Open Access Article
Open Access ArticleRetracted Article: A hybrid upconversion nanoprobe for ratiometric detection of aliphatic biogenic amines in aqueous medium†
Shilpi
Jaiswal
 ,
Subhankar
Kundu
,
Subhankar
Kundu
 ,
Sujoy
Bandyopadhyay‡
and
Abhijit
Patra
,
Sujoy
Bandyopadhyay‡
and
Abhijit
Patra
 *
*
Department of Chemistry, Indian Institute of Science Education and Research, Bhopal, Bhopal Bypass Road, Bhauri, Bhopal 462066, Madhya Pradesh, India. E-mail: abhijit@iiserb.ac.in
First published on 1st April 2021
Abstract
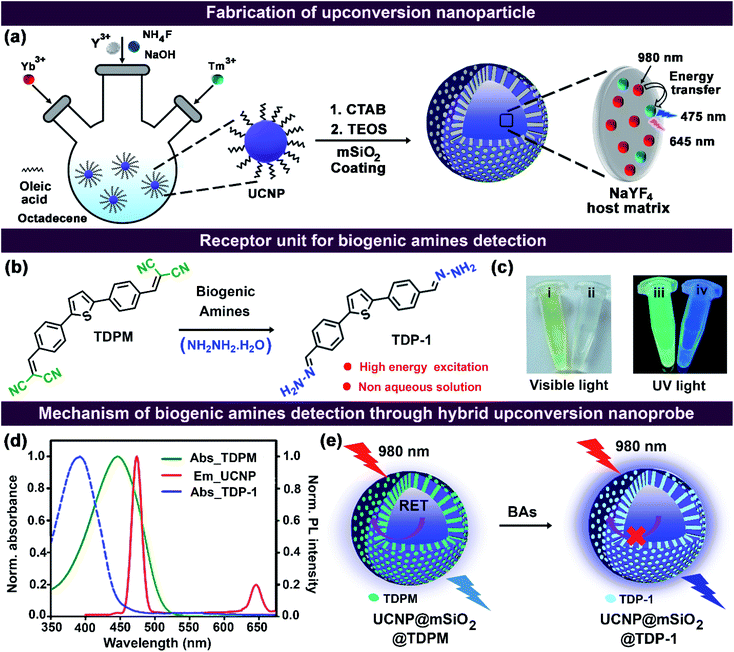
We fabricated an inorganic–organic hybrid upconversion nanoprobe for the ratiometric detection of aliphatic biogenic amines in water. The hybrid nanoprobe comprises a thiophene-based acceptor–π-donor–π-acceptor organic fluorescent dye, TDPM, and near-infrared light-absorbing upconversion nanoparticles (UCNPs). The organic dye was loaded into a mesoporous silica-coated UCNP (UCNP@mSiO2) matrix to circumvent the issues of water insolubility and higher energy excitation. Yb3+ and Tm3+-doped UCNPs exhibited dual emission bands at 475 and 645 nm upon excitation with a 980 nm laser. The significant spectral overlap between the absorption and the emission bands of TDPM and UCNPs, respectively, at 475 nm led to resonance energy transfer (RET) from the UCNPs to TDPM resulting in the quenching of the UCNP emission. In contrast, ‘turn-on’ emission was noticeable with the addition of aliphatic biogenic amines due to an inhibition of the RET. The emission at 645 nm remained unaffected during the energy transfer process making the hybrid probe a versatile platform for the ratiometric detection of different aliphatic biogenic amines. Furthermore, we explored the sensing of aliphatic biogenic amines in adulterated milk and rotten fish. The unique material attributes demonstrated in the current study hold promise for further development of real-time sensors and switches based on hybrid upconversion nanoprobes.
