Open Access Article
Open Access ArticleResponse of G protein-coupled receptor CED-1 in germline to polystyrene nanoparticles in Caenorhabditis elegans†
Yunhan
Yang
,
Wenting
Dong
,
Qiuli
Wu
 * and
Dayong
Wang
* and
Dayong
Wang
 *
*
Medical School, Southeast University, Nanjing 210009, China. E-mail: qlwu@seu.edu.cn; dayongw@seu.edu.cn
First published on 17th February 2021
Abstract
The deposition of a certain amount of nanopolystyrene (NPS) can be observed in the gonad of Caenorhabditis elegans. However, we still know little about the response of germline towards NPS exposure. In the germline of C. elegans, NPS (1–1000 μg L−1) increased the expression levels of two G protein-coupled receptors (GPCRs), namely PAQR-2 and CED-1. Moreover, susceptibility to NPS toxicity was observed in ced-1(RNAi) worms, which suggested that the protective response of germline was mediated by GPCR CED-1. In the germline, five proteins (CED-10, VPS-34, SNX-1, RAB-7, and RAB-14) functioned as downstream targets of GPCR CED-1 in controlling NPS toxicity. Furthermore, these five targets in the germline regulated NPS toxicity by affecting the activities of p38 MAPK and insulin signaling pathways in intestinal cells. Therefore, we raised a GPCR CED-1-mediated signaling cascade in the germline in response to NPS exposure, which is helpful for understanding the molecular basis of the germline in response to NPS exposure.
1. Introduction
Based on the observations of ubiquity and degradation into smaller particles (microplastics (MP) or nanoplastics (NP)), it has been gradually recognized that plastic pollution is already a serious environmental concern.1,2 Moreover, a growing number of literature on exposure to MP or NP have indicated their adverse effects on biota.3,4 Exposure to MP or NP could potentially induce damage to development, movement activity, immune system, reproductive capacity, etc.5–8 A few determining factors, including particle size, exposure concentration, exposure duration, species, and polymer type, can affect the toxicity induction of MP or NP.9 After the exposure, the determination of MP or NP toxicity was closely related to the activation of oxidative stress in organisms.10,11The NP particles are supposed to be abundant in different environments (such as aquatic environments) due to their role at the expected lower end of size distribution.12 The emergence of possible impacts due to NP exposure on environmental animals and humans has received increasing attention.13 Recently, the nematode Caenorhabditis elegans has been applied for the evaluation of NP toxicity.14,15 It is an animal model having sensitivity towards numerous environmental exposures.16 Potential exposure to NP particles induces at least reproductive toxicity, intestinal toxicity, and neurotoxicity in C. elegans.17–19 After exposure, the NP particles could not only be accumulated in the intestinal lumen but could also further be translocated and enriched in the gonad of nematodes.20,21
Bioavailable environmental toxicants normally induce the response by inhibiting or activating some G protein-coupled receptors (GPCRs) on the cytoplasmic membrane.22,23C. elegans is a powerful model to examine the molecular response of GPCRs towards toxicants.24 A certain number of GPCRs has been identified in different tissues to control stress response towards toxicants.25 For example, the intestinal GPCR of DAF-2 regulated nanoplastic toxicity by activating the downstream signaling cascade of AGE-1-AKT-1-DAF-16.26 Nevertheless, GPCRs in the germline dysregulated by the NP exposure are largely unclear in organisms.
The primary goal of this study is to determine GPCRs in the germline required for controlling response towards NP. Specific goals are: (i) to identify germline GPCRs dysregulated by NP exposure; (ii) to examine the role of germline GPCRs in controlling responses towards NP, and (iii) to determine the molecular basis for germline GPCRs in controlling NP toxicity. Nanopolystyrene (NPS) can be potentially used in at least food containers, packaging, textiles, and adhesives.27–29 Thus, we selected NPS as the model NP and C. elegans as the animal model. We hypothesized that specific GPCRs existed in the germline required for response towards NPS. We also raised the evidence that GPCR CED-1 mediated the response of worms in the germline to the NPS exposure.
