm (FWHM = 19.7 nm) respectively.
FTIR spectroscopy was used to characterize the surface chemistry of the three PNCs and to understand the surface–ligand interactions of surfactants (Fig. 3). For the amine-free and amine + TOP surface capped PNCs a peak around 1140 cm−1 is observed which is attributed to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration.46 The spectra show the characteristic CH2 stretching vibration at 2924 and bending vibration at 2854 cm−1, and NH2 bending vibration at 1636 cm−1, together with the C
O stretching vibration.46 The spectra show the characteristic CH2 stretching vibration at 2924 and bending vibration at 2854 cm−1, and NH2 bending vibration at 1636 cm−1, together with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1715 cm−1. In the case of the amine-free synthesis, the presence of TOP along with Pb(OA)2 may initiate the formation of TOPO by reaction with the carboxyl group of Pb(OA)2, which is proposed owing to the peak at 1140 cm−1 that could be attributed to the P
O stretching vibration at 1715 cm−1. In the case of the amine-free synthesis, the presence of TOP along with Pb(OA)2 may initiate the formation of TOPO by reaction with the carboxyl group of Pb(OA)2, which is proposed owing to the peak at 1140 cm−1 that could be attributed to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. Compared with the spectrum of pure TOPO, the shift in the absorption peak of P
O stretching vibration. Compared with the spectrum of pure TOPO, the shift in the absorption peak of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching from 1156 to 1140 cm−1 is attributed to bonding on the NC surface. The deformation bands at 1400–1440 cm−1 may be due to the presence of C–P interaction.46
O stretching from 1156 to 1140 cm−1 is attributed to bonding on the NC surface. The deformation bands at 1400–1440 cm−1 may be due to the presence of C–P interaction.46
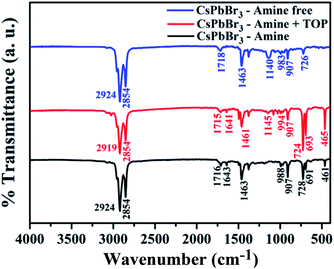 | ||
| Fig. 3 FTIR spectra of CsPbBr3 perovskite NCs synthesized using the three different surface capping groups. | ||
From the FTIR analysis, we may presume the presence of the respective surface ligands on the three different PNCs, but to get an actual insight into the surface chemistry we may require further detailed characterization.
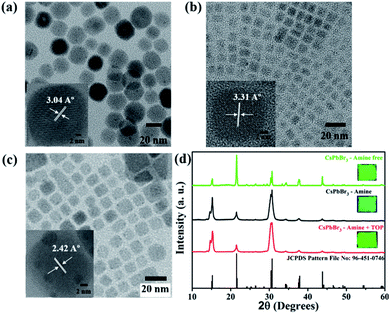
Transmission electron microscopy (TEM) was used to assess the structural properties of all the three CsPbBr3 PNCs and showed good dispersity and uniform size distribution (Fig. 4(a)–(c)). The TEM images further confirmed that there is no aggregation. Insets of Fig. 4(a)–(c) show high-resolution transmission electron microscopy images of a single nanocrystal with a scale bar of 2 nm, which show the presence of defined lattice fringes indicating the high crystallinity of the nanocrystals. The lattice spacing of amine-free, amine and amine + TOP capped CsPbBr3 NCs are 3.04, 3.31, and 2.42 Å, which correspond to the (121), (022) and (240) planes of the orthorhombic phase, respectively. The TEM image of the amine-free PNCs in Fig. 4(a) shows that the NCs have cubic morphology with an average particle size of 21.34 ± 2.8 nm. Fig. 4(b) and (c) present the transmission electron microscopy (TEM) images of the CsPbBr3 sample with amine capped and amine + TOP capped NCs. The as-synthesized CsPbBr3 NCs are cubic in shape and with varying surface ligands; the particle's morphology is largely maintained. In the case of amine and amine + TOP surfaces, the ligand capped CsPbBr3 PNCs have good colloidal stability with moderate monodispersity (the calculated average particle sizes of the samples are 8.53 ± 1.3 nm and 12.72 ± 2.1 nm respectively).