Introduction
Biogenic amines (BAs) are mostly generated due to the decarboxylation of amino acids through endogenous tissue metabolism or external microbial activity during food spoilage.1,2 BAs regulate cell growth, and elevated levels of BAs may indicate bacterial infection and cancer.3 On the other hand, aliphatic BAs, including spermine, cadaverine, and putrescine, are considered important biomarkers for monitoring food quality.3,4 Thus, the selective and sensitive detection of aliphatic BAs is crucial and relevant both from academic and industrial perspectives.5 Diverse methods like gas chromatography, high-performance liquid chromatography, electrolysis, and capillary electrophoresis have been employed to detect BAs.5–7 However, the sophisticated instrumentation and long operational time are some of the major concerns. In this context, colorimetric and fluorometric sensing attract the attention of the scientific community.8–12 Even though colorimetric detection is cost-effective, it has a lower sensitivity. In contrast, fluorescence-based detection is highly sensitive. However, low water solubility, less Stokes shift, and high energy excitation commonly encountered in strongly fluorescent organic molecules induce a bottleneck.13Recently, lanthanide-doped upconversion nanoparticles (UCNPs) have received considerable attention due to their unique features, such as low energy excitation (near-infrared), high energy emission (ultraviolet or visible), and high photochemical stability.14–17 However, surface modification is essential to improve the water dispersibility and overcome the non-specificity of pristine UCNPs for the detection of analytes. Considering these facts, the concept of hybrid nanoprobes developed through the judicious integration of small functional organic molecules with UCNPs has emerged as a smart sensory platform for detecting different biomolecules and toxic elements.18–22 The detection mechanism using hybrid upconversion nanoprobes mostly relies on the concept of energy transfer from UCNPs to organic fluorophores. In this context, the superiority of hybrid upconversion nanoprobes emerges from their large anti-Stokes shift emission, which rules out the possibility of co-excitation of both the donor (UCNPs) and acceptor (organic fluorophores) pairs, thus avoiding false-positive signals.20 Li and co-workers demonstrated a Yb3+-sensitized hybrid upconversion nanoprobe (UCNP@mSiO2@cyclodextrin@rhodamine B-derivative) for the ratiometric sensing of nitric oxide.21 Similarly, Zhang and co-workers reported a hybrid upconversion nanoprobe (UCNP@cyclodextrin@thiazole derivative) for the selective detection of Hg2+.22
Herein, we fabricated a hybrid upconversion nanoprobe using mesoporous silica-coated UCNPs (UCNP@mSiO2) and a thiophene-based acceptor–π-donor–π-acceptor (A–π-D–π-A) fluorescent organic dye, TDPM, for the detection of aliphatic BAs (Fig. 1). The surface modification of UCNPs through a mesoporous silica layer provided high physicochemical stability and water dispersibility and allowed further loading of fluorescent dyes. Consequently, the insolubility of TDPM in water and the higher energy excitation were circumvented by loading the dye into the UCNP@mSiO2 matrix. The resultant hybrid upconversion nanoprobe (UCNP@mSiO2@TDPM) was shown to exhibit ratiometric detection of aliphatic BAs in an aqueous medium. Furthermore, we employed the upconversion nanoprobe for the real-time detection of aliphatic BAs in adulterated milk and rotten fish. Different contaminants present in the test samples (fish, milk, and various food items) usually absorb in the UV-visible region. An advantage of the higher energy excitation of the developed probe is that the interference caused by such contaminants can be avoided.
Results and discussion
Design principle of the hybrid upconversion nanoprobe
The hybrid upconversion nanoprobe comprised UCNPs (NaYF4, Yb3+, and Tm3+) and a mesoporous silica (mSiO2) layer loaded with an organic dye, TDPM (Fig. 1). NaYF4, Yb3+, and Tm3+ were employed as the host matrix, sensitizer, and activator, respectively. The excited-state energy transfer from Yb3+ to Tm3+ under the excitation of 980 nm led to the generation of two distinct emission bands at 475 and 645 nm due to the 1G4 → 3H6 and 1G4 → 3F4 transitions, respectively.18,23 Furthermore, the silica coating on UCNPs reduced the surface quenching sites and enhanced the upconversion luminescence intensity.15,24 The appreciable overlap between the UCNP@mSiO2 emission band and the TDPM absorption band at 475 nm indicated the possibility of the resonance energy transfer (RET) process from the UCNPs to TDPM (Fig. 1d). A blue shift of the absorption band was noticeable upon the addition of the aliphatic BAs, including hydrazine (Fig. 1b–d). Hence, it was anticipated that the RET process would be inhibited upon the gradual addition of aliphatic BAs to the hybrid upconversion nanoprobe (Fig. 1d and e). Thus, the initial observations prompted us to fabricate the TDPM-loaded hybrid upconversion nanoprobe exhibiting promise for the ‘turn-on’ detection of aliphatic BAs in an aqueous medium under 980 nm excitation.Synthesis and characterization of the nanoprobe