2. Experimental section
NPS characterizations
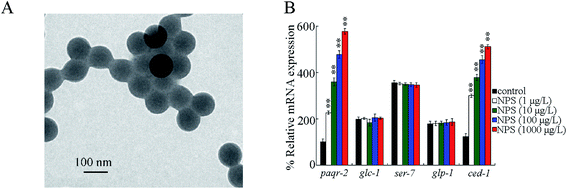
NPS (100 nm) suspended in water was purchased from Janus New-Materials Co. (Nanjing, China). The transmission electron microscopy (TEM) image of NPS before sonication is given in Fig. 1A, which shows the spherical morphology of NPS. Some other properties of this commercial NPS have been described in previous studies via Fourier transform infrared (FTIR) spectroscopy and Raman spectroscopy.30,31 The zeta potential measurement indicated the value of −9.143 ± 0.258 mV for NPS. The dynamic light scattering (DLS) measurement indicated that the size of NPS was 102.55 ± 3.8 nm. Working concentrations for NPS suspensions were 1–1000 μg L−1. Before the exposure, the working NPS suspensions were sonicated at 40 kHz (100 W) for 30 min. After sonication, the NPS suspensions did not show obvious aggregation for at least two days.26,30Strains and cultivation
The worm strains (unless otherwise described) and Escherichia coli strains (HT115 and OP50) were from the Caenorhabditis Genetics Center. Information for C. elegans strains is given in Table S1.†C. elegans was cultured as per standard procedure on nematode growth medium (NGM) agar seeded with OP50 in Petri dishes.32 To obtain a synchronized L1-larvae population, gravid worms were treated with a bleaching solution containing NaOH (0.45 M) and 2% HOCl (2%) to release enough eggs on NGM plates.33 Following that, the eggs were allowed to develop into L1 larvae.NPS exposure
The synchronized L1 larval worms were transferred and exposed in NPS suspensions added with OP50 (∼4 × 106 CFUs) to adult day-3 (approximately 6.5 days).30 The liquid PS-NP suspensions were used for exposure. NPS suspensions were refreshed daily during the exposure process by transferring worms into new liquid PS-NP suspensions. During the NPS exposure, we did not observe clear developmental defects.Assessment endpoints
The production of reactive oxygen species (ROS) was applied in order to reflect the induction of oxidative stress.34C. elegans was treated for 3 h with CM-H2DCFDA (1 μM) in darkness, followed by washing using M9 buffer. Fluorescent signals in C. elegans were observed at 510 nm (emission filter)/488 nm (excitation wavelength) under a laser scanning confocal microscope. In worms, the strongest ROS fluorescent signals are located in the intestine. Intestinal fluorescence intensities were analyzed by normalization against autofluorescence. For each exposure, 50 worms were determined.The locomotion behavior was used to indicate the possible neurotoxicity of NPS.35C. elegans was first washed with the M9 buffer, and after further recovery on the NGM plate for 1 min, their locomotion behaviors were analyzed under a dissecting microscope. To determine the head thrash, alteration in the direction of posterior bulb along the y-axis was analyzed, assuming that the traveling direction was along the x-axis. To determine the body bend, alteration in the bending direction at mid-body was analyzed. For each exposure, 40 worms were determined.
Brood size can reflect the reproductive capacity in worms.16 The brood size was counted as the number of offsprings at all stages beyond the egg under an optical microscope.31 For each exposure, 30 worms were examined.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The worms were first ground in liquid nitrogen. Total RNA was extracted using TRIZOL (Invitrogen) according to the manufacturer's protocol. The concentration and purity of extracted RNAs were further determined using a spectrophotometer. The complementary DNA (cDNA) was synthesized from the same amount of total RNAs in the reverse transcriptase reaction using a Superscript III first-strain synthesis system (Invitrogen). qPCR was carried out with the StepOnePlus™ real-time PCR system using gene-specific primers (Table S2†) and SYBR Green qRT-PCR master mix in order to determine the transcriptional expressions of examined genes. The relative mRNA expressions were examined by normalization against the mRNA of TBA-1, an alpha-tubulin protein. Three independent experiments were then carried out.RNA interference (RNAi)
