Open Access Article
Open Access ArticleTailoring silver nanoclusters via doping: advances and opportunities
Jie
Yang
 *a,
Runqiang
Pang
a,
Dongpo
Song
*a,
Runqiang
Pang
a,
Dongpo
Song
 a and
Man-Bo
Li
a and
Man-Bo
Li
 *bc
*bc
aSchool of Materials Science and Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, China. E-mail: yjolah@just.edu.cn
bInstitute of Physical Science and Information Technology, Anhui University, Hefei, Anhui 230601, P. R. China. E-mail: mbli@ahu.edu.cn
cHefei National Laboratory for Physical Sciences at the Microscale, Hefei, Anhui 230026, P. R. China
First published on 9th March 2021
Abstract
Atomically precise noble metal nanoclusters (especially Au and Ag) have been pursued due to their fascinating molecule-like properties. In spite of the significant progress on Au nanoclusters (NCs), the structure and property evolution of Ag NCs is still in high demand. Doping is a useful strategy for improving the physicochemical performances of Ag NCs. Herein we summarize the recent advances in tailoring silver NC structures and properties via doping. First, we reviewed the recent studies on the synthesis of hetero metal atom doped silver bimetallic nanoclusters, which are classified by the dopants, including Au, Pt, Pd, Cu, Ni and Cd. Second, the doping effects on their properties were reviewed, including the locations of hetero metal atoms, the influence on their stability, and the charge state evolution. Moreover, we highlighted the doping-dependent improvement of the photo-luminescence (PL) performance and catalytic activity of Ag NCs.
1. Introduction
Atomically precise metal nanoclusters (NCs) composed of a discrete number of metal atoms and monodisperse coordination ligands have raised immense scientific interest. They are unique materials, which are stable at a smaller scale (<2 nm) compared with nanoparticles and still retain molecule-like characteristics, such as luminescence, molecular magnetism, chirality, etc.1–6 Furthermore, their identified formulas and atomically precise structures provide atomic-level structure–property correlation and modification.As the two most outstanding species, gold and silver NCs showed different performances in stability. Improving the stability and enhancing their diverse properties is the major priority in studying Ag NCs. Adding hetero-metal atoms into the mono-metal kernel has been demonstrated to be an effective strategy to obtain stable and functional Ag NCs, where doping methods have been extensively carried out to tailor Ag NC properties in recent studies. Generally, doping with foreign atoms will tune the geometric and electronic structures of NCs and further influence their optical properties and catalytic activity. Moreover, doping endows Ag NCs with better thermal stability.7–12
To understand the fundamental mechanism for the rational design of functional doped Ag NCs, the compositions and structures of the nanoclusters have been investigated. The study of doped nanoclusters relies on their parent nanoclusters because they bear homologous structures. At the same time, the nature of the doped heteroatoms also plays a crucial role in tuning the structures and properties. Many promising metal atoms have been chosen and proved to be competent in tailoring Ag NCs, such as Au, Pt, Pd, Cu, etc., and several representative species of MxAgn−x NCs have been identified.8–15
Although many important advances have been made for gold nanoclusters, silver and its doped NCs are still not well understood. It is challenging to synthesize stable Ag NCs due to the easy oxidation of Ag under the zero-valent state. The breakthrough came from Ag44 with a determined crystal structure.16,17 Subsequently, some well determined Ag NCs with various stable sizes were prepared, such as Ag20,18,19 Ag21,19,20 Ag25,21 Ag29, (ref. 22) and so on. Meanwhile, significant advances have been achieved in recent studies, which focus on the hetero-metal atom doping of Ag NC templates. However, there are few reviews that focus on Ag and its doped NCs. In our previous review, we have summarized new advances in synthetic methods, new sizes, structural determination and properties of Ag NCs.23 In addition, in our latest review, we discussed luminescent Ag NCs and PL enhancement strategies.24 In this work, we will highlight some recent advances in foreign-atom doped Ag NCs with a focus on their synthesis and identified structures. Of note, in this review we only focus on bimetallic Ag NCs. Trimetallic Ag NCs are not included in this review. Moreover, we will discuss doping effects on their properties, including the location and stability (where the foreign atoms go: the surface or inside the kernel), the changes in the charge state, the PL enhancement and the improvement in catalytic activity and selectivity. Finally, we provide our perspectives.
2. Synthesis of doped silver bimetallic nanoclusters
The concept of doping emerging in the fields of metal nanoclusters has been clarified in previous reviews.8–10 Herein, doped Ag NCs are identified as MxAgn−x(SR)m (M = Au, Pt, Pd, Cu, etc.). The synthesis methods can be classified into four categories: (1) co-reduction of mixed metal-complexes, such as one-pot reduction of Ag–SR and M–SR mixed complexes, where the parent silver complexes and hetero-metal complexes are simultaneously reduced in one pot by the reductant; (2) doping homo-metal clusters with foreign metal salts or complexes, via galvanic exchange and anti-galvanic exchange, where hetero-metal salts or complexes react with Ag NCs via a redox process, and hetero-metals take the place of some Ag atoms, forming alloys; (3) inter-cluster reactions, where the reactions between Ag NCs and foreign metal NCs introduce foreign metals into Ag NCs, generating Ag bimetallic NCs; (4) the ligand-exchange method, where the structures of Ag bimetallic clusters can be converted by their reaction with introduced ligands. Atomically precise doping provides convenience for studying the composition and structure evolution. In addition, the number and nature of doping hetero-metal atoms and their doping locations (including centre, inner shell and outer shell) are also crucial factors, which need to be seriously considered. Different heteroatoms provide different electronic and geometric structures and diverse properties. This section summarizes the doping of different heteroatoms into silver nanoclusters, mainly including dopants like Au, Pt, and Pd, and non-noble metal atoms like Cu, Ni and Cd.According to the advances in Ag NCs, there are several typical Ag NC templates for preparing doped Ag NCs: the most famous types are based on Ag25, Ag29 and Ag44, and there a few studies that are also based on Ag20, Ag21 and some other stable sizes. Here, we will summarize doped Ag NCs according to the doped foreign atoms. We have reviewed hetero-metal atom doped Ag NCs in Table 1.