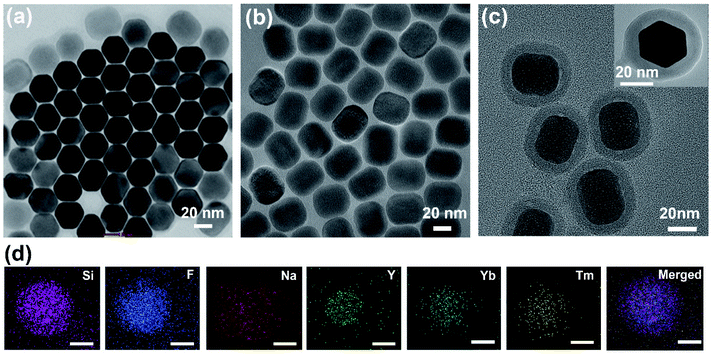
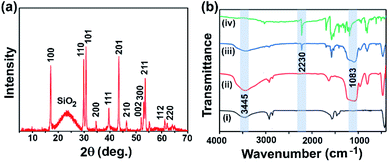
The oleic acid capped UCNPs were fabricated using the solvothermal method.25 The morphology and size of UCNPs were characterized using transmission electron microscopy (TEM; Fig. 2a and b). The TEM images showed a mixed hexagonal and cubic morphology of UCNPs (Fig. 2a and b).26 The average size of UCNPs was found to be 30 to 40 nm (the largest distance between two opposite faces) (Fig. 2a, b, and S1, ESI†). The lattice fringes in the high-resolution TEM images and the selected area electron diffraction (SAED) pattern confirmed the crystalline phase of UCNPs (Fig. S2, ESI†). Mesoporous silica was coated on the UCNP surface to improve the water dispersibility. It also enhanced the upconversion luminescence intensity.15,24Fig. 2c depicts the TEM images of silica-coated UCNPs with an average size of 40–50 nm. The energy dispersive X-ray (EDX) mapping using TEM suggested the homogeneous distribution of different ions in UCNP@mSiO2 (Fig. 2d).The powder X-ray diffraction (PXRD) pattern revealed a highly crystalline and hexagonal phase of UCNPs (Fig. S3,† JCPDS no. 16-0334).18,27 The broad new peak at 22° in the PXRD pattern for UCNP@mSiO2 confirmed the coating of amorphous silica on the UCNP surface (Fig. 3a).28 The thermal stability of both UCNPs and UCNP@mSiO2 was up to 330 °C, as indicated by the thermogravimetric analysis (TGA) curves (Fig. S4, ESI†). The surface area and porosity of UCNP@mSiO2 were estimated by using nitrogen adsorption and desorption isotherms at 77 K. The Brunauer–Emmett–Teller (BET) surface area of UCNP@mSiO2 was found to be 74 m2 g−1 with a total pore volume of 0.17 cm3 g−1 at P/P0 = 0.95. The hysteresis near P/P0 = 0.3 and the steady increase in nitrogen adsorption even at higher relative pressure signified the mesoporous nature of the SiO2 layer (Fig. S5a, ESI†). The pore size was calculated using the nitrogen sorption isotherm employing the nonlocal density functional theory (NLDFT) method. The major contribution of pore widths at 2.9 nm, along with the hierarchical distributions of mesopores, was observed (Fig. S5b, ESI†).
Afterward, TDPM was loaded into the mesoporous silica layer to fabricate a hybrid upconversion nanoprobe (UCNP@mSiO2@TDPM). In a typical dye loading procedure, 20 mg of UCNP@mSiO2 was dispersed into the solution of varying amounts of TDPM (0.05–0.35 mg in 3 mL DMSO). The resultant dispersions were sonicated for 1 h, followed by overnight stirring to obtain the hybrid nanoprobes. Furthermore, the hybrid upconversion nanoprobe (UCNP@mSiO2@TDPM) was washed multiple times with DMSO and water to ensure the removal of unloaded dye (Fig. S7, ESI†). No significant change in the size and morphology was observed for TDPM encapsulated nanoparticles (Fig. S1c and S1d, ESI†). The encapsulation of TDPM into the UCNP@mSiO2 matrix was monitored by Fourier-transform infrared (FTIR) spectroscopy. Fig. 3b shows the comparative FTIR profiles of UCNPs, UCNP@mSiO2, UCNP@mSiO2@TDPM, and TDPM. The broad peak at 3445 cm−1 was due to the stretching vibration of hydroxyl groups in oleic acid. The stretching vibrations of methylene groups in oleic acid were noticeable at 2924 and 2853 cm−1.28 The peak at 1565 cm−1 was attributed to the carbonyl functionality (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of oleic acid.28 The new peak at 1083 cm−1 was attributed to the stretching vibration of Si–O, which suggested the formation of a silica layer over UCNPs.28,29 The peak at 2230 cm−1 was attributed to the –C
O) of oleic acid.28 The new peak at 1083 cm−1 was attributed to the stretching vibration of Si–O, which suggested the formation of a silica layer over UCNPs.28,29 The peak at 2230 cm−1 was attributed to the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration frequency of TDPM. Thus, the peaks at 2230 and 1083 cm−1 in UCNP@mSiO2@TDPM confirmed the successful integration of TDPM and silica with UCNPs, respectively.