RNAi experiments were carried out by feeding C. elegans with bacteria (HT115) expressing double-stranded (ds) RNAs of PAQR-2, CED-1, CED-10, VPS-34, SNX-1, RAB-7 or RAB-14.36 Before the growth on NGM plates, HT115 was transferred into an LA broth (LB broth containing 100 μg L−1 ampicillin) with the addition of 5 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG). Then, the L1 larvae were cultured on RNAi plates with specific RNAi clones or an empty RNAi vector (L4440). The next generation was used for exposure to NPS. The DCL569 strain was a tool for the germline RNAi knockdown.37 The RNAi knockdown efficiency was confirmed by the qRT-PCR analysis.Constructs generation and transformation
For the construction of Pmex-5-ced-1, ced-1/Y47H9C.4a.1 was inserted in pPD95_77 with Pmex-5 (expressed in the germline) promoter. The transgene was performed by the coinjection of constructs (50 μg mL−1) and marker construct (Pdop-1::rfp, 50 μg mL−1) in the gonad.38 Related primers are given in Table S3.†Data analysis
Using the SPSS Statistics 19.0 software, we performed the data analysis, and the data were presented as the mean ± standard derivation (SD). The statistical significance of differences between treatments was examined using the one-way analysis of variance (ANOVA), followed by the post hoc test. Besides one-way ANOVA, two-way ANOVA was also carried out for comparing multiple factors.3. Results and discussion
Identification of germline GPCRs in response to NPS exposure
Previous studies have suggested that some genes encoding germline GPCRs (PAQR-2, GLC-1, SER-7, GLP-1 and CED-1) were potentially required for stress response.39–43 After the exposure, NPS (1–1000 μg L−1) did not influence glc-1, ser-7, and glp-1 expressions in the germline (Fig. 1B). However, exposure to NPS (1–1000 μg L−1) increased the expressions of paqr-2 and ced-1 in the germline (Fig. 1B). In NPS (1–1000 μg L−1) exposed C. elegans, an increase in the expression levels of paqr-2 and ced-1 in the germline was concentration-dependent (Fig. 1B).Germline RNAi knockdown of ced-1 led to susceptibility to NPS toxicity
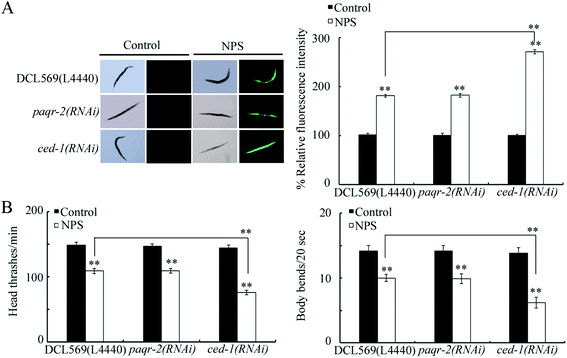
Using the DCL569 strain, the germline RNAi knockdown of paqr-2 or ced-1 could not result in a clear production of ROS, affected locomotion behavior, and influenced brood size under normal conditions (Fig. 2 and S1†). The germline RNAi knockdown of paqr-2 did not influence the NPS toxicity (Fig. 2 and S1†). Different from this, the toxicity of NPS exposure in inducing ROS production, inhibiting locomotion behavior, and reducing brood size was enhanced by the germline RNAi knockdown of ced-1 (Fig. 2 and S1†). Thus, the GPCR CED-1 function in the germline is to control the NPS toxicity. The efficiency for the germline RNAi knockdown of paqr-2 or ced-1 is shown in Fig. S2.†Target identification for germline CED-1 in controlling NPS toxicity
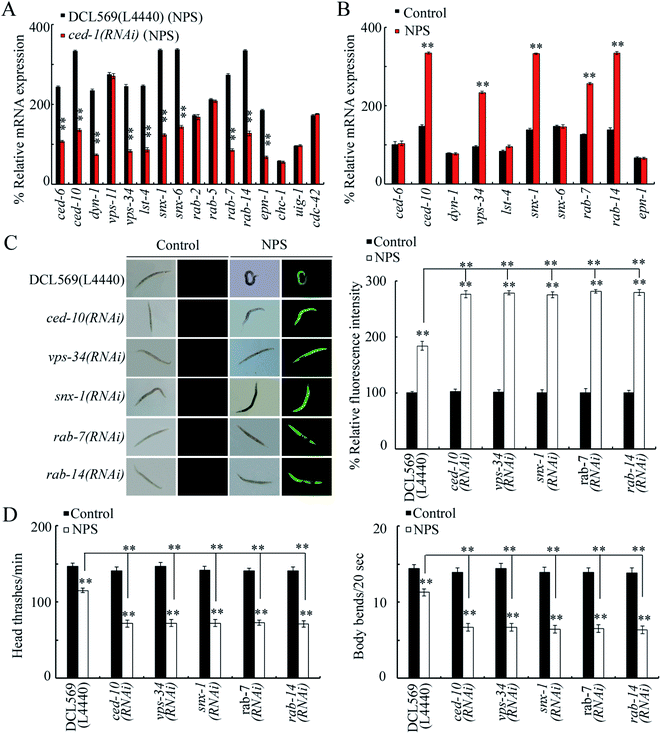
Potentially targeted genes of CED-1 have been raised during the control of different processes, and some of them can be expressed in the germline.44–49 Among 16 potential targeted genes for germline ced-1, the germline RNAi knockdown of ced-1 decreased the expression of CED-6, CED-10, DYN-1, VPS-34, LST-4, SNX-1, SNX-6, RAB-7, RAB-14, and EPN-1 in NPS-exposed DCL569 nematodes (Fig. 3A). Among these 10 candidate genes, NPS (1 μg L−1) further increased the expression levels of CED-10, VPS-34, SNX-1, RAB-7, and RAB-14 in wild-type nematodes (Fig. 3B). In NPS exposed DCL569 worms, the germline RNAi knockdown of ced-10, vps-34, snx-1, rab-7, or rab-14 caused severe production of ROS, suppression