X-ray diffraction patterns of all three CsPbBr3 films made of the three different surface group capped NCs are shown in Fig. 4(d). The X-ray diffraction patterns confirmed that the PNCs are highly crystalline with the orthorhombic phase which is in good match with the JCPDS pattern file: 96-451-0746. The NC films were prepared by drop-casting the colloidal solutions of CsPbBr3 NCs on a cleaned glass substrate and their respective photographs were taken under UV light irradiation with excitation wavelength at 365 nm.
Due to their large extinction coefficient in the visible range (∼106 M−1 cm−1)46,49 and low exciton binding, cesium lead halide (CsPbX3) PNCs provide an excellent opportunity for light absorption and subsequent photoinduced charge separation. Because of low electron and hole trapping which is typical of PNCs, facile electron and hole transfer has been observed in unmodified nanocrystals. A variety of organic syntheses have been demonstrated with CsPbBr3 NCs as efficient photoredox catalysts.41,49 But the presence of long-chain surface capping groups affects the charge transfer from CsPbBr3 NCs to the electron/hole acceptor. The surface ligand shell plays a critical role in stabilizing the crystalline phase of halide perovskite NCs. Due to their more ionic nature, these materials often suffer from phase transformation or dissolution.50 Moreover, we overcome the problem of charge separation and stability in PNCs by a simple and controllable method that involves the judicious choice of suitable surface capping groups. Here we have investigated different surface ligand capped PNCs to study their photo redox activity and performed a photoinduced electron transfer (PET) experiment with the standard electron acceptor, benzoquinone (BQ) molecule.
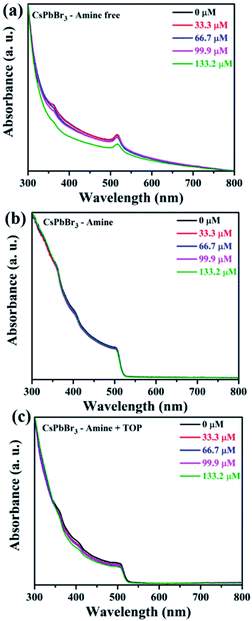
Firstly, we investigated the influence of benzoquinone addition on the absorption spectra of CsPbBr3 nanocrystals capped with the three different ligand systems. Toluene is used as a solvent for the dispersion of both nanocrystals and benzoquinone. The concentration of all three PNC samples was determined from absorption spectroscopy using the reported extinction coefficients.51Fig. 5(a)–(c) show the absorption spectra of CsPbBr3 prepared with OA/TOP (amine-free), OA/OAm ligands, and OA/OAm/TOP ligands. The benzoquinone was added incrementally to each PNC solution (same concentration of PNCs) and the absorption spectra were recorded at several concentrations.
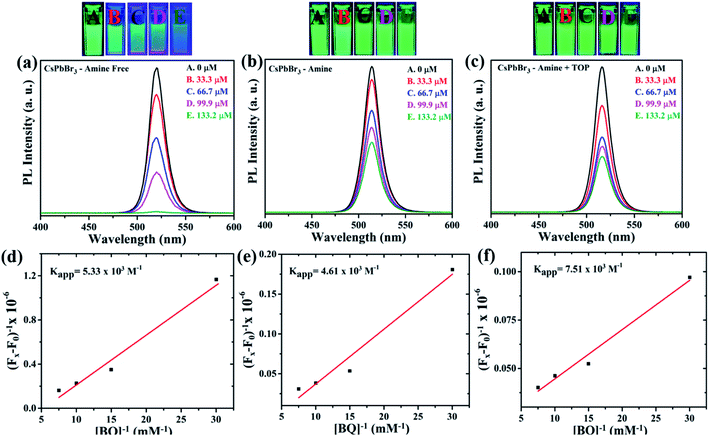
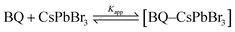
The amine-free CsPbBr3 PNCs with OA/TOP ligands showed a large degree of scattering and upon addition of BQ, there is not much change observed (Fig. 5(a)). The dynamic nature of surface capping ligands hinders their complete dispersion and thus gives rise to a scattering effect. On the other hand, for the amine and amine + TOP capped CsPbBr3 PNCs there was no scattering (Fig. 5(b) and (c)) with no obvious changes in absorption upon sequential addition of BQ. The absence of scattering of these two PNCs is due to oleylamine, the presence of which results in good dispersion of the NCs in solution. Fig. 6(a)–(c) show the photoluminescence (PL) spectra of the nanocrystals with OA/TOP (amine-free), OA/OAm ligands, and OA/OAm/TOP ligands, respectively with the stepwise addition of benzoquinone. In each case, the PL of CsPbBr3 PNCs is quenched upon the addition of benzoquinone. On the top panel of Fig. 6, the change in PL intensity can be seen from the cuvettes (without and with BQ) under UV light irradiation. We analysed the fluorescence quenching data by considering the equilibrium between benzoquinone and CsPbBr3 (reaction (1)).