N stretching vibration frequency of TDPM. Thus, the peaks at 2230 and 1083 cm−1 in UCNP@mSiO2@TDPM confirmed the successful integration of TDPM and silica with UCNPs, respectively.
Optical properties of hybrid upconversion nanoprobes
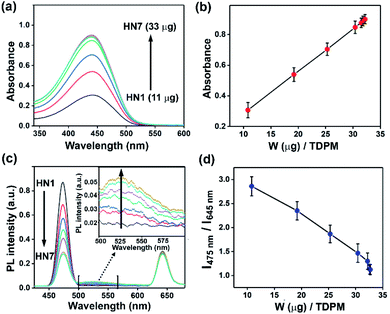
TDPM-loaded hybrid upconversion nanoprobes were designated as HN1 to HN7, indicating the varied amount of encapsulated dye (HN1: 11 μg to HN7: 33 μg, per 20 mg of UCNP@mSiO2). Repeated washing, along with centrifugation, followed by absorption measurements, confirmed the absence of any free dye in the aqueous dispersion of the hybrid nanoprobe (Fig. S7 and S8, ESI†). The TDPM loading amount was estimated through UV-Vis absorption spectroscopy (Fig. S9, ESI†). The increasing amount of TDPM loading into the UCNP@mSiO2 matrix was reflected by the gradual rise in absorbance (Fig. 4a). The absorbance saturation for HN7 suggested a maximum dye loading capacity of 33 μg for UCNP@mSiO2 (Fig. 4b). The spectral overlap between the UCNP emission and the TDPM absorption inferred the possibility of energy transfer from Tm3+ to TDPM dye, encapsulated in the porous SiO2 matrix (Fig. 1d). Furthermore, to demonstrate the RET process, the upconversion luminescence was recorded for the aqueous dispersions of hybrid nanoprobes (HN1–HN7; Fig. 4c). The gradual decrease of UCNP emission (Tm3+) at 475 nm and the increase of TDPM emission at 500–580 nm ascertained the RET process (Fig. 4c). Interestingly, the emission peak of Tm3+ at 645 nm remained unchanged, providing scope to monitor the ratiometric change in fluorescence by tuning the energy transfer process (Fig. 4d).Detection of aliphatic biogenic amines
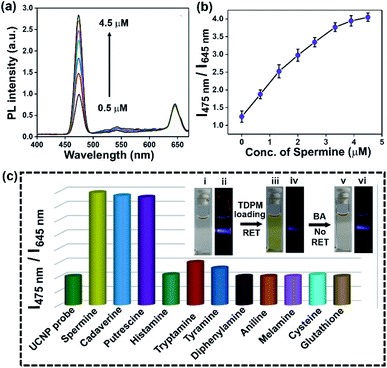
The potential of the hybrid upconversion nanomaterial to detect aliphatic BAs in an aqueous medium was probed by monitoring the relative intensity of UCNP emission. The upconversion luminescence of UCNP@mSiO2@TDPM (HN7) at 475 nm was recovered upon subsequent addition of spermine (aliphatic BA) due to the inhibition of RET (Fig. 5a). The mass spectrometric analysis revealed the chemical transformation of TDPM into the corresponding imine (analogous to TDP-1 obtained upon treatment with hydrazine,30Fig. 1b and Scheme S3, Fig. S10 and S11, ESI†) in the presence of spermine (Fig. S12 and S13, ESI†). The spectral mismatch between the absorption of the resultant imine and the UCNP emission was the reason behind the inhibition of the energy transfer leading to a ‘turn on’ upconversion luminescence response. The peak at 645 nm was used as a reference for the ratiometric detection (I475 nm/I645 nm) of aliphatic BAs (Fig. 5b). In contrast, TDPM alone was unsuitable for detecting aliphatic BAs in an aqueous medium due to its insolubility (Sec. 4.5, Fig. S14 and S15, ESI†). Furthermore, the RET process was probed through the decay analysis for UCNP (donor) emission at 475 nm using a time-correlated single-photon counting (TCSPC) set-up (Sec. 4.6, Fig. S16 and Scheme S4, ESI†). We observed a decrease in the lifetime of Tm3+ (donor, λem = 475 nm) from 250 to 169 μs in the hybrid upconversion nanoprobe upon the gradual increase of the TDPM loading (HN1 to HN7) compared to that of the pristine mesoporous silica-coated UCNPs (Fig. S16, ESI†). The results unambiguously ascertained the energy transfer from UCNPs to TDPM. Moreover, the concomitant increase in the decay time for Tm3+ emission was observed upon gradual addition of a biogenic amine (spermine, 0.5–4.5 μM) to the dispersion of the hybrid nanoprobe (HN7), indicating an inhibition of the energy transfer process.The selectivity of aliphatic BA detection was assessed by comparing the upconversion luminescence response of UCNP@mSiO2@TDPM in the presence of a diverse range of analytes, including aliphatic biogenic amines, aromatic biogenic amines, and metal ions (Fig. S17–S19, ESI†). A significant enhancement of the ratiometric upconversion luminescence signal was noticeable upon the addition of aliphatic BAs (Fig. 5c). Both the electronic and the steric factors of amines governed the sensitivity of the hybrid upconversion nanoprobes. The higher nucleophilicity and less steric hindrance of aliphatic BAs and biogenic polyamines like spermine than that of their aromatic counterpart might be the reason behind the observed sensing behaviour (Table S1, ESI†).31 The limit of detection (LOD) for spermine was found to be 0.11 ± 0.02 μM (Fig. S20, ESI†). Additionally, the LOD was calculated for other aliphatic biogenic amines like cadaverine and putrescine (Fig. S21 and S22, ESI†).