in the locomotion behavior, and reduction in the brood size (Fig. 3C, D and S3†), indicating the susceptibility of ced-10(RNAi), vps-34(RNAi), snx-1(RNAi), rab-7(RNAi), and rab-14(RNAi) animals towards NPS toxicity. These findings implied the potential function of CED-10, VPS-34, SNX-1, RAB-7, and RAB-14 as targets for the germline CED-1 in controlling responses towards NPS exposure. ced-10 encodes a small GTPase, vps-34 encodes a VPS protein, snx-1 encodes a BAR domain-containing sorting nexin, rab-7 encodes a GTPase, and rab-14 also encodes a GTPase. The efficiency for the germline RNAi knockdown of ced-10, vps-34, snx-1, rab-7 or rab-14 is shown in Fig. S4.†Genetic interaction between CED-1 and CED-10, VPS-34, SNX-1, RAB-7, or RAB-14 in the germline to control response to NPS exposure
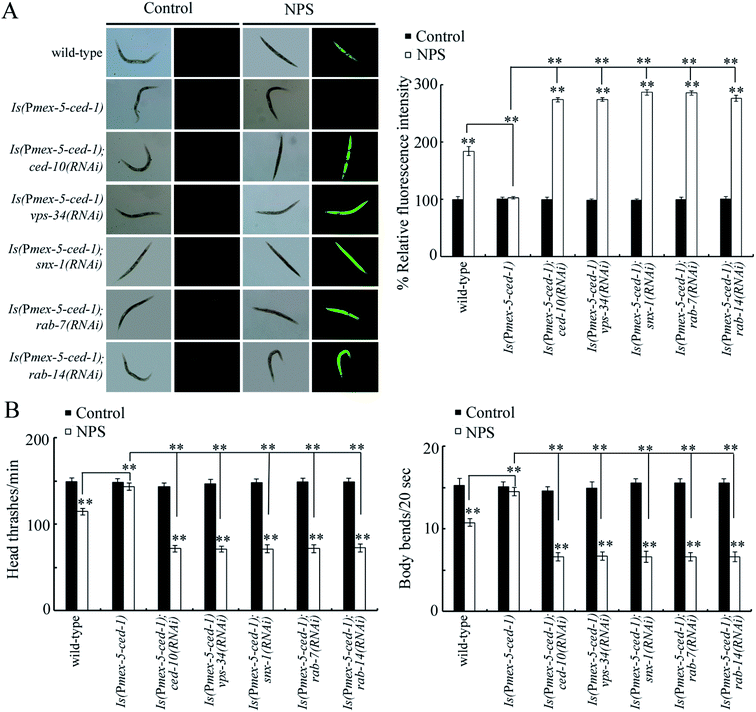
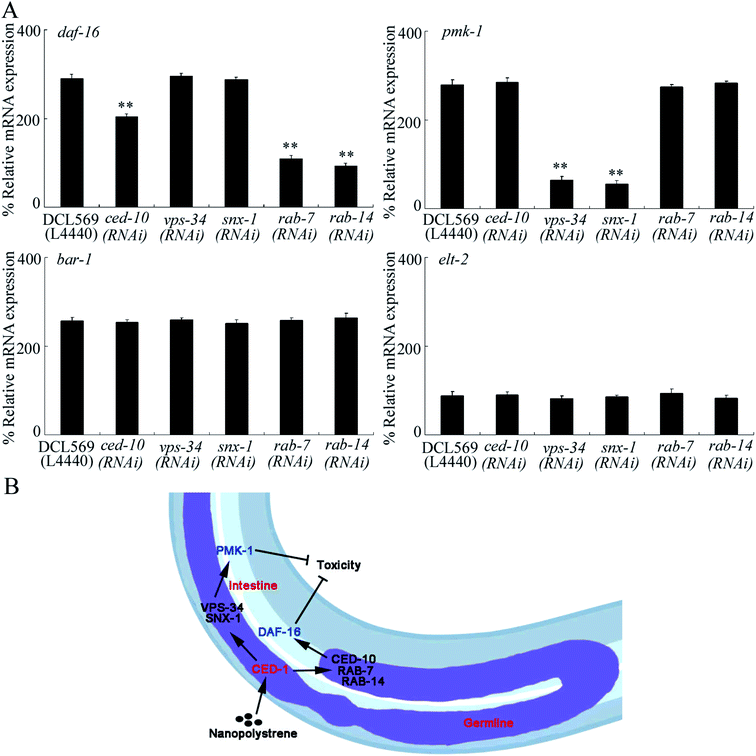
To confirm the role of CED-10, VPS-34, SNX-1, RAB-7 or RAB-14 as the targets of the germline CED-1 in controlling NPS toxicity, we examined the genetic interaction between CED-1 and CED-10, VPS-34, SNX-1, RAB-7, or RAB-14 in the germline. The germline overexpression of CED-1 significantly suppressed the production of ROS, enhanced the locomotion behavior, and increased the brood size in NPS exposed worms (Fig. 4 and S5†), indicating the resistance of animals overexpressing germline CED-1 (Is(Pmex-5-ced-1)) to the NPS toxicity. Moreover, the RNAi knockdown of ced-10, vps-34, snx-1, rab-7, or rab-14 could cause a clear production of ROS, inhibition of the locomotion behavior, and reduction in the brood size in NPS exposed Is(Pmex-5-ced-1) worms (Fig. 4 and S5†), demonstrating that CED-10, VPS-34, SNX-1, RAB-7, and RAB-14 acted downstream of germline CED-1 to control the NPS toxicity.Effect of the germline RNAi knockdown of ced-10, vps-34, snx-1, rab-7, or rab-14 on the expressions of genes encoding intestinal signaling
In C. elegans, the insulin, Wnt, p38 MAPK, and ELT-2 signaling pathways functioned in intestinal cells to control NPS toxicity.26,30,50,51 In the insulin signaling pathway, DAF-16 is a FOXO transcriptional factor; in the Wnt signaling pathway, BAR-1 is a β-catenin transcriptional factor; and in the p38 MAPK signaling pathway, PMK-1 is a p38 MAPK. In insulin, Wnt, and p38 MAPK signaling pathways, only PMK-1/p38 MAPK and signaling cascades of DAF-2-AGE-1-AKT-1-DAF-16 and GSK-3-BAR-1 were found to be in response to NPS (1 μg L−1) exposure.26,30,50 After NPS exposure, the germline RNAi knockdown of ced-10, vps-34, snx-1, rab-7 or rab-14 could not influence the expression levels of BAR-1 and ELT-2 (Fig. 5A). In contrast, the germline RNAi knockdown of ced-10, rab-7 or rab-14 decreased the DAF-16 expression, and the germline RNAi knockdown of vps-34 or snx-1 decreased PMK-1 expression (Fig. 5A). Moreover, we found that the germline RNAi knockdown of ced-1 could further decrease the expression of DAF-16 and PMK-1 in NPS-exposed DCL569 worms (Fig. S6†). Nevertheless, after the NPS exposure, the germline RNAi knockdown of ced-1 could not affect the expressions of BAR-1 and ELT-2 (Fig. S6†).During the regulation of stress response, the germline is an important organ.25 In C. elegans, the bioavailable NPS particles were not only accumulated in the intestinal lumen but were also translocated and deposited in some reproductive organs, such as the gonad.20,21 This observation implied that the NPS particles can directly activate the response of C. elegans in the germline to NPS exposure. Nevertheless, there is still no direct evidence to support this assumption. Here, we found that NPS (1–1000 μg L−1) increased the expressions of two GPCRs in the germline (PAQR-2 and CED-1) (Fig. 1B). That is, a certain number of GPCRs can be activated in the germline by NPS exposure. This provides evidence for the existence of direct molecular response in the germline to NPS exposure in C. elegans.