| Dopants | Templates | Formula | Synthesis methods | Ref. |
|---|---|---|---|---|
| Au | Ag25 | [Ag24Au(2,4-SPhMe2)18]− | Galvanic reaction | 26 |
| [Au1Ag24(Dppm)3(SR)17]2+ | Co-reduction | 27 | ||
| Ag29 | Au1Ag28(LA)123− | Co-reduction & galvanic reaction | 28 | |
| Ag29−xAux(BDT)12(TPP)4 | Co-reduction | 29 | ||
[Au4Ag23(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CBut)10(dppf)4Cl7](PF6)2 CBut)10(dppf)4Cl7](PF6)2 |
Co-reduction | 30 | ||
[Au5Ag24(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-p-But)16(dppf)4Cl4](PF6)3 CC6H4-p-But)16(dppf)4Cl4](PF6)3 |
Co-reduction | 30 | ||
| Ag44 | [Ag32Au12(SR)30]4− | 17 | ||
| M4AuxAg44−x(p-MBA)30 | Co-reduction | 31 | ||
| Ag44−xAux(SR)304− (x = 0–12). | Inter-cluster reaction | 32 | ||
| Ag20 | [Au1+xAg20−x{Se2P(OiPr)2}12]+ | Galvanic reaction | 33 | |
| [AuAg19{S2P(OnPr)2}12] | Galvanic reaction | 34 | ||
| [Au@Ag20{S2P(OnPr)2}12]+ | Galvanic reaction | 34 | ||
| Ag33 | [Ag33AuS2(PhCH2S)18(CF3COO)9(DMF)6] | Co-reduction | 35 | |
| Giant sizes | [Ag46Au24(SR)32](BPh4)2, Ag267−xAux(SR)80 | Co-reduction | 36 and 37 | |
| Pt | Ag25 | [PtAg24(SR)18]2− | Galvanic reaction | 38 |
| Pt2Ag23Cl7(PPh3)10 | Co-reduction | 39 | ||
| Pt1Ag12(dppm)5(SPhMe2)2 | Ligand-exchange | 40 | ||
| Ag29 | [PtAg28(BDT)12(TPP)4]4− | Ligand-exchange | 42 | |
| PtAg28(S-Adm)18(PPh3)4 | Ligand-exchange & co-reduction | 41 and 46 | ||
| [PtAg28(BDT)12(PPh3)4]4− | Ligand-exchange | 44 | ||
| Other sizes | Pt1Ag31(S-Adm)16(DPPM)3Cl3 | Galvanic reaction | 47 | |
| [Pt3Ag33(PPh3)12Cl8]+ | Galvanic reaction | 48 | ||
| PtAg20(dtp)12, Pt2Ag33(dtp)17, Pt3Ag44(dtp)22 | Co-reduction | 49 | ||
| Pd | Ag25 | [PdAg24(SR)18]2− | Co-reduction | 25 |
| Ag29 | [PdAg28(S-Adm)18(PPh3)4]2+ | Co-reduction | 50 | |
| Ag21 | [PdAg20{S2P(OnPr)2}12] | Co-reduction | 51 | |
| [Pd6Ag14(S){S2P(OnPr)2}12] | Co-reduction | 51 | ||
| Cu | [Ag28Cu12(SR)24]4− | Co-reduction | 52 | |
| [Ag61Cu30(SAdm)38S3]BPh4 | Co-reduction | 53 | ||
| Ni | Ag25 | [NiAg24(SPhMe2)18]0, [NiAg24(SPhMe2)18]2− | Co-reduction | 14 |
| Ag29 | [NiAg28(BDT)12]4−, [NiAg28(BDT)12]3− | Ligand-exchange | 55 | |
| Cd | Cd12Ag32(SePh)36 | Co-reduction | 54 |
2.1 Au doping
Silver and gold have nearly identical atomic radii, which are 2.89 and 2.88 Å, respectively. Gold is much more stable in the zero-valent state. Gold doped Ag NCs have been well studied and compared with their Ag NC analogue. The Au dopant can occupy both positions, i.e., the centre core and the kernel shell.[Ag25(SR)18]− was reported in 2015 by Osman M. Bakr et al.21 At the same time, Zheng's group gave a report on homologous gold doped Ag NCs: Ag24M(SR)18,25 and then Bakr et al. prepared [Ag24Au(2,4-SPhMe2)18]− in 2016.26 Zhu and his co-workers prepared [Au1Ag24(Dppm)3(SR)17]2+, which contained 6 free valence electrons.27
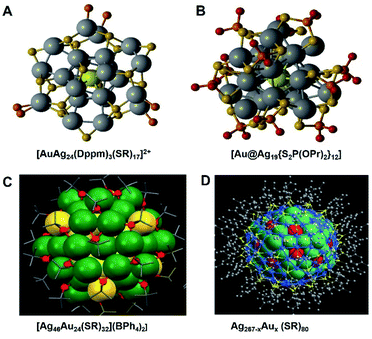
This work bridges the gap in the understanding of 6e Ag24M(SR)18 NCs. As Ag25 fits the super atom model, there are two reasonable charges of M@Au24: 0 or 2 charge states, which correspond to 6e and 8e, respectively. They synthesized this Au1Ag24via the co-reduction method, where Ag and Au ions were reduced in one pot. The formula has been identified as 1 Au and 24 Ag protected by 17 cyclohexyl mercaptan and 3 Dppm via ESI-MS measurements. The [Au1Ag24(Dppm)3(SR)17]2+ contains open icosahedral Au1Ag12 surrounded by a Ag12(Dppm)3(SR)15 ring motif and two thiols, see Fig. 1A. In the Au1Ag12 kernel, there is a distortion, which leads to longer Au–Ag and Ag–Ag bond lengths that range from 2.730 to 3.068 Å and from 2.851 to 2.982 Å, respectively.
 | ||
| Fig. 1 Crystal structure of Au doped Ag Bimetallic NCs. Redrawn (A and B) and reproduced (C and D) from ref. 27, 34, 36 and 37. Copyright 2019 and 2018 Royal Society of Chemistry. Copyright 2015 American Association for the Advancement of Science. Copyright 2018 Springer Nature. | ||
There are more varieties of gold-doped Ag29 NCs, and they contain different numbers of surface ligands. Groot's group tried to synthesize Au1Ag28(LA)123− (LA = lipoic acid) via the co-reduction approach and galvanic reactions, and the products show a preference for mono-doped clusters.28 In their work, they predicted the central location of the doped Au by X-ray absorption spectroscopy (XAS). Cui29 and his co-workers prepared Ag29−xAux(BDT)12(TPP)4 (x = 1–5), and they identified the reasonable substitution site of x Au by using DFT and TD-DFT calculations. Of note, according to the calculations, the energy barrier of center doping is about 10 kcal mol−1, which is less than that of the others, matching the experimental results that it is the most stable one. They further discussed the crucial influences of the heteronuclear Au–Ag bonds on the electronic structures and optical performance. We will discuss the photoluminescence enhancement in the next section. In Wang's work,30 they claimed that surface ligands play a vital role and can adjust the doping pattern in Ag alloy NCs. There was only a subtle change of groups between the two alkynyl ligands, which resulted in the formation of two entirely different bimetallic NCs: [Au4Ag23(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CBut)10(dppf)4Cl7](PF6)2 and [Au5Ag24(C
CBut)10(dppf)4Cl7](PF6)2 and [Au5Ag24(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-p-But)16(dppf)4Cl4](PF6)3. The synthesis was carried out by the method of co-reduction. Au4Ag23 and Au5Ag24 have different geometric structures and electronic properties. They both have an icosahedral Au@Ag12 core, and the outer motifs are Au3Ag11(C
CC6H4-p-But)16(dppf)4Cl4](PF6)3. The synthesis was carried out by the method of co-reduction. Au4Ag23 and Au5Ag24 have different geometric structures and electronic properties. They both have an icosahedral Au@Ag12 core, and the outer motifs are Au3Ag11(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CBut)10(dppf)4Cl7 in Au4Ag23 and Au4Ag12(C
CBut)10(dppf)4Cl7 in Au4Ag23 and Au4Ag12(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-p-But)16(dppf)4Cl4 in Au5Ag24, respectively. The total number of valence electrons in Au4Ag23 is eight, which belongs to the spherical superatomic series, while the Au@Ag12 kernel of Au5Ag24 is an oblate ellipsoid, and the number of valence electrons is 6, which can fit the electronic configuration [Sσ]2[Pσ]4. This work provides new insights into the unexpected role of the ligand in doping reactions.
CC6H4-p-But)16(dppf)4Cl4 in Au5Ag24, respectively. The total number of valence electrons in Au4Ag23 is eight, which belongs to the spherical superatomic series, while the Au@Ag12 kernel of Au5Ag24 is an oblate ellipsoid, and the number of valence electrons is 6, which can fit the electronic configuration [Sσ]2[Pσ]4. This work provides new insights into the unexpected role of the ligand in doping reactions.