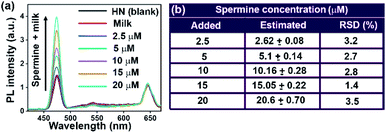
Detection of spermine in milk and fish
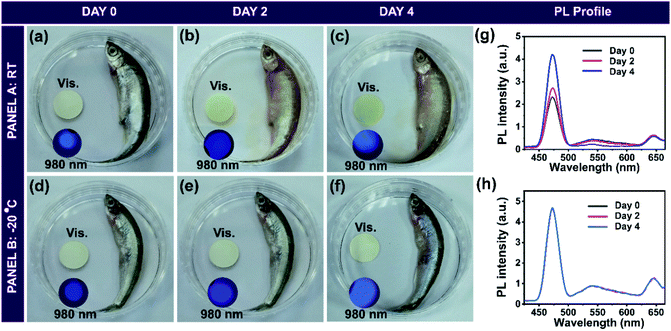
We employed the upconversion nanoprobe for the sensing of spermine in spiked milk to evaluate the practical applicability of the developed hybrid nanomaterial. Fresh milk was treated with trichloroacetic acid following earlier reports.32 The upconversion luminescence intensity was monitored before and after adding the pre-treated milk sample (50 μL) into the aqueous dispersion of UCNP@mSiO2@TDPM (100 mg/3 mL). The negligible change in the luminescence signal indicated the absence of aliphatic BAs in the treated milk samples (Fig. 6a and S23, ESI†). Furthermore, the milk sample was spiked with varying concentrations of spermine. A progressive enhancement in the upconversion luminescence intensity was observed upon the addition of the spiked milk samples (Fig. 6a). The amount of spermine in the spiked milk samples was estimated to be within the range of 100 to 104%, with the relative standard deviation (RSD) values in the range of 1.4 to 3.5% (Fig. 6b and Table S2, ESI†). The detection limit of spermine (0.11 μM) and cadaverine (0.13 μM) using the hybrid upconversion nanoparticles suggests that the probe can detect the minimum concentration of cadaverine (0.13 mg kg−1, 1.2 μM) and spermine (0.86 mg kg−1, 4.2 μM) found in fresh milk.33 Hence, the UCNP@SiO2@TDPM probe can be used as a ratiometric sensor for monitoring the freshness of milk samples (Sec. 5, ESI†).In addition, the hybrid upconversion nanoprobe was employed for monitoring the freshness of raw fish. Rotten fish contains an elevated level of BAs.34 The fish sample and pellet made up of UCNP@mSiO2@TDPM were stored in Petri dishes separately at room temperature (RT ∼ 25 °C) and −20 °C for 0 to 4 days (Fig. 7). The excess BAs in rotten fish after storage for 4 days led to the chemical transformation of TDPM. An increase in the upconversion luminescence was observed under the illumination of 980 nm for the pellet kept at room temperature. It was due to the formation of biogenic amines from the rotten fish in 4 days resulting in the inhibition of the RET process (Panel A, Fig. 7a–c). In contrast, no significant visual change in upconversion luminescence was noticeable for the pellet with fish stored at −20 °C after 4 days (Panel B, Fig. 7d–f). The results were also corroborated through upconversion luminescence intensity vs. wavelength plots (Fig. 7g and h). Thus, UCNP@mSiO2@TDPM can be used as a marker for monitoring the freshness of fish and food beverages (Tables S3 and S4, ESI†).