For the two candidate germline GPCRs, the phenotype analysis indicated that only RNAi knockdown of ced-1 affected the toxicity of NPS (Fig. 2 and S1†). The susceptibility could be observed in ced-1(RNAi) worms (Fig. 2 and S1†). Initially, CED-1 was identified to mediate the degradation and engulfment of cell corpses,52,53 which indicates that CED-1 has the ability to recognize apoptotic cells by acting as a phagocyte receptor.54 Due to this biological role, CED-1 is also required for the removal of neuronal debris during neuronal regeneration.55 Moreover, CED-1 is involved in innate immunity control by activating the expression of genes for unfolded protein response.39 In this study, our results further demonstrated that GPCR CED-1 in the germline can control the stress response by mediating a protective response towards certain toxicants such as NPS (Fig. 5B).
During the control of NPS toxicity, we provided several lines of evidence to prove the roles of CED-10, VPS-34, SNX-1, RAB-7, and RAB-14 as downstream targets activated by GPCR CED-1 in the germline in controlling NPS toxicity. First, the genes encoding these five proteins could be increased by NPS exposure (Fig. 3B) and decreased in NPS exposed ced-1(RNAi) worms (Fig. 3A). Second, the susceptibility to NPS toxicity could be detected in ced-10(RNAi), vps-34(RNAi), snx-1(RNAi), rab-7(RNAi), and rab-14(RNAi) worms (Fig. 3C, D and S3†). More importantly, the resistance of Is(Pmex-5-ced-1) worms towards NPS toxicity was inhibited by the RNAi knockdown of ced-10, vps-34, snx-1, rab-7, and rab-14 (Fig. 4 and S5†). During the removal of cell corpses, CED-10 linked different engulfment pathways by functioning downstream of CED-7, CED-6 and CED-1.56 During the phagolysosome formation, RAB-7 was a downstream effector of CED-1.57 VPS-34 and RAB-14 functioned in an ordered manner downstream of CED-1 to control phagosome maturation.46,49,58 SNX-1 (a nexin) was required in driving the degradation of cell corpses in the signaling cascade initiated by CED-1.48 Here, we further raised the signaling cascade of CED-1-CED-10/VPS-34/SNX-1/RAB-7/RAB-14 in the germline to control NPS toxicity, which strengthened the understanding of the molecular basis for the germline in response to exposure to toxicants such as NPS (Fig. 5B). Nevertheless, the molecular basis for the germline in response to NPS exposure was still very limited. Future work on the elucidation of molecular signals mediated by germlinez CED-10, VPS-34, SNX-1, RAB-7 and RAB-14 in controlling NPS toxicity is needed.
In C. elegans, during the degradation and engulfment of the apoptotic cell, CED-6 (an adaptor protein) and DYN-1 (a GTPase dynamin) mediate the CED-1 function.52,59 At the early step, LST-4 promoted the phagosome maturation process.46 SNX-6 is another nexin in the signaling cascade initiated by CED-1 to regulate the degradation of cell corpses.48 EPN-1 (an adaptor epsin) functioned in the same CED-1 pathway to control cell corpse engulfment.45 We further observed that besides the expression levels of CED-10, VPS-34, SNX-1, RAB-7, and RAB-14, the expression levels of CED-6, DYN-1, LST-4, SNX-6, and EPN-1 were also inhibited in 100 nm NPS exposed ced-1(RNAi) worms (Fig. 3A). In C. elegans, the smaller NPS particles induced more severe toxic effects than polystyrene having large sizes.60 Doses (≤1 μg L−1) have been raised as predicted environmental doses for NP particles.61 Thus, CED-6, DYN-1, LST-4, SNX-6 and EPN-1 may also potentially function downstream of the germline GPCR CED-1 to control the toxicity of NPS with the smaller size and at the predicted environmental concentration.
Furthermore, we found the germline-intestine communication mediated by CED-6, DYN-1, LST-4, SNX-6, and EPN-1 during the control of NPS toxicity (Fig. 5B). In NPS-exposed nematodes, the expressions of DAF-16 and PMK-1 could be inhibited in ced-10(RNAi), vps-34(RNAi), snx-1(RNAi), rab-7(RNAi) or rab-14(RNAi) worms (Fig. 5A). Previous studies have indicated that the functions of both p38 MAPK and insulin signaling pathways in controlling NPS toxicity were only restricted in the intestinal cells.28,30 Therefore, CED-1 activated signaling cascade in the germline could control the NPS toxicity by affecting the activities of p38 MAPK and insulin signaling pathways in the intestinal cells. That is, besides the neuron-intestine communication,62,63 the germline-intestine communication is further required for controlling NPS toxicity.
4. Conclusions
Together, we examined the GPCRs in the germline in response to NPS exposure. We detected two GPCRs in the germline (PAQR-2 and CED-1) activated by NPS (1–1000 μg L−1). After the NPS exposure, the increase in the germline GPCR CED-1 mediated a protective response. CED-1 mediated this protective response to NPS exposure by activating the downstream five targets (CED-10, VPS-34, SNX-1, RAB-7, and RAB-14). Moreover, these five germline targets further controlled the NPS toxicity by affecting the functions of p38 MAPK and insulin signaling pathways in the intestine. Our findings strengthened the understanding of the molecular basis for the germline in response to NPS exposure.Conflicts of interest
There are no conflicts to declare.References
- D. K. A. Barnes, F. Galgani, R. C. Thompson and M. Barlaz, Accumulation and fragmentation of plastic debris in global environments, Philos. Trans. R. Soc., B, 2009, 364, 1985–1998 CrossRef CAS PubMed
.
- S. Lambert and M. Wagner, Characterisation of nanoplastics during the degradation of polystyrene, Chemosphere, 2016, 145, 265–268 CrossRef CAS PubMed
.
- Y. Chae and Y. J. An, Effects of micro- and nanoplastics on aquatic ecosystems: current research trends and perspectives, Mar. Pollut. Bull., 2017, 124, 624–632 CrossRef CAS PubMed
.