 | (1) |
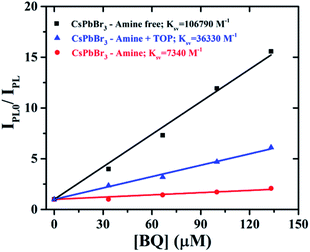
The binding interaction between BQ and CsPbBr3 PNCs was studied using the double reciprocal plot following the Benesi–Hildebrand equation.52Kapp in the above equation is the apparent association constant between BQ and CsPbBr3 PNCs. Using the double reciprocal plot of the emission quenching data, Kapp is evaluated. We found that OA/TOP (amine-free), OA/OAm (amine) ligands, and OA/OAm/TOP (amine + TOP) ligand capped PNCs have an association constant Kapp of ∼5.3 × 103, ∼4.6 × 103 M−1, and ∼7.5 × 103 M−1 respectively. The apparent association constant (Kapp) values for three different surface chemistry based PNCs with benzoquinone molecule are in the same order. The similar Kapp values indicate a relatively similar binding between nanocrystals and BQ molecules for all the three PNC samples. Despite the long capping ligands of all the three CsPbBr3 PNCs, their surface is still accessible for interaction with redox-active molecules. The photoluminescence (PL) decay using time-correlated single-photon counting (TCSPC) has been used to probe the effect of BQ on the radiative excited state of CsPbBr3. Fig. 7 shows the decay of the photoluminescence monitored at the emission maximum for PNCs with the three different surface chemistries. The native PL lifetime (i.e., without BQ) is the highest for amine-free OA/TOP-capped CsPbBr3 (![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) avg ∼ 17.03 ns). For amine and amine + TOP capped CsPbBr3, the native lifetime is lower. In each case, sequential addition of BQ decreases the PL lifetime which indicates electron transfer from the excited CsPbBr3 to the surface-bound BQ. In the case of amine-free PNCs, the rapid quenching of the CsPbBr3 excited state by BQ indicates that a fast electron transfer process dominates (eqn (2)). Furthermore, the photo-induced electron transfer (PET) process is quantitatively analyzed with the help of time-resolved PL and steady-state PL of the CsPbBr3 NCs with various concentrations of BQ molecules. The PET process depends on the reduction potential of the CsPbBr3 NCs and the permeability of the surface binding ligands.53 As shown in Fig. 6(a) and 7(a), the PL and TCSPC spectra of amine-free CsPbBr3 NCs, we find that the PL properties of these PNCs are dynamically quenched by BQ when compared to amine-CsPbBr3 NCs and amine + TOP-CsPbBr3 NCs as shown in Fig. 6(b), 7(b) and 6(c), 7(c) respectively. The Stern–Volmer equation has been used to study the charge transfer process (eqn (3)) as shown in Fig. 8.