Conclusion
In summary, an inorganic–organic hybrid upconversion nanoprobe (UCNP@mSiO2@TDPM) was fabricated for the ratiometric detection of aliphatic biogenic amines in aqueous medium. The probe consisted of mesoporous silica-coated upconversion nanoparticles [UCNP(NaYF4, Yb3+, Tm3+)@mSiO2] and a thiophene containing π-conjugated organic molecule, TDPM. A chemical transformation of TDPM into the corresponding imine form was noticeable upon the addition of aliphatic biogenic amines. The significant overlap between the UCNP@mSiO2 emission and TDPM absorption was used to demonstrate the resonance energy transfer process. A decrease in the upconversion emission intensity at 475 nm was noticeable upon increasing the concentration of TDPM loaded into UCNP@mSiO2. In contrast, the energy transfer ceased in the presence of aliphatic biogenic amines. Consequently, a gradual increase of the 475 nm emission band along with an unaltered 645 nm band of the hybrid upconversion nanoprobe offered a viable platform for the ratiometric detection of aliphatic biogenic amines in an aqueous medium. Moreover, we demonstrated the detection of aliphatic biogenic amines in adulterated milk and rotten fish, signifying the potential for further development of diverse hybrid upconversion nanoprobes as real-time sensors and switches.Experimental section
Fabrication of upconversion nanoparticles (UCNPs)
The fabrication of upconversion nanoparticles was carried out following a reported procedure.25 0.78 mmol of YCl3·6H2O, 0.20 mmol of YbCl3·6H2O, and 0.02 mmol of TmCl3 were dissolved in a minimum volume of water to make a homogeneous solution. The solution was taken into a three-neck round-bottom (RB) flask and was heated up to 120 °C. Subsequently, 6 mL of oleic acid and 15 mL of 1-octadecene were added to the RB flask and the solution was heated to 160 °C for 2 h. Afterward, the reaction mixture was brought to room temperature. Then, a methanol solution of 4 mmol of ammonium fluoride and 2.5 mmol of sodium hydroxide was added to the reaction mixture, followed by 30 min stirring. The mixture was heated up to 110 °C. Furthermore, the solution was degassed and kept under a nitrogen atmosphere for 10 min. Finally, the temperature was slowly increased to 280 °C, and heating was continued for 120 min. Then the reaction mixture was cooled to room temperature. The as-prepared UCNPs (NaYF4, Yb3+, and Tm3+) were collected by centrifugation at 6000 rpm for 15 min and washed multiple times with hexane and ethanol.Fabrication of silica-coated UCNPs (UCNP@mSiO2)
10 mg of UCNPs were dispersed in 2 mL of cyclohexane. Then, the dispersion was added into an aqueous solution of CTAB (0.1 g in 20 mL of water) and was vigorously stirred at room temperature to evaporate cyclohexane. Furthermore, a solution containing water (20 mL), ethanol (3 mL), and NH4OH (150 μL, 30%) was added to the above reaction mixture. Then, 150 μL tetraethyl orthosilicate (TEOS) was added dropwise, and the resultant mixture was stirred for 48 h. The product was centrifuged at 6000 rpm for 10 min and washed multiple times with ethanol.25Loading of TDPM into the UCNP@mSiO2 matrix
The loading of TDPM into the UCNP@mSiO2 matrix was carried out by a simple stirring and centrifugation method. In a typical dye loading procedure, 20 mg of silica-coated UCNPs was taken into seven different RB flasks. Then, different amounts of TDPM (0.05, 0.1, 0.15, 0.20, 0.25, 0.30, and 0.35 mg in 3 mL DMSO) were added into the respective RB flasks. The mixture was sonicated for 1 h and kept stirring overnight. Finally, the different suspensions were centrifuged at 6000 rpm for 10 min and washed multiple times with DMSO and water.Milk sample treatment
2 mL of 20% trichloroacetic acid was added to 10 mL of milk sample for protein coagulation.32 Afterward, the milk sample was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The supernatant was then filtered through a 0.22 μm membrane to remove all the interfering substances in the milk. Furthermore, the milk sample was spiked with varying concentrations of spermine from 2.5 μM to 20 μM.
000 rpm for 30 min. The supernatant was then filtered through a 0.22 μm membrane to remove all the interfering substances in the milk. Furthermore, the milk sample was spiked with varying concentrations of spermine from 2.5 μM to 20 μM.
Conflicts of interest
There is no conflict to declare.Acknowledgements
Financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, (No. 01(2878)/17/EMR-II), and BRNS, DAE (No. 37(2)/14/06/2016-BRNS/37020), and infrastructural support from IISERB, and the FIST-supported TEM facility to the Department of Chemistry, IISERB, are gratefully acknowledged. SJ and SK thank CSIR and UGC, respectively, for fellowships.References
- (a) A. R. Shalaby, Food Res. Int., 1996, 29, 675 CrossRef CAS; (b) A. Naila, S. Flint, G. Fletcher, P. Bremer and G. Meerdink, J. Food Sci., 2010, 75, 139 CrossRef PubMed.