The Ag44(SR)30 is the first reported Ag NC with a determined structure. In 2013, both Bigioni and Zheng reported the structure of [Ag44(p-MBA)30]4− and [Ag44(SR)30]4− (SR = SPhF, SPhF2, or SPhCF3), respectively.16,17 And in the same work of Zheng's group, several [Ag32Au12(SR)30]4− (SR = SPhF, SPhF2, or SPhCF3) alloys have also been obtained.17 Further, Bigioni et al.31 have mapped the possible compositions of Ag alloy NCs which take M4AuxAg44−x(p-MBA)30 (M = counteraction, x = 0–12) as the model, while other groups also reported the synthesis of [Ag44−xAux(SR)30]4− alloy NCs. In the work of Pradeep and his co-workers,32 they prepared Au doped Ag NCs via an inter-cluster reaction between Ag44(SPhF2)304− and Au25(PET)18−:
| Ag44(SPhF2)304− + Au25(PET)18− → Ag44−xAux(SR)304− (x = 0–12). |
Apart from the Au doped Ag NCs, by using the common Ag NC templates, several other stable sizes of Ag alloys have been reported. Liu et al. synthesized Ag20 and Ag21 NCs, where S and Se were used as donor ligands, respectively.18–20 In their studies, they also reported alloys based on these Ag20 and Ag21 templates. They obtained [Au1+xAg20−x{Se2P(OiPr)2}12]+ (x = 0–2) alloy NCs by performing galvanic exchange on the [Ag20{Se2P(OiPr)2}12] template.33 It's noteworthy that the first dopant Au leads to a metal nuclearity increase from 20 to 21, whereas subsequent Au-atom doping maintains the total number of metal atoms. This is different from the reported mechanism for Au doped Ag NCs we mentioned before. The single crystals of [Au@Ag20{Se2P(OiPr)2}12]+ and [Au@Au2Ag18{Se2P(OiPr)2}12]+ were successfully obtained and their structures were determined. The [Au@Ag20{Se2P(OiPr)2}12]+ bears a T symmetry, which is similar to the [AuAg20{Se2P(OEt)2}12]+ and [Au@Au2Ag18{Se2P(OiPr)2}12]+ that possess C1 symmetries, while their parent [Ag20{Se2P(OiPr)2}12] has a C3 symmetry. There are two types of S-donor Au doping Ag alloys: AuAg19 and AuAg20.34 Firstly, [AuAg19{S2P(OnPr)2}12] was prepared via a galvanic exchange route based on the Ag20 template, see Fig. 1B. And it was further transformed to higher nuclearity [Au@Ag20{S2P(OnPr)2}12]+ by reaction with Ag+ salt at low temperature. These studies give a prospect for further study of core-size controlled preparation of the doped Ag NCs.
Zang's35 group synthesized 3-electron Ag34 NCs and Au doped Ag33Au alloy NCs via the one-pot reduction method. Ag33Au has a similar framework to Ag34, where the doping Au atom takes the place of the core Ag atom. However, Ag33Au showed vigorous stability compared to Ag34. Furthermore, some larger sized Au doped Ag NCs have been reported, for instance, Au24Ag46 (ref. 36) and AuxAg267−x,37 see Fig. 1C and D. The [Au24Ag46(SR)32](BPh4)2 contains a Ag2@Au18@Ag20 kernel which is protected by a chiral Ag24Au6(SR)32 outer shell.36 Unlike the other Agn−xAux(SR)m alloy NCs we discussed above, the doped Au atoms didn't occupy the centre of the core of the Au24Ag46 NCs. The spherical (AuAg)267(SR)80 is generated with trigonal-prismatic (AuAg)45(2,4-SPhMe2)27 (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) via co-crystallization. The Ag267−xAux (SR)80 (ref. 37) contains a four concentric-shell icosahedral structure of Ag@M12@M42@M92@Ag120(SR)80 (M = Au or Ag) with Ih symmetry. The centre atom of the core is Ag, the same as that in the [Ag46Au24(SR)32](BPh4)2.
1) via co-crystallization. The Ag267−xAux (SR)80 (ref. 37) contains a four concentric-shell icosahedral structure of Ag@M12@M42@M92@Ag120(SR)80 (M = Au or Ag) with Ih symmetry. The centre atom of the core is Ag, the same as that in the [Ag46Au24(SR)32](BPh4)2.
2.2 Pt doping
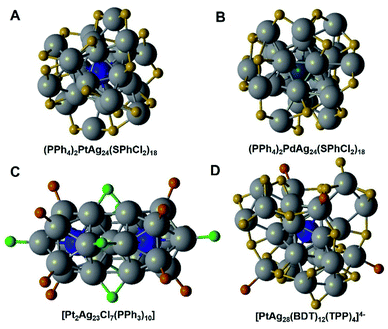
Platinum doping in Ag NCs has been demonstrated to be feasible. According to the reported studies, most of the Pt–Ag alloys are synthesized based on the Ag25 and Ag29 templates.Zheng et al. have synthesized a doped Ag NC [MAg24(SR)18]2−, in which the dopant could be Pd or Pt.38 They adopted and improved the one-pot synthesis method of [Ag44(SR)30]4−, by adding PdCl2 or K2PtCl4, to prepare [MAg24(SR)18]2−. The [MAg24(SR)18]2− (M = Pd, Pt) NC bears a M@Ag12 core capped by six distorted dimeric –RS–Ag–SR–Ag–SR– staple motifs, see Fig. 2A and B. Basset and his co-workers have synthesized a rod-like Pt2Ag23Cl7(PPh3)10via a one pot co-reduction method.39 It contains two PtAg12 icosahedra, which are self-assembled into a rod-shape by vertex-sharing, see Fig. 2C. Electronic structure calculations further illustrate the origin of Pt centre-doping-dependent enhancement of stability. Zhu and his co-workers obtained Pt1Ag12(dppm)5(SPhMe2)2 NCs by using the ligand-exchange method with Pt2Ag23Cl7(PPh3)10.40 After first etching with a HS–PhMe2 ligand, Pt2Ag23Cl7(PPh3)10 transformed to PtAg24(SPhMe2)18, where the rod-like PtAg12 icosahedra split into one PtAg12 icosahedron capped with six Ag2(SR)3 dimeric motifs. After that, the PtAg24(SPhMe2)18 NC was etched into a smaller mono-icosahedral PtAg12 nanocluster by a dppm ligand. However, PtAg12 NCs are not as stable as Pt2Ag23 and PtAg24, and it is difficult to obtain their single crystals. Wu's group reported a PtAg26(2-EBT)18(PPh3)6 alloy, in which a bipyramid Pt@Ag14 kernel is surrounded by six dimeric (–SR–Ag–SR–Ag–PR3–) staple motifs.41 Both PtAg26 and PtAg24 are 8e superatoms, and they share highly similar electronic structures.
 | ||
| Fig. 2 Crystal structure of Pt (A, C and D) and Pd (B) doped Ag bimetallic NCs. Redrawn with permission of ref. 38, 39 and 42. Copyright 2015 and 2017 American Chemical Society. Copyright 2017 Royal Society of Chemistry. | ||
There are several studies that reported the synthesis of PtAg28 NCs. Unlike Au1Ag28 NCs, PtAg28 NCs were prepared via the ligand-exchange approach and more kernel structure types were formed, including icosahedral and FCC structures. Basset et al. synthesized [PtAg28(BDT)12(TPP)4]4− by the reaction of Pt2Ag23Cl7 (TPP)10 with a BDTH2 (1,3-benzenedithiol) ligand.42 The Pt dopant in PtAg28 also occupies the center position of the icosahedron core. PtAg28 possesses a closed-shell electronic configuration, showing enhanced stability, see Fig. 2D. Of note, they further doped Au atoms into these bimetallic NCs, and found out that the Au atom takes the place of the Ag atom, forming [PtAuAg27(BDT)12]4− trimetallic NCs. Kang et al.43 prepared PtAg28(S-Adm)18(PPh3)4 NCs via etching Pt1Ag24(SPhMe2)18 with the 1-adamantanethiolate and triphenyl phosphine ligands. The Pt atom in the PtAg28(S-Adm)18(PPh3)4 also occupies the center position. This NC bears a face-centered cubic (FCC) PtAg12 kernel, which is different from that of the doped Ag NCs that we discussed above. PtAg28(S-Adm)18(PPh3)4 exhibited better thermal-stability and stronger luminescence than Pt1Ag24(SPhMe2)18. In subsequent research, chemists tried to identify the relationship between the icosahedron and FCC structures. Bakr et al.44 obtained [PtAg28(BDT)12(PPh3)4]4− NCs with a FCC structure by the ligand exchange method, using dithiolate 1,3-benzenedithiolate to replace the bulky mono-thiolate 1-adamantanethiolate in the icosahedron [PtAg28(S-Adm)18(PPh3)4]2+. Zhu and his co-workers45 reported the reversible transformation between the icosahedral and FCC structures: the 1-adamantanethiolate capped PtAg28 with a FCC kernel was transformed to its isomer with an icosahedral kernel in the presence of cyclohexanethiolate. Interestingly, the reverse process was also observed by the addition of 1-adamantanethiolate. In the latest work of Huang's group,46 they synthesized [PtAg28(S-Adm)18(PPh3)4]2+ by a one-pot wet chemical method. It was a breakthrough in preparing PtAg28 alloy NCs. Compared with the previous ligand-exchange method, they achieved high symmetry with the FCC kernel in high yield.