avg ∼ 17.03 ns). For amine and amine + TOP capped CsPbBr3, the native lifetime is lower. In each case, sequential addition of BQ decreases the PL lifetime which indicates electron transfer from the excited CsPbBr3 to the surface-bound BQ. In the case of amine-free PNCs, the rapid quenching of the CsPbBr3 excited state by BQ indicates that a fast electron transfer process dominates (eqn (2)). Furthermore, the photo-induced electron transfer (PET) process is quantitatively analyzed with the help of time-resolved PL and steady-state PL of the CsPbBr3 NCs with various concentrations of BQ molecules. The PET process depends on the reduction potential of the CsPbBr3 NCs and the permeability of the surface binding ligands.53 As shown in Fig. 6(a) and 7(a), the PL and TCSPC spectra of amine-free CsPbBr3 NCs, we find that the PL properties of these PNCs are dynamically quenched by BQ when compared to amine-CsPbBr3 NCs and amine + TOP-CsPbBr3 NCs as shown in Fig. 6(b), 7(b) and 6(c), 7(c) respectively. The Stern–Volmer equation has been used to study the charge transfer process (eqn (3)) as shown in Fig. 8.
| [CsPbBr3–BQ] + hν → [CsPbBr3(h–e)–BQ] → [CsPbBr3(h)–BQ˙] | (2) |
IPL0/IPL = 1 + KSV[BQ] = 1 + Kq![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) 0[BQ]. 0[BQ]. | (3) |
![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) 0 is the intensity-average lifetime constant from the bi-exponential fit to the CPbBr3 NCs without any electron acceptor. All the quenching constants are mentioned in Table 1. Amine-free PNCs have the highest KSV when compared to the conventional amine-based and amine + TOP capped CsPbBr3 NCs. The bimolecular quenching constant Kq, of amine-free PNCs, is nearly 8.8 times higher when compared to the amine-CsPbBr3 NCs. The larger KSV value for amine-free CsPbBr3 NCs is due to their enhanced ligand permeability which impacts their redox potential. Thus, we can hypothesize that surface chemistry plays a vital role in enhancing the charge separation but further studies are needed to understand the effect of various other factors.
0 is the intensity-average lifetime constant from the bi-exponential fit to the CPbBr3 NCs without any electron acceptor. All the quenching constants are mentioned in Table 1. Amine-free PNCs have the highest KSV when compared to the conventional amine-based and amine + TOP capped CsPbBr3 NCs. The bimolecular quenching constant Kq, of amine-free PNCs, is nearly 8.8 times higher when compared to the amine-CsPbBr3 NCs. The larger KSV value for amine-free CsPbBr3 NCs is due to their enhanced ligand permeability which impacts their redox potential. Thus, we can hypothesize that surface chemistry plays a vital role in enhancing the charge separation but further studies are needed to understand the effect of various other factors.
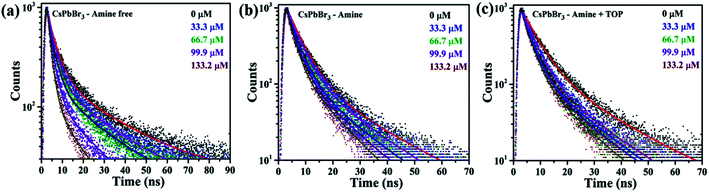 | ||
| Fig. 7 Time-resolved PL spectra, the solid lines are the fit with the bi-exponential function: (a) amine-free (b) amine and (c) amine + TOP (λex = 370 nm). | ||
| CsPbBr3 NCs | K SV (mM−1) |
![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) 0 (ns)
0 (ns) |
K q (M−1 ns−1) | K q improvement |
|---|---|---|---|---|
| Amine-free | 106.79 | 17.03 | 6270 | 8.8 |
| Amine + TOP | 36.33 | 11.68 | 3110 | 2.01 |
| Amine | 7.34 | 10.30 | 712 | 1 |