- J. Saavedra, S. Stoll and V. I. Slaveykova, Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton, Environ. Pollut., 2019, 252, 715–722 CrossRef CAS PubMed
.
- S. B. Sjollema, P. Redondo-Hasselerharm, H. A. Leslie, M. H. S. Kraak and A. D. Vethaak, Do plastic particles affect microalgal photosynthesis and growth?, Aquat. Toxicol., 2016, 170, 259–261 CrossRef CAS PubMed
.
- L. Canesi, C. Ciacci, E. Bergami, M. P. Monopoli, K. A. Dawson, S. Papa, B. Canonico and I. Corsi, Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus, Mar. Environ. Res., 2015, 111, 34–40 CrossRef CAS PubMed
.
- L. G. A. Barboza, L. R. Vieira and L. Guilhermino, Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): changes in behavioural responses and reduction of swimming velocity and resistance time, Environ. Pollut., 2018, 236, 1014–1019 CrossRef CAS PubMed
.
- Y. Jin, J. Xia, Z. Pan, J. Yang, W. Wang and Z. Fu, Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish, Environ. Pollut., 2018, 235, 322–329 CrossRef CAS PubMed
.
- T. Kögel, Ø. Bjorøy, B. Toto, A. M. Bienfait and M. Sanden, Micro- and nanoplastic toxicity on aquatic life: determining factors, Sci. Total Environ., 2020, 709, 136050 CrossRef PubMed
.
- Y. Yu, H. Chen, X. Hua, Y. Dang, Y. Han, Z. Yu, X. Chen, P. Ding and H. Li, Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans, Sci. Total Environ., 2020, 726, 138679 CrossRef CAS PubMed
.
- T. Zheng, D. Yuan and C. Liu, Molecular toxicity of nanoplastics involving in oxidative stress and desoxyribonucleic acid damage,
J. Mol. Recognit.
, 2019, 32, e2804 CrossRef CAS PubMed
.
- A. Cózar, F. Echevarría, J. Ignacio González-Gordillo, X. Irigoien, B. Úbeda, S. Hernández-León, A. T. Palma, S. Navarro, J. Garcia-de-Lomas, A. Ruiz, M. L. Fernandez-de-Puelles and C. M. Duarte, Plastic debris in the open ocean?, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10239–10244 CrossRef PubMed
.
- R. Lehner, C. Weder, A. Petri-Fink and B. Rothen-Rutishauser, Emergence of nanoplastic in the environment and possible impact on human health, Environ. Sci. Technol., 2019, 53, 1748–1765 CrossRef CAS PubMed
.
- H. M. Kim, D. K. Lee, N. P. Long, S. W. Kwon and J. H. Park, Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans, Environ. Pollut., 2019, 246, 578–586 CrossRef CAS PubMed
.
- S. W. Kim, D. Kim, S. Jeong and Y. An, Size-dependent effects of polystyrene plastic particles on the nematode Caenorhabditis elegans as related to soil physicochemical properties, Environ. Pollut., 2020, 258, 113740 CrossRef CAS PubMed
.
-
D.-Y. Wang, Exposure Toxicology in Caenorhabditis elegans, Springer Nature Singapore Pte Ltd, 2020 Search PubMed
.
- M. Qu, Y.-X. Qiu, Y. Kong and D.-Y. Wang, Amino modification enhances reproductive toxicity of nanopolystyrene on gonad development and reproductive capacity in nematode Caenorhabditis elegans, Environ. Pollut., 2019, 254, 112978 CrossRef CAS PubMed
.
- M. Qu and D.-Y. Wang, Toxicity comparison between pristine and sulfonate modified nanopolystyrene particles in affecting locomotion behavior, sensory perception, and neuronal development in Caenorhabditis elegans, Sci. Total Environ., 2020, 703, 134817 CrossRef CAS PubMed
.
- L. Lei, S. Wu, S. Lu, M. Liu, Y. Song, Z. Fu, H. Shi, K. H. Raley-Susman and D. He, Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans, Sci. Total Environ., 2018, 619–620, 1–8 CrossRef CAS PubMed
.
- L. Zhao, M. Qu, G. Wong and D.-Y. Wang, Transgenerational toxicity of nanopolystyrene particles in the range of μg/L in nematode Caenorhabditis elegans, Environ. Sci.: Nano, 2017, 4, 2356–2366 RSC
.
- M. Qu, K.-N. Xu, Y.-H. Li, G. Wong and D.-Y. Wang, Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles, Sci. Total Environ., 2018, 643, 119–126 CrossRef CAS PubMed
.
- K. Gamo, S. Kiryu-Seo, H. Konishi, S. Aoki, K. Matsushima, K. Wada and H. Kiyama, G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity, J. Neurosci., 2008, 28, 11980–11988 CrossRef CAS PubMed
.
- C. Jee, J. Lee, J. P. Lim, D. Parry, R. O. Messing and S. L. McIntire, SEB-3, a CRF receptor-like GPCR, regulates locomotor activity states, stress responses and ethanol tolerance in Caenorhabditis elegans, Genes, Brain Behav., 2013, 12, 250–262 CrossRef CAS PubMed
.
-
D.-Y. Wang, Molecular Toxicology in Caenorhabditis elegans, Springer Nature Singapore Pte Ltd, 2019 Search PubMed
.
-
D.-Y. Wang, Target Organ Toxicology in Caenorhabditis elegans, Springer Nature Singapore Pte Ltd, 2019 Search PubMed
.