Although most of the studies on Pt doped Ag NCs are based on templates of Ag25 or Ag29, some other sized Pt–Ag alloy NCs were investigated as well. Kang et al.47 prepared Pt1Ag31(S-Adm)16(DPPM)3Cl3via the reaction between Pt1Ag28(SAdm)18(PPh3)4 and Ag2(DPPM)Cl2 complexes. The Ag2(DPPM)Cl2 substitutes the Ag–PPh3 units, leading to the size growth of the NCs. Although it maintains the same composition as the Pt1Ag12 kernel, the FCC configuration in the Pt1Ag28 changes to an icosahedral configuration. The Pt1Ag12 kernel is protected by a three Ag6(SR)6(DPPM)Cl assembly via sharing Ag2(SR)3 edges. Pt1Ag31(S-Adm)16(DPPM)3Cl3 exhibited a different UV absorption compared to Pt1Ag28(SAdm)18(PPh3)4, so it is easy to distinguish them. [Pt3Ag33(PPh3)12Cl8]+ with three icosahedral PtAg12 building blocks, where adjacent PtAg12 units share a vertex with silver atoms in a cyclic manner, was reported by Zhu's group.48 It can be classified as the same homologous series with rod-like Ag23Pt2Cl7(PPh3)10 and Pt1Ag12(dppm)5(SPhMe2)2 NCs. It exhibits growing evolution from single icosahedral PtAg12 to rod-like bi-icosahedral PtAg12, and then to a cyclic tri-icosahedral PtAg12. Comparing the UV absorption and PL excitation of Pt3Ag33 with those of Pt1Ag12 and Pt2Ag23, it was found that there is a continuous red shift with the assembly of more PtAg12 units. Furthermore, Liu and his co-workers49 obtained three bimetallic Pt doped Ag NCs: PtAg20(dtp)12, Pt2Ag33(dtp)17, and Pt3Ag44(dtp)22 (dtp: dipropyl dithiophosphate) via the one pot co-reduction approach, which are 8e, 16e and 22e super molecules, respectively. Similar to Pt1Ag12, Pt2Ag23 and Pt3Ag33, these three homoleptic PtAg20(dtp)12, Pt2Ag33(dtp)17, and Pt3Ag44(dtp)22 contain one, two and three centered icosahedral Pt@Ag12 units, respectively. Pt2Ag33(dtp)17 with 16 electrons contains two noninteracting 8-electron vertex-sharing icosahedral super molecules, and its electronic structure is isolobal to Ne2. The Pt@Ag12 units in Pt3Ag44(dtp)22 are assembled linearly by vertex-sharing, and its electronic structure is isolobal to the triiodide ion. These inter-icosahedral bonding interactions provide evidence and chances for deeper understanding and further investigation of super molecular alloys.
2.3 Pd doping
Similar to Pt doped Ag NCs, palladium doped Ag NCs are usually prepared based on Ag20, Ag25 and Ag29 NCs, and the Pd dopant also prefers center doping. As we discussed above, Zheng's group25 used the one pot method to prepare [PdAg24(SR)18]2−. It has a centred icosahedral Pd@Ag12 core stabilized by six distorted dimeric staple –RS–Ag–SR–Ag–SR– motifs, which is similar to Au25(SR)18. They carefully compared the structures of [PdAg24(SR)18]2− and Au25(SR)18, and found that there were several distortions in PdAg24. Moreover, the distorted dimeric staple motifs change the surface chemical environment. Due to the interactions between neighbouring staple units on the surface, they bear shorter Ag…S distances (3.006 Å in average) than Au25(SR)18. Therefore, the structure of [PdAg24(SR)18]2− can be considered as an icosahedral Pd@Ag12 core capped by four triangular surface [(AgSR)3(SR)3/2] units.Huang et al.50 prepared [PdAg28(S-Adm)18(PPh3)4]2+ with a FCC kernel surrounded by a tetrahedron-like Ag16S18P4 shell via the one pot approach. The outer shells contain four Ag4S6P1 staple motifs sharing six S, which is the same as that of the PtAg28 we mentioned above. Of note, PdAg28 reacted with AuPPh3Cl, forming AuAg28 bimetallic NCs instead of trimetallic alloy NCs. This observation is quite different from that of the reaction between PtAg28 and the Au complex. Liu51 and his co-workers prepared [PdAg20{S2P(OnPr)2}12] based on the [Ag21{S2P(OiPr)2}12]+ template, by the co-reduction method. It has an 8e [PdAg12]4+ superatomic core capped by eight Ag+ in C2 symmetry. DFT calculations show that the center displacement energy of Pd is more than 20 kcal mol−1 that of other positions. Moreover, they obtained [Pd6Ag14(S){S2P(OnPr)2}12] by increasing the amount of [Pd{S2P(OnPr)2}2]. Its structure is completely different from PdAg20, even though they exist in the reaction solution at the same time. The [Pd6Ag14(S){S2P(OnPr)2}12] has a sulfide-centered Pd6Ag2 rhombohedron core capped by twelve silver atoms with S6 symmetry, and it displays a smaller electrochemical HOMO–LUMO gap. Compared with the common icosahedron or FCC core, this octahedral hexa-palladium(0) kernel embedded within a silver(I) cluster is a novel structure type in alloy NCs.
2.4 Cu doping
Cu(0) nanoclusters are rarely reported due to their easy oxidation. There is only a little preliminary progress on copper doped Ag NCs. Zheng's group reported the first Ag–Cu bimetallic alloy, [Ag28Cu12(SR)24]4−.52 It has a chiral metal framework with T symmetry, and its kernel is arranged in a three-concentric shell structure composed of Ag4@Ag24@Cu12(SR)24, where a two-shell Ag4@Ag24 core is capped by four nearly planar Cu3(SR)6 staple motifs, see Fig. 3A. The Cu atoms take the place of the Ag atoms in the outer shell, and these 28 Ag atoms are arranged in a distorted FCC pattern, which results in a distorted HCP Ag–Cu interface. Its chiral separation and asymmetric synthesis was conducted by ion pairing with chiral quaternary ammonium salts. In the synthesis process, they used achiral counter cations (nBu4N+), and hence the as-prepared cluster was a racemic mixture. Furthermore, this racemic mixture can be separated into enantiomers by using chiral quaternary ammonium salts, such as N-benzylcinchonidinium chloride (BCDC) and N-benzylcinchoninium chloride (BCNC), thereby obtaining optically pure enantiomers separately. This asymmetric synthesis and ion paring chiral enantio-separation provides effective routes for preparing chiral metal NCs.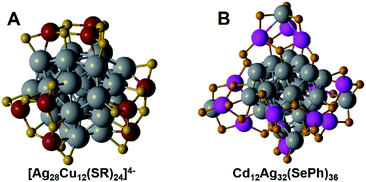 | ||
| Fig. 3 Crystal structure of Pd (A) and Cd (B) doped Ag Bimetallic NCs. Redrawn with permission of ref. 52 and 54. Copyright 2016 and 2019 American Chemical Society. | ||
Zhu's53 group reported kernel-doping of Cu atoms in Ag NCs, and the preparation of a giant bimetal NC [Ag61Cu30(SAdm)38S3]BPh4 which possesses a rare valence of 46 free electrons. Ag61Cu30 (Ag13@Cu30@Ag48(SAdm)38S3) also has a three-shell framework, where the Cu30 is in the middle layer. The icosahedral Ag13 kernel is enclosed by a Cu30 icosidodecahedron, where each Ag atom connects five pentagonal Cu atoms, and is further capped by an Ag48(SAdm)38S3 outer shell. Doping active metal atoms in the kernel of metal NCs offers new insights into the atomically controlled synthesis of metal alloy NCs.
2.5 Other hetero-metal doping
Apart from the above-mentioned Ag alloy nanoclusters doped with Au, Pt, Pd and Cu, several other metal doped Ag alloy NCs, such as Ni and Cd doped Ag alloy NCs, were also reported.Hyeon and Zheng et al.54 reported a Cd doped Ag nanocluster Cd12Ag32(SePh)36 by a phosphine-assisted process, see Fig. 3B. Cd occupied the outer shell positions, and formed an asymmetric two-shell Ag4@Ag24@Cd3Ag(SePh)9 framework. The Ag4@Ag24 kernel contains a Ag4 tetrahedron, which is encapsulated by four Ag6 facets. This Ag28 kernel in Cd12Ag32(SePh)36 is similar to the core in [Cu12Ag28(SR)24]4−.52 In the outer layer of Cd3Ag(SePh)9, there are three CdSe4 tetrahedra, where three Se bind with one Ag to form a AgSe3 cap-like structure. According to the electronic structure analysis and DFT calculations, it is a 20e superatom, which exhibits a chiral optical response.