Conclusions
In summary, we investigated the impact of the surface chemistry of CsPbBr3 PNCs on the excited state interactions and interfacial charge transfer to a redox molecule. We compared the charge transfer process with CsPbBr3 PNCs synthesized with OA/OAm, OA/TOP (amine-free), and OA/OAm/TOP as surface capping ligands. The surface chemistry based binding interaction between the electron acceptor (BQ) and CsPbBr3 PNCs was studied. All three ligands based CsPbBr3 PNC system exhibits a similar apparent association constant. More interestingly, we found that the surface chemistry has a substantial influence on charge transfer efficiency. The amine-free CsPbBr3 PNCs (OA + TOP as ligands) exhibited the highest photoinduced electron transfer (PET) efficiency which was 9 times higher than that of the conventional amine/oleic acid-based CsPbBr3 system. Our work highlights that through controlling the surface ligand shells we can tune the photoredox activity of CsPbBr3 PNCs. Thus, we envisioned that this work will provide new insights for the future design of halide perovskite nanomaterials suitable for photocatalytic reactions.Author contributions
S. A. conducted all syntheses, under the guidance of N. M. S. A. and V. G. V. D. analysed the optical spectroscopy data under the guidance of N. M. S. A. and N. M. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the HRTEM FACILITY at the SRMIST set up with support from MNRE (Project no. 31/03/2014-15/PVSER&D), Government of India.References
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed
.
- G. Almeida, O. J. Ashton, L. Goldoni, D. Maggioni, U. Petralanda, N. Mishra, Q. A. Akkerman, I. Infante, H. J. Snaith and L. Manna, J. Am. Chem. Soc., 2018, 140, 14878–14886 CrossRef PubMed
.
- M. Li, X. Zhang, Y. Du and P. Yang, J. Lumin., 2017, 190, 397–402 CrossRef CAS
.
- M. Li, X. Zhang, T. Dong, P. Wang, K. Matras-Postolek and P. Yang, J. Phys. Chem. C, 2018, 122, 28968–28976 CrossRef CAS
.
- A. Swarnkar, R. Chulliyil, V. K. Ravi, M. Irfanullah, A. Chowdhury and A. Nag, Angew. Chem., 2015, 127, 15644–15648 CrossRef
.
- V. G. V. Dutt, S. Akhil and N. Mishra, ChemNanoMat, 2020, 6, 1730–1742 CrossRef CAS
.
- S. Akhil, V. G. V. Dutt and N. Mishra, ChemNanoMat DOI:10.1002/cnma.202100002
.
- B. A. Koscher, J. K. Swabeck, N. D. Bronstein and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 6566–6569 CrossRef CAS PubMed
.
- F. Di Stasio, S. Christodoulou, N. Huo and G. Konstantatos, Chem. Mater., 2017, 29, 7663–7667 CrossRef
.
- H. Lin, C. Zhou, Y. Tian, T. Siegrist and B. Ma, ACS Energy Lett., 2018, 3, 54–62 CrossRef CAS
.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Science, 2017, 358, 745–750 CrossRef CAS PubMed
.
- S. Bai, Z. Yuan and F. Gao, J. Mater. Chem. C, 2016, 4, 3898–3904 RSC
.
- G. Rainò, G. Nedelcu, L. Protesescu, M. I. Bodnarchuk, M. V. Kovalenko, R. F. Mahrt and T. Stöferle, ACS Nano, 2016, 10, 2485–2490 CrossRef PubMed
.
- K. Chen, S. Schünemann, S. Song and H. Tüysüz, Chem. Soc. Rev., 2018, 47, 7045–7077 RSC
.
- E. Yassitepe, Z. Yang, O. Voznyy, Y. Kim, G. Walters, J. A. Castañeda, P. Kanjanaboos, M. Yuan, X. Gong, F. Fan, J. Pan, S. Hoogland, R. Comin, O. M. Bakr, L. A. Padilha, A. F. Nogueira and E. H. Sargent, Adv. Funct. Mater., 2016, 26, 8757–8763 CrossRef CAS
.