- H.-M. Shao, Z.-Y. Han, N. Krasteva and D.-Y. Wang, Identification of signaling cascade in the insulin signaling pathway in response to nanopolystyrene particles, Nanotoxicology, 2019, 13, 174–188 CrossRef CAS PubMed
.
- P. Sen, Y. Xiong, Q. Zhang, S. Park, W. You, H. Ade, M. W. Kudenov and B. T. O'Connor, Shear-enhanced transfer printing of conducting polymer thin films, ACS Appl. Mater. Interfaces, 2018, 10, 31560 CrossRef CAS PubMed
.
- H. Gelbke, M. Banton, C. Block, G. Dawkins, R. Eisert, E. Leibold, M. Pemberton, I. M. Puijk, A. Sakoda and A. Yasukawa, Risk assessment for migration of styrene oligomers into food from polystyrene food containers, Food Chem. Toxicol., 2019, 124, 151–167 CrossRef CAS PubMed
.
- M. A. Abdallah, M. Sharkey, H. Berresheim and S. Harrad, Hexabromocyclododecane in polystyrene packaging: a downside of recycling?, Chemosphere, 2018, 199, 612–616 CrossRef CAS PubMed
.
- M. Qu, Y.-Q. Liu, K.-N. Xu and D.-Y. Wang, Activation of p38 MAPK signaling-mediated endoplasmic reticulum unfolded protein response by nanopolystyrene particles, Adv. Biosyst., 2019, 3, 1800325 CrossRef CAS PubMed
.
- Y.-H. Yang, H.-H. Du, G.-S. Xiao, Q.-L. Wu and D.-Y. Wang, Response of intestinal Gα subunits to nanopolystyrene in nematode Caenorhabditis elegans, Environ. Sci.: Nano, 2020, 7, 2351–2359 RSC
.
- S. Brenner, The genetics of Caenorhabditis elegans, Genetics, 1974, 77, 71–94 CrossRef CAS PubMed
.
- Y.-H. Yang, Q.-L. Wu and D.-Y. Wang, Epigenetic response to nanopolystyrene in germline of nematode Caenorhabditis elegans, Ecotoxicol. Environ. Saf., 2020, 206, 111404 CrossRef PubMed
.
- Y.-X. Qiu, Y.-Q. Liu, Y.-H. Li and D.-Y. Wang, Intestinal mir-794 responds to nanopolystyrene by linking insulin and p38 MAPK signaling pathways in nematode Caenorhabditis elegans, Ecotoxicol. Environ. Saf., 2020, 201, 110857 CrossRef CAS PubMed
.
- H.-L. Liu, D. Li, R.-J. Zhang, L.-M. Sun and D.-Y. Wang, Lipid metabolic sensors of MDT-15 and SBP-1 regulated the response to simulated microgravity in the intestine of Caenorhabditis elegans, Biochem. Biophys. Res. Commun., 2020, 528, 28–34 CrossRef CAS PubMed
.
- L.-M. Sun, W.-J. Li, D. Li and D.-Y. Wang, microRNAs involved in the control of toxicity on locomotion behavior induced by simulated microgravity stress in Caenorhabditis elegans, Sci. Rep., 2020, 10, 17510 CrossRef CAS PubMed
.
- L. Zou, D. Wu, X. Zang, Z. Wang, Z. Wu and D. Chen, Construction of a germline-specific RNAi tool in C. elegans, Sci. Rep., 2019, 9, 2354 CrossRef PubMed
.
- D. Li, Y.-J. Yuan and D.-Y. Wang, Regulation of response to nanopolystyrene by intestinal microRNA mir-35 in nematode Caenorhabditis elegans, Sci. Total Environ., 2020, 736, 139677 CrossRef CAS PubMed
.
- K. A. Haskins, J. F. Russell, N. Gaddis, H. K. Dressman and A. Aballay, Unfolded protein response genes regulated by CED-1 are required for Caenorhabditis elegans innate immunity, Dev. Cell, 2008, 15, 87–97 CrossRef CAS PubMed
.
- K. Singh, M. Y. Chao, G. A. Somers, H. Komatsu, M. E. Corkins, J. Larkins-Ford, T. Tucey, H. M. Dionne, M. B. Walsh, E. K. Beaumont, D. P. Hart, S. Lockery and A. C. Hart,
C. elegans notch signaling regulates adult chemosensory response and larval molting quiescence, Curr. Biol., 2011, 21, 825–834 CrossRef CAS PubMed
.
- R. Ghosh, E. C. Andersen, J. A. Shapiro, J. P. Gerke and L. Kruglyak, Natural variation in a chloride channel subunit confers avermectin resistance in C. elegans, Science, 2012, 335, 574–578 CrossRef CAS PubMed
.
- E. Svensk, R. Devkota, M. Ståhlman, P. Ranji, M. Rauthan, F. Magnusson, S. Hammarsten, M. Johansson, J. Boren and M. Pilon,
Caenorhabditis elegans PAQR-2 and IGLR-2 protect against glucose toxicity by modulating membrane lipid composition, PLoS Genet., 2016, 12, e1005982 CrossRef PubMed
.
- R. Pocock and O. Hobert, Hypoxia activates a latent circuit for processing gustatory information in C. elegans, Nat. Neurosci., 2010, 13, 610–614 CrossRef CAS PubMed
.
- L. J. Neukomm, S. Zeng, A. P. Frei, P. A. Huegli and M. O. Hengartner, Small GTPase CDC-42 promotes apoptotic cell corpse clearance in response to PAT-2 and CED-1 in C. elegans, Cell Death Differ., 2014, 21, 845–853 CrossRef CAS PubMed
.
- Q. Shen, B. He, N. Lu, B. Conradt, B. D. Grant and Z. Zhou, Phagocytic receptor signaling regulates clathrin and epsin mediated cytoskeletal remodeling during apoptotic cell engulfment in C. elegans, Development, 2013, 140, 3230–3243 CrossRef CAS PubMed
.
- D. Chen, Y. Jian, Z. Liu, Y. Zhang, J. Liang, X. Qi, H. Du, W. Zou, L. Chen, Y. Chai, G. Ou, L. Miao, Y. Wang and C. Yang, Clathrin and AP2 are required for phagocytic receptor-mediated apoptotic cell clearance in Caenorhabditis elegans, PLoS Genet., 2013, 9, e1003517 CrossRef CAS PubMed
.
- L. J. Neukomm, A. Nicot, J. M. Kinchen, J. Almendinger, S. M. Pinto, S. Zeng, K. Doukoumetzidis, H. Tronchère, B. Payrastre, J. F. Laporte and M. O. Hengartner, The phosphoinositide phosphatase MTM-1 regulates apoptotic cell corpse clearance through CED-5-CED-12 in C. elegans, Development, 2011, 138, 2003–2014 CrossRef CAS PubMed
.
- N. Lu, Q. Shen, T. R. Mahoney, X. Liu and Z. Zhou, Three sorting nexins drive the degradation of apoptotic cells in response to Ptdlns(3)P signaling, Mol. Biol. Cell, 2011, 22, 354–374 CrossRef CAS PubMed
.
- J. M. Kinchen, K. Doukoumetzidis, J. Almendinger, L. Stergiou, A. Tosello-Trampont, C. D. Sifri, M. O. Hengartner and K. S. Ravichandran, A novel pathway for phagosome maturation during engulfment of apoptotic cells, Nat. Cell Biol., 2008, 10, 556–566 CrossRef CAS PubMed
.
- H.-M. Shao, Y. Kong and D.-Y. Wang, Response of intestinal signaling communication between nucleus and peroxisome to nanopolystyrene at predicted environmental concentration, Environ. Sci.: Nano, 2020, 7, 250–261 RSC
.
- H.-M. Shao and D.-Y. Wang, Long-term and low-dose exposure to nanopolystyrene induces a protective strategy to maintain functional state of intestine barrier in nematode Caenorhabditis elegans, Environ. Pollut., 2020, 258, 113649 CrossRef CAS PubMed
.
- X. Yu, S. Odera, C. H. Chuang, N. Lu and Z. Zhou,
C. elegans dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells, Dev. Cell, 2006, 10, 743–757 CrossRef CAS PubMed
.
- Z. Zhou, E. Hartwieg and H. R. Horvitz, CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans, Cell, 2001, 104, 43–56 CrossRef CAS PubMed
.
- X. Wang, W. Li, D. Zhao, B. Liu, Y. Shi, B. Chen, H. Yang, P. Guo, X. Geng, Z. Shang, E. Peden, E. Kage-Nakadai, S. Mitani and D. Xue,
Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor, Nat. Cell Biol., 2010, 12, 655–664 CrossRef CAS PubMed
.
- H. Chiu, Y. Zou, N. Suzuki, Y. Hsieh, C. Chuang, Y. Wu and C. Chang, Engulfing cells promote neuronal regeneration and remove neuronal debris through distinct biochemical functions of CED-1, Nat. Commun., 2018, 9, 4842 CrossRef PubMed
.
- J. M. Kinchen, J. Cabello, D. Klingele, K. Wong, R. Feichtinger, H. Schnabel, R. Schnabel and M. O. Hengartner, Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans, Nature, 2005, 434, 93–99 CrossRef CAS PubMed
.
- X. Yu, N. Lu and Z. Zhou, Phagocytic receptor CED-1 initiates a signaling pathway for degrading engulfed apoptotic cells, PLoS Biol., 2008, 6, e61 CrossRef PubMed
.
- P. Guo, T. Hu, J. Zhang, S. Jiang and X. Wang, Sequential action of Caenorhabditis elegans Rab GTPases regulates phagolysosome formation during apoptotic cell degradation, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18016–18021 CrossRef CAS PubMed
.
- H. P. Su, K. Nakada-Tsukui, A. Tosell-Trampont, Y. Li, G. Bu, P. M. Henson and K. S. Ravichandran, Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP), J. Biol. Chem., 2002, 277, 11772–11779 CrossRef CAS PubMed
.
- Y.-X. Qiu, Y.-Q. Liu, Y.-H. Li, G.-J. Li and D.-Y. Wang, Effect of chronic exposure to nanopolystyrene on nematode Caenorhabditis elegans, Chemosphere, 2020, 256, 127172 CrossRef CAS PubMed
.
- R. Lenz, K. Enders and T. G. Nielsen, Microplastic exposure studies should be environmentally realistic, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4121–E4122 CrossRef CAS PubMed
.
- H.-L. Liu, R.-J. Zhang and D.-Y. Wang, Response of DBL-1/TGF-β signaling-mediated neuron-intestine communication to nanopolystyrene in nematode Caenorhabditis elegans, Sci. Total Environ., 2020, 745, 1141047 Search PubMed
.
- S.-T. Wang, H.-L. Liu, Y.-Y. Zhao, Q. Rui and D.-Y. Wang, Dysregulated mir-354 enhanced the protective response to nanopolystyrene by affecting the activity of TGF-β signaling pathway in nematode Caenorhabditis elegans, NanoImpact, 2020, 20, 100256 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00867b |
| This journal is © The Royal Society of Chemistry 2021 |