Ni doped Ag NCs were synthesized based on Ag25 and Ag29. The synthesis of NiAg24 (ref. 14) was carried out by the co-reduction method while the NiAg28 (ref. 55) was prepared by the ligand-exchange method. For NiAg24, they obtained a neutral 6e [NiAg24(SPhMe2)18]0 and an 8e [NiAg24(SPhMe2)18]2−. The neutral NiAg24 is much more stable than the dianionic form. The crystal structure of [NiAg24(SPhMe2)18]2− is determined to contain a centered icosahedral NiAg12 core surrounded by six SR–Ag–SR–Ag–SR staple motifs. This structure is similar to the Pt and Pd doped MAg24. For NiAg28,55 the ESI analysis suggested two molecules with different charge states: [NiAg28(BDT)12]4− and [NiAg28(BDT)12]3−. In this work, Pradeep et al. also prepared [NiAg24(DMBT)18]2−. However, these Ni–Ag alloy NCs were less stable than their parent Ag NCs. Further work focusing on the preparation of stable Ni doped Ag NCs and understanding their geometric and electronic structures is needed.
3. Doping effects on properties
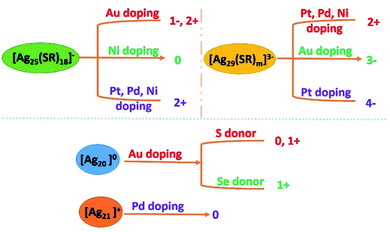
As we discussed in our previous studies, Ag NCs exhibit relatively poor stability compared to Au NCs, which makes their further study and applications challenging. Doping methods have been demonstrated to be efficient in tuning the properties of Ag NCs. The doping of Ag NCs with hetero atoms brings changes in constituent structure and electronic structure, which further influence their properties including PL, catalytic activity, chirality, etc.7,83.1 Doping location and stability
The atoms of Au, Pt, Pd, Cu and Ni have been doped into the NCs of Ag20, Ag21, Ag25, Ag29 and Ag44, etc. In general, most of the hetero atom doped Ag NCs exhibit enhanced stability compared to their parent Ag NCs. Bakr et al. showed that the [Ag24Au(SR)18]− undergoes minimal degradation in five days under ambient conditions, while pure Ag25 suffers obvious degradation after 24 h.56Benefiting from the better stability, many Ag alloy NCs are reported with a determined crystal structure. The crystal structures of these doped Ag alloy NCs were studied using a single crystal X-ray diffractometer (SC-XRD). Different dopants prefer to occupy different locations in the Ag kernel due to their unique nature, see Fig. 4. Studying the reported structures of these Ag alloy NCs, we find some rules in doping locations. The Pt, Pd and Ni heteroatoms prefer to occupy the centre core position, while Au sometimes occupies the outer shell positions in giant Ag alloy NCs. For instance, the Au doped Ag alloy NCs Ag46Au24(SR)32](BPh4)236 contain a Ag2@Au18@Ag20 kernel. And the centre position is also occupied by a Ag atom in the Ag267−xAux(SR)80 NC kernel. Unlike the relatively stable noble metals, the Cu dopant is very active, and centre doping is full of challenges. Accordingly, Cu prefers to occupy the outer shell positions. In the case of [Ag28Cu12(SR)24]4−,52 its kernel is arranged in a three-concentric shell framework composed of Ag4@Ag24@Cu12(SR)24, and the Cu atoms occupy the outermost shell to protect the two Ag4@Ag24 kernels. For [Ag61Cu30(SAdm)38S3]BPh4,53 Cu30 is arranged in the middle layer of the kernel, which is connected with the icosahedral Ag13 kernel and outer shell Ag48(SAdm)38S3.
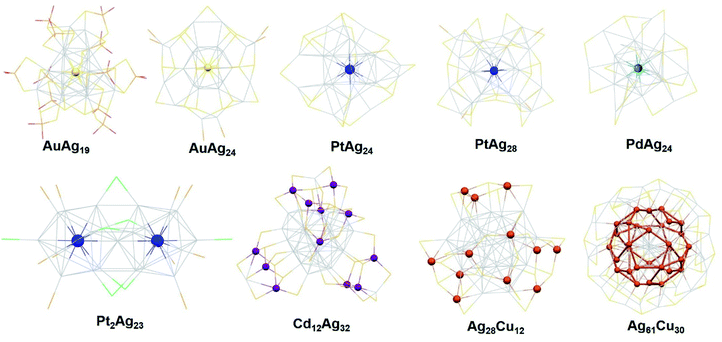 | ||
| Fig. 4 Dopant locations in the crystal structures of hetero atom doped Ag Bimetallic NCs. Redrawn with permission of ref. 27, 34, 38, 42, 49, 52, 53 and 54. Copyright 2017, 2018 and 2019 Royal Society of Chemistry. Copyright 2015, 2016, 2017, 2019 and 2020 American Chemical Society. | ||
Density functional theory (DFT) calculations and the TD-DFT method were used to study the geometric and electronic structures and further theoretically validate the optimal location of the dopants in the Ag alloy nanocluster. Dolg and Cui et al.29 studied the structures of Ag29−xAux (x = 1–5) NCs with possible isomers by performing DFT calculations. In their work, they claim that the Au atom prefers to occupy the central position. For instance, in the case of x = 1, the most stable isomer is the one in which Au is located in the central Ag13 icosahedral core. And when x = 2, the most stable isomer is the one in which two Au dopants occupy the central and vertical positions. Furthermore, they found that the Ag29−xAux (x = 1–5) NCs exhibit increased stability with the increase of Au–Ag bonds. In the work of Basset et al.,42 they used TDDFT UV-vis spectra to analyze the optimal location of Pt in the [PtAg28(BDT)12(P(CH3)3)4]4− NC, see Fig. 5A. They compared the simulated curves of PtAg28 with different Pt locations based on the experimental spectrum. Accordingly, the Pt center doped curve is well matched with the experimental spectrum as they have similar main peaks and no low-energy features. The center doped [PtAg28(BDT)12(P(CH3)3)4]4− NC shows higher thermodynamic stability. In Kappes's work, they monitored the reaction process via high-resolution trapped ion mobility mass spectrometry (TIMS), and they figured out the doping path of the gold atoms,32 see Fig. 5B. Firstly, Au atoms displace Ag atoms in the outer staples of Ag44(SPhF2)304− and further migrate to the icosahedron core. At the same time, they theoretically analyzed the isomeric structures and collision cross section (CCS) values via DFT calculations and trajectory method calculations, respectively. They claimed that the most stable isomer is the centre doped Au12Ag32(SR)30 based on the DFT calculations. In addition to the approach we mentioned above, the extended X-ray absorption fine structure (EXAFS) and the X-ray absorption near edge structure (XANES) methods were used in combination with electronic structure calculations to confirm the location of the Au dopants in the Au-doped Ag29 clusters.28 And NMR measurements were also used to predict the Au location in the Ag29(BDT)12(TPP)4 cluster,56 see Fig. 5C. Overall, the SC-XRD, TIMS, EXAFS, XANES, DFT and TD-DFT methods were used to analyze the crystal structures of the Ag alloy NCs, which provide experimental and theoretical evidence to understanding the doping process. Although several structures of the Ag alloy NCs have been reported, further studies still need to focus on the preparation of Ag alloy NCs with more stable sizes and new types of dopants.