- M. Meyns, M. Perálvarez, A. Heuer-Jungemann, W. Hertog, M. Ibáñez, R. Nafria, A. Genç, J. Arbiol, M. V. Kovalenko, J. Carreras, A. Cabot and A. G. Kanaras, ACS Appl. Mater. Interfaces, 2016, 8, 19579–19586 CrossRef CAS PubMed
.
- Q. A. Akkerman, M. Gandini, F. Di Stasio, P. Rastogi, F. Palazon, G. Bertoni, J. M. Ball, M. Prato, A. Petrozza and L. Manna, Nat. Energy, 2016, 2, 1–7 Search PubMed
.
- P. Ramasamy, D.-H. Lim, B. Kim, S.-H. Lee, M.-S. Lee and J.-S. Lee, Chem. Commun., 2016, 52, 2067–2070 RSC
.
- Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang, Z. Yi, J. Li, X. Xie, Y. Wang, Y. Li, D. Fan, D. B. L. Teh, A. H. All, O. F. Mohammed, O. M. Bakr, T. Wu, M. Bettinelli, H. Yang, W. Huang and X. Liu, Nature, 2018, 561, 88–93 CrossRef CAS PubMed
.
- Z.-C. Kong, J.-F. Liao, Y.-J. Dong, Y.-F. Xu, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, ACS Energy Lett., 2018, 3, 2656–2662 CrossRef CAS
.
- J. Hou, S. Cao, Y. Wu, Z. Gao, F. Liang, Y. Sun, Z. Lin and L. Sun, Chem.–Eur. J., 2017, 23, 9481–9485 CrossRef CAS PubMed
.
- G. Gao, Q. Xi, H. Zhou, Y. Zhao, C. Wu, L. Wang, P. Guo and J. Xu, Nanoscale, 2017, 9, 12032–12038 RSC
.
- C. S. Ponseca, E. M. Hutter, P. Piatkowski, B. Cohen, T. Pascher, A. Douhal, A. Yartsev, V. Sundström and T. J. Savenije, J. Am. Chem. Soc., 2015, 137, 16043–16048 CrossRef CAS PubMed
.
- A. Giampietri, G. Drera and L. Sangaletti, Adv. Mater. Interfaces, 2017, 4, 1700144 CrossRef
.
- C. de Weerd, L. Gomez, H. Zhang, W. J. Buma, G. Nedelcu, M. V. Kovalenko and T. Gregorkiewicz, J. Phys. Chem. C, 2016, 120, 13310–13315 CrossRef CAS
.
- K. Zheng, Q. Zhu, M. Abdellah, M. E. Messing, W. Zhang, A. Generalov, Y. Niu, L. Ribaud, S. E. Canton and T. Pullerits, J. Phys. Chem. Lett., 2015, 6, 2969–2975 CrossRef CAS PubMed
.
- Q. Hu, E. Rezaee, Q. Dong, H. Shan, Q. Chen, L. Wang, B. Liu, J.-H. Pan and Z.-X. Xu, Sol. RRL, 2019, 3, 1800264 CrossRef
.
- M. Kulbak, D. Cahen and G. Hodes, J. Phys. Chem. Lett., 2015, 6, 2452–2456 CrossRef CAS PubMed
.
- J. B. Hoffman, G. Zaiats, I. Wappes and P. V. Kamat, Chem. Mater., 2017, 29, 9767–9774 CrossRef CAS
.
- E. M. Sanehira, A. R. Marshall, J. A. Christians, S. P. Harvey, P. N. Ciesielski, L. M. Wheeler, P. Schulz, L. Y. Lin, M. C. Beard and J. M. Luther, Sci. Adv., 2017, 3, eaao4204 CrossRef PubMed
.
- Y. Hu, F. Bai, X. Liu, Q. Ji, X. Miao, T. Qiu and S. Zhang, ACS Energy Lett., 2017, 2, 2219–2227 CrossRef CAS
.
- S. Xiang, Z. Fu, W. Li, Y. Wei, J. Liu, H. Liu, L. Zhu, R. Zhang and H. Chen, ACS Energy Lett., 2018, 3, 1824–1831 CrossRef CAS
.