- (a) H. Zhong, C. Liu, W. Ge, R. Sun, F. Huang and X. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 22875 CrossRef CAS PubMed; (b) R. Jia, W. Tian, H. Bai, J. Zhang, S. Wang and J. Zhang, Nat. Commun., 2019, 10, 795 CrossRef CAS PubMed.
- (a) B. Bao, L. Yuwen, X. Zheng, L. Weng, X. Zhu, X. Zhan and L. Wang, J. Mater. Chem., 2010, 20, 9628 RSC; (b) R. R. Kothur, B. A. Patel and P. J. Cragg, Chem. Commun., 2017, 53, 9078 RSC.
- (a) M. H. SillaSantos, Int. J. Food Microbiol., 1996, 29, 213 CrossRef CAS; (b) Y. Özogul and F. Özogul, in Biogenic Amines in Food: Analysis, Occurrence and Toxicity, The Royal Society of Chemistry, 2020 Search PubMed.
- A. Önal, Food Chem., 2007, 103, 1475 CrossRef.
- R. Draisci, G. Volpe, L. Lucentini, A. Cecilia, R. Federico and G. Palleschi, Food Chem., 1998, 62, 225 CrossRef CAS.
- T. Esatbeyoglu, A. Ehmer, D. Chaize and G. Rimbach, J. Agric. Food Chem., 2016, 64, 2105 CrossRef CAS PubMed.
- A. P. Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef PubMed.
- (a) H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79 RSC; (b) X. Zhang, J. Yin and J. Yoon, Chem. Rev., 2014, 114, 4918 CrossRef CAS PubMed; (c) D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105 RSC.
- (a) M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185 RSC; (b) S. Singh, D. Kim, H. Seo, S. W. Choa and K. H. Ahn, Chem. Soc. Rev., 2015, 44, 4367 RSC; (c) J. Oh, H. Rhee and J. Hong, in Synthetic Receptors for Biomolecules: Design Principles and Applications, The Royal Society of Chemistry, 2015 Search PubMed; (d) J. Zhou and H. Ma, Chem. Sci., 2016, 7, 6309 RSC; (e) B. Liu, J. Zhuang and G. Wei, Environ. Sci.: Nano, 2020, 7, 2195 RSC.
- (a) A. Deshmukh, S. Bandyopadhyay, A. James and A. Patra, J. Mater. Chem. C, 2016, 4, 4427 RSC; (b) P. Pallavi, V. Kumar, M. W. Hussain and A. Patra, ACS Appl. Mater. Interfaces, 2018, 10, 44696 CrossRef CAS PubMed; (c) A. Weißenstein, C. R. Saha-Möller and F. Würthner, Chem.–Eur. J., 2018, 24, 8009 CrossRef PubMed.
- (a) V. Kumar, B. Sk, S. Kundu and A. Patra, J. Mater. Chem. C, 2018, 6, 12086 RSC; (b) V. Kumar, S. Kundu, B. Sk and A. Patra, New J. Chem., 2019, 43, 18582 RSC; (c) C. Guo, A. C. Sedgwick, T. Hirao and J. L. Sessler, Coord. Chem. Rev., 2021, 427, 213560 CrossRef CAS PubMed.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395 CrossRef CAS PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161 CrossRef CAS PubMed.
- (a) W. Zheng, P. Huang, D. Tu, E. Ma, H. Zhu and X. Chen, Chem. Soc. Rev., 2015, 44, 1379 RSC; (b) M. H. Chan and R. S. Liu, Nanoscale, 2017, 9, 18153 RSC; (c) M. Safdar, A. Ghazy, M. Lastusaari and M. Karppinen, J. Mater. Chem. C, 2020, 8, 6946 RSC.
- (a) B. Gu and Q. Zhang, Adv. Sci., 2018, 5, 1700609 CrossRef PubMed; (b) S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Nat. Commun., 2018, 9, 2415 CrossRef PubMed; (c) G. Tessitore, G. A. Mandl, M. G. Brik, W. Park and J. A. Capobianco, Nanoscale, 2019, 11, 12015 RSC; (d) Y. Wong, S. Pang, M. Tsang, Y. Liu, H. Huang, S. Yu and J. Hao, Nanoscale Adv., 2019, 1, 265 RSC.
- Z. Li, T. Liang, S. Lv, Q. Zhuang and Z. Liu, J. Am. Chem. Soc., 2015, 137, 11179 CrossRef CAS.