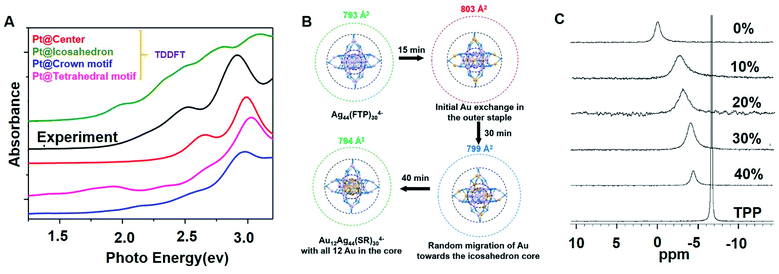 | ||
| Fig. 5 (A) TDDFT UV-vis spectra of [PtAg28(BDT)12(P(CH3)3)4]4− NCs for different Pt positions compared with the experimental spectrum. (B) Schematic of Au atom migration from the outer staple to the inner core. (C) NMR spectra of pure TPP, Ag29(BDT)12(TPP)4, and Ag29−xAux(BDT)12(TPP)4 clusters. Reproduced with permission of ref. 42, 32 and 56. Copyright 2019 American Chemical Society. Copyright 2017 Royal Society of Chemistry. Copyright 2016 Wiley-VCH. | ||
3.2 Charge state
Metal atoms show peculiarity in their outer electron arrangements and activities. Take Au and Ag as examples, both of them contain a single electron in the outermost S orbital. However, they exhibit different charge states: Ag exhibits the 1+ charge state, while Au exhibits 1+ or 3+ charge states. The addition of dopant hetero metal atoms would affect the electron cloud density and direction, and the value of the wave function would need to be recalculated. Therefore, doping would induce charge stripping and lead to diverse valence states. The [Ag25SR18]− NCs exhibit four charge states after doping with different foreign metal atoms, including 1−, 0, 2− and 2+. The Au doped Ag25SR18 alloy NCs display 1− and 2+ charge states: [Ag24Au(SPhMe2)18]− and [Au1Ag24(Dppm)3(SR)17]2+, see Scheme 1.27 And with the Pt and Pd dopants,38 a 2− charge state is seen: [MAg24(SR)18]2− (M = Pd, Pt). With the Ni dopant, 0 and 2− charge states of the NCs (neutral 6e [NiAg24(SPhMe2)18]0 and 8e [NiAg24(SPhMe2)18]2−) were obtained,14 see Scheme 1. In the case of Ag29 cluster templates, three charges, 2+ 3− and 4−, were observed. The charge state remains at 3− after Au doping, while Pt doping alters the charge state to 2+ or 4− ([PtAg28(S-Adm)18(PPh3)4]2+ and [PtAg28(BDT)12(TPP)4]4−).42,45,46 Pd doping results in a 2+ charge state, [PdAg28(S-Adm)18(PPh3)4]2+,50 and Ni would convert the charge state to 4− or maintain at 3−: [NiAg28(BDT)12]4− and [NiAg28(BDT)12]3−.55 In the Ag44(SR)30 template, the charge state remains the same. The charge states of Ag20 and Ag21 are illustrated in Scheme 1. Doping reduced charge stripping will bring more opportunities in their reaction activities. What we need to consider is how to understand the mechanisms and further explain the charge stripping.3.3 PL properties
The most anticipated property of silver nanoclusters is their photoluminescence (PL) property. However, considering their further application in a wide range of fields, there is an urgent need for the synthesis of NCs with high quantum yield (QY) and long life. We have discussed the strategies for enhancing the luminescence of Ag NCs in our latest review; these properties are governed by their constitutions and structures.24 There are two ways to improve the PL performance: modifying the metal kernel or tailoring the surface ligands. Doping is one of the most proven methods to improve the PL performance of Ag NCs. Bakr et al.56 synthesized Ag29−xAux(BDT)12(TPP)4 (x = 1–5) NCs by doping Au into Ag29(BDT)12(TPP)4 clusters. The Au doped Ag29 clusters achieved 26-fold PL QY enhancement, from 0.9% (Ag29) to 19% (30% Au dopants) and 24% (40% Au dopants).Cui et al.29 employed fs–ns TA spectroscopy to explore the electronic origins of PL enhancement. According to the TA spectra, there is an obvious shift in the GSB position at 435 nm for the Au doped Ag29 clusters. Moreover, the ESA curve displayed a change in the range of 485–800 nm after Au doping. Unlike the parent Ag29 NCs, after Au doping, the alloy shows improved time constants, where doping brings a longer lifetime, up to 612.65 ns (20% Au dopants) and 890.71 ns (40% Au dopants). The PL enhancement of the clusters depends on the nature of the excited state (S1), which is highly affected by the doping amount of the Au atoms, see Fig. 6A. They used DFT and TD-DFT methods to explore the origin of the PL enhancement of Au doped Ag29−xAux (x = 1–5) clusters, in the lowest S1 and S0 electronic states. Generally, when the doping number of Au is 1 or 2, the PL performance of the Ag29−xAux clusters is very close to that of the undoped Ag29 cluster. When it is highly doped, with the doping number of Au going up to more than 3, there is an obvious increase in the PL intensity. This reveals that the crucial effects on the PL enhancement originate from Au dopants. Furthermore, they figured out that the Au–Ag bond played an important role in increasing the stability and governing the PL intensity. However, the Au amount effects cannot be generalized. In Mattoussi's work, the PL intensity does not simply increase with the increase of Au amount.57
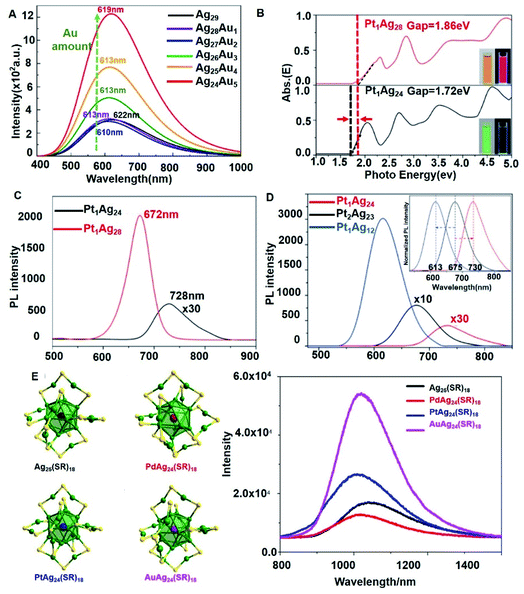 | ||
| Fig. 6 (A) TD-DFT computed emission spectra of Ag29−xAux (x = 0–5) nanoclusters. (B and C) The spectra on the energy scale (B) and PL (C) of the Pt1Ag24 and Pt1Ag28 NCs. Insets show (B): digital photographs of each nanocluster in CH2Cl2 solution under visible and UV light. (D) The PL spectra of the Pt2Ag23, Pt1Ag24 and Pt1Ag12 nanoclusters. Inset: the peak shifts in normalized PL spectra. (E)Structures and photoluminescence spectra of MAg24(SR)18 (M = Ag/Pd/Pt/Au) NCs in the crystal state. Adapted with permission from ref. 29, 43, 40 and 58. Copyright 2018 Wiley-VCH. Copyright 2017 Royal Society of Chemistry. Copyright 2017 American Chemical Society. | ||
Pt doped Ag NCs also exhibit a noticeable PL enhancement. Basset et al. reported an ∼2.3 fold PL intensity increase in Pt doped Ag29 NCs, [PtAg28(BDT)12(TPP)4]4−.42 Moreover, an ∼80 nm red shift of the PL peak with broadening appeared after doping, where the broadening was attributed to the partial replacement of TPP ligands with solvent DMF molecules. They highlighted the solvent-effect on PL enhancement of the PtAg28 alloy, whose surface was exposed to the solvent. In Zhu's work,43 they obtained Pt1Ag28(SR)18(PPh3)4 by the ligand exchange method based on Pt1Ag24(SPhMe2)18. They compared the energy scales and PL of these two alloy NCs, see Fig. 6B and C. The energy gaps of Pt1Ag24 and Pt1Ag28 are 1.72 eV and 1.86 eV, respectively. Moreover, the solution color of Pt1Ag24 is green whereas that of Pt1Ag28 is orange, and the PL of Pt1Ag28 can be observed by the naked eye under UV light in the dark. In addition, the QYs of these two Pt doped Ag NCs are very different: the QY of Pt1Ag24 is only 0.1% while that of Pt1Ag28 is up to 4.9% (about a 50-fold enhancement). The PL peak of Pt1Ag24 is at 728 nm, and it shifts to 672 nm when the Pt1Ag28 is formed. They also compared the energy gaps and the PL spectra of Pt1Ag12(dppm)5(SPhMe2)2, Pt1Ag24(SPhMe2)18 and Pt2Ag23(PPh3)10Cl7.40 The PL emission of Pt2Ag23 is at 675 nm, while it shifted to 730 nm and 613 nm when it was converted to Pt1Ag24 and Pt1Ag12, respectively, see Fig. 6D. Blue-shifts appeared in Pt2Ag23 and Pt1Ag24 when they were converted to Pt1Ag12, which can be assigned to the increase of the optical energy gap. However, the red shift of the PL emission on the conversion of Pt2Ag23 to Pt1Ag24 points out that the energy gap cannot fully represent the energy of PL due to the dramatic electronic perturbation and high vibration loss. Moreover, the PLQY displays a 28 times increase from 0.2 to 5.5% by re-assembling from Pt2Ag23 to Pt1Ag12. The PL spectra of Pt1Ag28 with different kernel structures (FCC and icosahedron) are very similar.45 The PL QY of Pt1Ag28 (icosahedron) is only 2.7% at room temperature, while that of Pt1Ag28(FCC) is 4.9%. However, the PL of Pt1Ag28 (icosahedron) is temperature-dependent. It emits bright-red light at low temperature, and the QY reaches 100% at 98 K or lower temperature. Moreover, the PL intensity increases dramatically at low temperature.