- K. Chen, X. Deng, G. Dodekatos and H. Tüysüz, J. Am. Chem. Soc., 2017, 139, 12267–12273 CrossRef CAS PubMed
.
- H. Lu, X. Chen, J. E. Anthony, J. C. Johnson and M. C. Beard, J. Am. Chem. Soc., 2019, 141, 4919–4927 CrossRef CAS PubMed
.
- X. Luo, R. Lai, Y. Li, Y. Han, G. Liang, X. Liu, T. Ding, J. Wang and K. Wu, J. Am. Chem. Soc., 2019, 141, 4186–4190 CrossRef CAS PubMed
.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Nat. Energy, 2016, 2, 1–8 Search PubMed
.
- Y.-F. Xu, M.-Z. Yang, B.-X. Chen, X.-D. Wang, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, J. Am. Chem. Soc., 2017, 139, 5660–5663 CrossRef CAS PubMed
.
- M. Ou, W. Tu, S. Yin, W. Xing, S. Wu, H. Wang, S. Wan, Q. Zhong and R. Xu, Angew. Chem., 2018, 130, 13758–13762 CrossRef
.
- S. Schünemann, M. van Gastel and H. Tüysüz, ChemSusChem, 2018, 11, 2057–2061 CrossRef PubMed
.
- H. Huang, H. Yuan, J. Zhao, G. Solís-Fernández, C. Zhou, J. W. Seo, J. Hendrix, E. Debroye, J. A. Steele, J. Hofkens, J. Long and M. B. J. Roeffaers, ACS Energy Lett, 2019, 4, 203–208 CrossRef CAS
.
- X. Zhu, Y. Lin, Y. Sun, M. C. Beard and Y. Yan, J. Am. Chem. Soc., 2019, 141, 733–738 CrossRef CAS PubMed
.
- Y. Li, Q. Shu, Q. Du, Y. Dai, S. Zhao, J. Zhang, L. Li and K. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 451–460 CrossRef CAS PubMed
.
- S. W. Tong, N. Mishra, C. L. Su, V. Nalla, W. Wu, W. Ji, J. Zhang, Y. Chan and K. P. Loh, Adv. Funct. Mater., 2014, 24, 1904–1910 CrossRef CAS
.
- N. Mishra, V. G. Vasavi Dutt and M. P. Arciniegas, Chem. Mater., 2019, 31, 9216–9242 CrossRef CAS
.
- H. Lu, X. Zhu, C. Miller, J. San Martin, X. Chen, E. M. Miller, Y. Yan and M. C. Beard, J. Chem. Phys., 2019, 151, 204305 CrossRef PubMed
.
- K. Wu, G. Liang, Q. Shang, Y. Ren, D. Kong and T. Lian, J. Am. Chem. Soc., 2015, 137, 12792–12795 CrossRef CAS PubMed
.
- S. Akhil, V. G. V. Dutt and N. Mishra, Chem.–Eur. J., 2020, 26, 17195–17202 CrossRef CAS PubMed
.
- V. G. V. Dutt, S. Akhil and N. Mishra, CrystEngComm, 2020, 22, 5022–5030 RSC
.
- X. Zhu, Y. Lin, J. San Martin, Y. Sun, D. Zhu and Y. Yan, Nat. Commun., 2019, 10, 2843 CrossRef PubMed
.
- J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Chem. Rev., 2019, 119, 3296–3348 CrossRef CAS PubMed
.
- J. De Roo, M. Ibáñez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. Van Driessche, M. V. Kovalenko and Z. Hens, ACS Nano, 2016, 10, 2071–2081 CrossRef CAS PubMed
.
- J. T. DuBose and P. V. Kamat, J. Phys. Chem. C, 2020, 124, 12990–12998 CrossRef CAS
.
- K. E. Knowles, M. Tagliazucchi, M. Malicki, N. K. Swenson and E. A. Weiss, J. Phys. Chem. C, 2013, 117, 15849–15857 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00091h |
| This journal is © The Royal Society of Chemistry 2021 |