- (a) S. Wu, X. J. Kong, Y. Cen, J. Yuan, R. Q. Yu and X. Chu, Nanoscale, 2016, 8, 8939 RSC; (b) O. Dukhno, F. Przybilla, M. Collot, A. Klymchenko, V. Pivovarenko, M. Buchner, V. Muhr, T. Hirsch and Y. Mély, Nanoscale, 2017, 9, 11994 RSC; (c) Y. Liu, Q. Ouyang, H. Li, Z. Zhang and Q. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 18314 CrossRef CAS PubMed; (d) M. K. Mahata and K. T. Lee, Nanoscale Adv., 2019, 1, 2372 RSC.
- E. Jo, J. Byun, H. Mun, D. Bang, J. H. Son, J. Y. Lee, L. P. Lee and M. Kim, Anal. Chem., 2018, 90, 716 CrossRef CAS PubMed.
- N. Wang, X. Yu, K. Zhang, C. A. Mirkin and J. Li, J. Am. Chem. Soc., 2017, 139, 12354 CrossRef CAS PubMed.
- B. Gu, Y. Zhou, X. Zhang, X. Liu, Y. Zhang, R. Marks, H. Zhang, X. Liu and Q. Zhang, Nanoscale, 2016, 8, 276 RSC.
- J. Peng, W. Xu, C. L. Teoh, S. Han, B. Kim, A. Samanta, J. C. Er, L. Wang, L. Yuan, X. Liu and Y. Chang, J. Am. Chem. Soc., 2015, 137, 2336 CrossRef CAS PubMed.
- (a) A. Kar and A. Patra, Nanoscale, 2012, 4, 3608 RSC; (b) S. Liu, L. Zhang, T. Yang, H. Yang, K. Y. Zhang, X. Zhao, W. Lv, Q. Yu, X. Zhang, Q. Zhao, X. Liu and W. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 11013 CrossRef CAS PubMed; (c) A. Kar, S. Kundu and A. Patra, ChemPhysChem, 2015, 16, 505 CrossRef CAS PubMed; (d) B. Kumar, V. S. S. Rathnam, S. Kundu, N. Saxena, I. Banerjee and S. Giri, ChemNanoMat, 2018, 4, 583 CrossRef CAS.
- M. K. Gnanasammandhan, N. M Idris, A. Bansal, K. Huang and Y. Zhang, Nat. Protoc., 2016, 4, 688 CrossRef PubMed.
- (a) H. Mai, Y. Zhang, R. Si, Z. Yan, L. Sun, L. You and C. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS PubMed; (b) R. Arppe, T. Näreoja, S. Nylund, L. Mattsson, S. Koho, J. M. Rosenholm, T. Soukka and M. Schäferling, Nanoscale, 2014, 6, 6837 RSC.
- T. Ma, Y. Ma, S. Liu, L. Zhang, T. Yang, H. Yang, W. Lv, Q. Yu, W. Xu, Q. Zhao and W. Huang, J. Mater. Chem. C, 2015, 3, 6616 RSC.
- R. Han, J. Shi, Z. Liu, Y. Hou and Y. Wang, ACS Biomater. Sci. Eng., 2018, 4, 3478 CrossRef CAS PubMed.
- (a) M. Wang, C. Mia, Y. Zhang, J. Liu, F. Lia, C. Mao and S. Xu, J. Phys. Chem. C, 2009, 113, 19021 CrossRef CAS PubMed; (b) A. Sedlmeier and H. H. Gorris, Chem. Soc. Rev., 2015, 44, 1526 RSC.
- (a) M. Sun, J. Guo, Q. Yang, N. Xiao and Y. Li, J. Mater. Chem. B, 2014, 2, 1846 RSC; (b) X. Yang, Y. Liu, Y. Wu, X. Ren, D. Zhang and Y. Ye, Sens. Actuators, B, 2017, 253, 488 CrossRef CAS.
- S. Jindal, V. K. Maka and J. N. Moorthy, J. Mater. Chem. C, 2020, 8, 11449 RSC.
- P. Leng, F. Zhao, B. Yin and B. Ye, Chem. Commun., 2015, 51, 8712 RSC.
- D. M. Linares, M. C. Martin, V. Ladero, M. A. Alvarez and M. Fernandez, Food Sci. Nutr., 2011, 51, 691 CAS.
- J. Donthuan, S. Yunchalard and S. Srijaranai, Anal. Methods, 2014, 6, 1128 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of fabrication and characterization of the hybrid upconversion nanoprobe, structural features, optical properties, and comparative analysis of biogenic amine detection. See DOI: 10.1039/d0na00995d |
| ‡ Present address: Dr Sujoy Bandyopadhyay, Assistant Professor, Department of Chemistry, Indrashil University, Rajpur, Gujarat 382740, India. |
| This journal is © The Royal Society of Chemistry 2021 |