Wu and his co-workers58 studied a series of MAg24(SR)18 NCs, which were doped with different hetero atoms: Au, Pt and Pd. They compared the PL spectra of these Ag alloy NCs, see Fig. 6E. The Au doped MAg24(SR)18 showed the highest PL intensity.
To sum up, heteroatoms play a positive role in tuning the PL properties of Ag NCs. Doping leads to an obvious enhancement in the PL intensity and QY. It would provide various opportunities to their potential applications in the fields of bio-labelling, biomedicine, ion probing and so on. To synthesize and modify these functional alloy Ag NCs at the atomically precise level, we need to figure out the PL mechanism and the origin of PL enhancement. In previous studies, the origin of the PL enhancement was investigated via TA spectroscopy and DFT and TD-DFT methods. To date, the PL mechanism still needs deeper investigation. As we discussed above, many features can influence the PL performance, such as the dopant species, the doping amount, the alloy kernel structures, the solvent, the temperature and so on. However, the effects cannot be explained with a unified mechanism. Further study needs to focus on the fundamental PL mechanisms of these alloy NCs.
3.4 Catalytic properties
As we discussed above, doping tunes the electronic structure of Ag NCs and influences their catalytic properties. Zhu's group studied the catalytic performance of CNT-supported metal nanoclusters Au24Ag46 NC.36 Compared with undoped NCs, this bimetallic Au24Ag46 NC takes advantage of both silver (high selectivity for benzaldehyde) and gold (high conversion) and showed higher conversion and selectivity for epoxides.Zhu et al.59 carried out centre doping of Ag25 NCs with different foreign atoms (Au, Pt and Pd), and further studied their influence on the catalytic performance in the carboxylation reaction of terminal alkynes with CO2, see Fig. 7. The doping atoms indeed contribute to the catalytic properties, and the enhancement factors depend on the nature of the foreign atoms. The elegant work from Zhu and co-workers revealed the crucial structure of the Au@Ag kernel of the Au1Ag24(SR)18 NC, which showed high activity and stability in catalyzing CF3-ketone alkynylation. Their further studies demonstrated that the Au12Ag32(SR)30 NCs, bearing a Au@Ag kernel, also showed high-activity, which confirmed their conclusion.61 In Qin's work,60 CeO2 supported Au13Ag16L24 NCs showed high catalytic activity and selectivity to A3-coupling reactions. In all, the doped Ag NCs show great potential for catalytic application, where the dopants enhance the catalytic activity and selectivity. DFT calculations help to analyse the effect of the dopants on the catalytic process and understand the improvement factors.
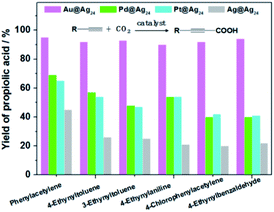 | ||
| Fig. 7 The catalytic carboxylation of terminal alkynes with CO2 over Au@Ag24, Pd@Ag24, Pt@Ag24 and Ag@Ag24 clusters, respectively. Adapted with permission from ref. 59. Copyright 2018 Wiley-VCH. | ||
4. Conclusions and outlook
In conclusion, we have summarized the recent studies on tailoring Ag NCs via doping with hetero metal atoms. Doping is based on Ag NC templates, and classified by the dopants: Au, Pt, Pd, Cu and other hetero atoms. The synthetic methods and the structures of bimetallic Ag NCs are highlighted. Furthermore, the doping effects on the properties of Ag NCs have been concluded, including the location of dopants, charge states, PL properties and catalytic properties. Doping improves or diversifies the properties of Ag NCs, providing versatile opportunities for their applications.For future perspectives, we outline the following aspects:
(1) To date, only a few sizes of Ag bimetallic NCs have been reported, and these reports are mainly concentrated upon the templates of Ag25 and Ag29. Studies on trimetallic Ag NCs are scarcer. The synthesis methods for preparing hetero atom doped Ag NCs are limited, and most of them are carried out by the ligand exchange approach. Exploring facile synthesis methods for preparing new sizes of doped Ag NCs is required.
(2) Although the locations of doping atoms have been analysed via SCXRD measurements and DFT calculations and the diffusion process has been monitored by using in situ UV-vis absorption spectroscopy in combination with ESI mass and tandem mass spectrometry,62 the fundamental physical mechanisms are not well understood. To control the doping location and design doped Ag NCs with atomic precision, the occupation mechanism needs to be further studied.
(3) As we discussed in our latest review, understanding the PL mechanism of Ag NCs is a big challenge for researchers. Although obvious PL intensity enhancement, PLQY increment and longer lifetime performance by doping Ag NCs were observed and the TA method or DFT calculations were applied to explain the experimental results, the universal mechanism of the PL properties is not clear. More studies on the PL mechanism of doped Ag NCs are needed.
(4) Doping improves the properties of Ag NCs, bringing opportunities for practical applications. For bio-application, we need to seriously consider the toxicity of these doped Ag NCs. As we discussed above, the addition of some dopants would lead to an open shell in the kernel, and expose the metals to the solution. This may increase the risk of toxicity. Therefore, the biological properties such as biocompatibility and toxicity of doped Ag NCs should be further explored before their application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Y. acknowledges the support from the National Natural Science Foundation of China (Grant No. 51801077). M.-B. L. acknowledges the support from the National Natural Science Foundation of China (Grant No. 92061110), and the Hefei National Laboratory for Physical Sciences at the Microscale (Grant No. KF2020102).Notes and references
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346 CrossRef CAS PubMed.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338 CrossRef CAS PubMed.
- T. Udayabhaskararao and T. Pradeep, J. Phys. Chem. Lett., 2013, 4, 1553 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208 CrossRef CAS PubMed.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120(2), 526 CrossRef CAS PubMed.
- R. Jin, Nanoscale, 2015, 7, 1549 RSC.
- C. P. Joshi, M. S. Bootharaju and O. M. Bakr, J. Phys. Chem. Lett., 2015, 6, 3023 CrossRef CAS PubMed.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Acc. Chem. Res., 2018, 51, 3094 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443 RSC.
- T. Kawawaki, Y. Imai, D. Suzuki, S. Kato, I. Kobayashi, T. Suzuki, R. Kaneko, S. Hossain and Y. Negishi, Chem.–Eur. J., 2020, 26, 16149 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422 RSC.
- S. Wang, Q. Li, X. Kang and M. Zhu, Acc. Chem. Res., 2018, 51, 2784 CrossRef CAS PubMed.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922 CrossRef CAS PubMed.
- M. Kim, K. L. D. M. Weerawardene, W. Choi, S. M. Han, J. Paik, Y. Kim, M.-G. Choi, C. M. Aikens and D. Lee, Chem. Mater., 2020, 32(23), 10216 CrossRef CAS.
- S. Sharma, K. K. Chakrahari, J.-Y. Saillard and C. W. Liu, Acc. Chem. Res., 2018, 51, 2475 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399 CrossRef CAS PubMed.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Hakkinen and N. Zheng, Nat. Commun., 2013, 4, 2422 CrossRef PubMed.
- R. S. Dhayal, Y.-R. Lin, J.-H. Liao, Y.-J. Chen, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Chem.–Eur. J., 2016, 22, 9943 CrossRef CAS PubMed.
- W.-T. Chang, P.-Y. Lee, J.-H. Liao, K. K. Chakrahari, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2017, 56, 10178 CrossRef CAS PubMed.
- R. S. Dhayal, J.-H. Liao, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J. Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 3702 CrossRef CAS PubMed.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. D. Gobbo, R. G. AbdulHalim, M. Eddaoudi, D.-e. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970 CrossRef CAS PubMed.
- J. Yang and R. Jin, ACS Mater. Lett., 2019, 1, 482 CrossRef CAS.
- J. Yang and R. Jin, J. Phys. Chem. C, 2020, 125, 2619 CrossRef.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Hakkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 11880 CrossRef CAS PubMed.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922 CrossRef CAS PubMed.
- Y. Li, M. Zhou, S. Jin, L. Xiong, Q. Yuan, W. Du, Y. Pei, S. Wang and M. Zhu, Chem. Commun., 2019, 55, 6457 RSC.
- M. v. d. Linden, A. J. v. Bunningen, L. Amidani, M. Bransen, H. Elnaggar, P. Glatzel, A. Meijerink and F. M. F. de Groot, ACS Nano, 2018, 12, 12751 CrossRef PubMed.
- X.-Y. Xie, P. Xiao, X. Cao, W.-H. Fang, G. Cui and M. Dolg, Angew. Chem., Int. Ed., 2018, 57, 9965 CrossRef CAS PubMed.
- Y. Du, Z.-J. Guan, Z.-R. Wen, Y.-M. Lin and Q.-M. Wang, Chem.–Eur. J., 2018, 24, 16029 CrossRef CAS PubMed.
- B. E. Conn, A. Atnagulov, B. Bhattarai, B. Yoon, U. Landman and T. P. Bigioni, J. Phys. Chem. C, 2018, 122, 13166 CrossRef CAS.
- A. Baksi, E. K. Schneider, P. Weis, K. R. Krishnadas, D. Ghosh, H. Hahn, T. Pradeep and M. M. Kappes, J. Phys. Chem. C, 2019, 123, 46 Search PubMed , 28477.
- W.-T. Chang, S. Sharma, J.-H. Liao, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Chem.–Eur. J., 2018, 24, 14352 CrossRef CAS PubMed.
- Y.-R. Lin, P. V. V. N. Kishore, J.-H. Liao, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Nanoscale, 2018, 10, 6855 RSC.
- X.-J. Xi, J.-S. Yang, J.-Y. Wang, X.-Y. Dong and S.-Q. Zang, Nanoscale, 2018, 10, 21013 RSC.
- S. Wang, S. n Jin, S. Yang, S. Chen, Y. Song, J. Zhang and M. Zhu, Sci. Adv., 2015, 1, e1500441 CrossRef PubMed.
- J. Yan, S. Malola, C. Hu, J. Peng, B. Dittrich, B. K. Teo, H. Häkkinen, L. Zheng and N. Zheng, Nat. Commun., 2018, 9, 3357 CrossRef PubMed.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 11880 CrossRef CAS PubMed.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, M. Harb, N. Maity, A. Shkurenko, M. R. Parida, M. N. Hedhili, M. Eddaoudi, O. F. Mohammed, O. M. Bakr, L. Cavallo and J.-M. Basset, J. Am. Chem. Soc., 2017, 139(3), 1053 CrossRef CAS PubMed.
- X. Kang, L. Xiong, S. Wang, Y. Pei and M. Zhu, Chem. Commun., 2017, 53, 12564 RSC.
- L. He, J. Yuan, N. Xia, L. Liao, X. Liu, Z. Gan, C. Wang, J. Yang and Z. Wu, J. Am. Chem. Soc., 2018, 140, 3487 CrossRef CAS PubMed.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, M. Harb, M. R. Parida, M. N. Hedhili, O. F. Mohammed, O. M. Bakr, L. Cavallo and J.-M. Basset, Nanoscale, 2017, 9, 9529 RSC.
- X. Kang, M. Zhou, S. Wang, S. Jin, G. Sun, M. Zhu and R. Jin, Chem. Sci., 2017, 8, 2581 RSC.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, A. Shkurenko, A. M. El-Zohry, O. F. Mohammed, M. Eddaoudi, O. M. Bakr, L. Cavallo and J.-M. Basset, Chem. Mater., 2018, 30, 2719 CrossRef CAS.
- X. Kang, L. Huang, W. Liu, L. Xiong, Y. Pei, Z. Sun, S. Wang, S. Wei and M. Zhu, Chem. Sci., 2019, 10, 8685 RSC.
- X. Lin, C. Liu, K. Sun, R. Wu, X. i. Fu and J. Huang, Nano Res., 2019, 12, 309 CrossRef CAS.
- X. Kang, S. Jin, L. Xiong, X. Wei, M. Zhou, C. Qin, Y. Pei, S. Wang and M. Zhu, Chem. Sci., 2020, 11, 1691 RSC.
- S. Yang, J. Chai, Y. Lv, T. Chen, S. Wang, H. Yu and M. Zhu, Chem. Commun., 2018, 54, 12077 RSC.
- T.-H. Chiu, J.-H. Liao, F. Gam, I. Chantrenne, S. Kahlal, J.-Y. Saillard and C. W. Liu, J. Am. Chem. Soc., 2019, 141, 12957 CrossRef CAS PubMed.
- X. Lin, H. Cong, K. Sun, X. Fu, W. Kang, X. Wang, S. Jin, R. Wu, C. Liu and J. Huang, Nano Res., 2020, 13, 366 CrossRef CAS.
- S. Kumar Barik, T.-H. Chiu, Y.-C. Liu, M.-H. Chiang, F. Gam, I. Chantrenne, S. Kahlal, J.-Y. Saillard and C. W. Liu, Nanoscale, 2019, 11, 14581 RSC.
- J. Yan, H. Su, H. Yang, C. Hu, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2016, 138, 12751 CrossRef CAS PubMed.
- X. Zou, Y. Li, S. Jin, X. Kang, X. Wei, S. Wang, X. Meng and M. Zhu, J. Phys. Chem. Lett., 2020, 11, 2272 CrossRef CAS PubMed.
- M. S. Bootharaju, H. Chang, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. Zheng and T. Hyeon, J. Am. Chem. Soc., 2019, 141, 8422 CrossRef CAS PubMed.
- E. Khatun, P. Chakraborty, B. R. Jacob, G. Paramasivam, M. Bodiuzzaman, W. A. Dar and T. Pradeep, Chem. Mater., 2020, 32, 611 CrossRef CAS.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A.-H. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749 CrossRef CAS PubMed.
- D. Mishra, V. Lobodin, C. Zhang, F. Aldeek, E. Lochner and H. Mattoussi, Phys. Chem. Chem. Phys., 2018, 20, 12992 RSC.
- X. L. Liu, J. Yuan, C. Yao, J. Chen, L. Li, X. Bao, J. Yang and Z. Wu, J. Phys. Chem. C, 2017, 121, 13848 CrossRef CAS.
- Y. Liu, X. Chai, X. Cai, M. Chen, R. Jin, W. Ding and Y. Zhu, Angew. Chem., Int. Ed., 2018, 130, 9923 CrossRef.
- Z. Qin, S. Sharma, C. Wan, S. Malola, W. Xu, H. Häkkinen and G. Li, Angew. Chem., Int. Ed., 2021, 133, 983 CrossRef.
- L. Sun, K. She, H. Sheng, Y. i. Yun, Y. Song, D. Pan, Y. Du, H. Yu, M. Chen and M. Zhu, J. Catal., 2019, 378, 220 CrossRef CAS.
- K. Zheng, V. Fung, X. Yuan, D. Jiang and J. Xie, J. Am. Chem. Soc., 2019, 141, 18977 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |