Open Access Article
Open Access ArticleInvestigating the remediation potential of iron oxide nanoparticles in Cu-polluted soil–plant systems: coupled geochemical, geophysical and biological approaches†
E.
Demangeat
 *a,
M.
Pédrot
a,
A.
Dia
*a,
M.
Pédrot
a,
A.
Dia
 a,
M.
Bouhnik-Le-Coz
a,
M.
Bouhnik-Le-Coz
 a,
P.
Roperch
a,
G.
Compaoré
a and
F.
Cabello-Hurtado
*b
a,
P.
Roperch
a,
G.
Compaoré
a and
F.
Cabello-Hurtado
*b
aUniv Rennes, CNRS, Géosciences Rennes, UMR 6118, 35000 Rennes, France. E-mail: ed.demangeat@gmail.com
bUniv Rennes, CNRS, ECOBIO, UMR 6553, 35000 Rennes, France. E-mail: francisco.cabello@univ-rennes1.fr
First published on 22nd February 2021
Abstract
Although the use of iron oxide nanoparticles (IONPs) has high potential in remediation and agriculture, a major hindrance to their use includes the risk of contamination of soil and water resources with underexplored effects of IONPs on biota. The fate, phytotoxicity and remediation potential of IONPs are investigated with soil column experiments using 7 nm-sized magnetite (Fe3O4) nanoparticles (magnNPs) and sunflower (Helianthus annuus). Control soil, magnNP-containing soil (10 g magnNPs per kg soil), copper-polluted soil (500 mg Cu per kg soil) and copper-polluted soil containing magnNPs (10 g magnNPs per kg soil and 500 mg Cu per kg soil) support sunflower growth for 57 and 95 days. In magnNP-exposed plants, the occurrence of magnNPs does not affect the growth of the vegetative aerial parts and photosynthetic efficiency. Decreased lipid peroxidation indicates an enhanced antioxidant enzymatic response of magnNP-exposed plants. In plants grown in Cu- and magnNP–Cu-soils, the physiological and biochemical impacts of excess copper are clearly identified, resulting in growth retardation, decreased pigment contents and photosynthetic efficiency, and increased lipid peroxidation and peroxidase (POD) activities. Based on magnetic susceptibility, a higher amount of magnNPs is detected after 57 days in the roots of magnNP-exposed plants (1400 mg kg−1) than in the roots of magnNP–Cu-exposed plants (920 mg kg−1). In the latter, magnNP internalization is likely hampered because of the plants' physiological responses to Cu toxicity. At the working Cu and magnNP concentrations, magnNPs neither decrease Cu accumulation in the plant tissues nor alleviate the overall growth retardation of sunflowers and certain phytotoxic effects induced by excess Cu. However, this study highlights several positive environmental aspects relative to magnNP use, including the harmless effects of magnNPs on sunflowers (1% magnNPs in soil) and the ability of magnNPs to influence Cu mobility in the soil (which could be even more pronounced at lower Cu concentration).
1. Introduction
The increasing production and use of engineered nanoparticles (NPs) has led to the significant occurrence of these new materials in the environment.1 The unique properties of NPs, particularly their high reactivity (high surface-to-volume ratio), have fuelled their employment in the technological, medical, agricultural and environmental fields.2–7 As a result, their direct intentional (agrochemical spraying) or unintentional deposition (aerial deposition, urban waste) in the environment has provided multiple ways for NPs to enter ecosystems, through the air, soil or water.8 Amongst these NPs, iron oxide nanoparticles (IONPs) may originate from their use in the environmental sector, especially for soil and water decontamination, where IONPs show promising potential for metal and metalloid removal.9,10 As a consequence, considerable research is needed to assess the impacts and fate of IONPs in the environment and the interactions that ensue from the presence of IONPs in natural media. Considering the key role played by plants within ecosystems, special attention has been paid to elucidate the effects of IONPs on plants.11–13One of the first challenges to deciphering the physiological and biochemical responses of plants to IONP exposure is to understand the complex interplay in between IONP properties, biota and soil. Abiotic (pH, salinity, ionic strength, temperature, light, etc.) and biotic conditions (related to plant roots or rhizosphere microfauna and microbial, bacterial and fungal activities)8,14–16 are important and need to be considered as they can influence IONP persistence in a medium, as well as IONP mobility, bioavailability, uptake and toxicity in plants.17 Previous studies showed that the uptake of IONPs by plants is directly related to the individual IONP size, surface charge, and surface coating.14 Besides, IONP concentration in the medium as well as the physico-chemical properties and composition of the medium, the plant species, the plant growth stage and the plant exposure route all together play a critical role in driving IONP uptake and IONP effects on the plants exposed.18–20
Because of these multiple interfering parameters, the effects of IONPs on plants are variable and often appear quite hazardous, ranging from positive to lethal.21,22 The adverse effects of IONPs on plants generally result from oxidative stress and/or from decreased cellular exchange with the media due to particle binding onto the cell surface.23 Once in the cell, metal nanoparticles could interfere with the electron transport chain of both chloroplasts and mitochondria, which may result in an oxidative burst, as observed by increased reactive oxygen species (ROS) concentration.24 Although ROS can act as secondary messengers in the stress signal transduction pathway, excessive ROS production can cause oxidative stress, which damages plants by oxidizing photosynthetic pigments, membrane lipids, proteins and nucleic acids.25,26 On the other hand, IONPs can indirectly impact plant metabolism. In particular, IONPs can react with pollutants in soils and soil solutions, such as heavy metals or pesticides and other contaminants, and thus modify their availability, transport and toxicity.
Along with the use of pesticides, fungicides, industrial effluents and wastewater irrigation, copper (Cu) is commonly found in agricultural soils where high Cu concentrations have raised great concern for sustainable agricultural production.27 For example, the use of the Bordeaux mixture has seriously increased Cu pollution in European vineyard soils (200–500 mg Cu per kg soil)28 compared to uncontaminated soils (3 to 100 mg Cu per kg soil).29 Although Cu is an essential micronutrient present in many plant proteins, excess Cu in soil results in phytotoxicity, including leaf chlorosis and stunted plant growth (particularly root growth). Copper phytotoxicity also leads to a reduced uptake and accumulation of other mineral nutrients, thus disturbing important plant biochemical processes.30,31 Copper was notably found to prevent Fe absorption, highlighting an antagonistic relationship between Cu and Fe uptake.31 In addition, different studies have described Cu affinity and adsorption onto IONPs32,33 suggesting that the presence of IONPs could affect the expected Cu phytotoxicity in polluted soil. On the other hand, Cu bioavailability depends upon soil physico-chemical parameters and CEC (cation exchange capacity) and the total soil Cu content.34 Thus, much of the Cu present in soils is not easily available to plants because of the tight binding of Cu2+ to soil organic matter and other colloids.35
Studies devoted to the interactions between plants and IONPs are at the heart of multiple research investigations, with important implications for agricultural practices. To exploit the potential benefits of IONPs for crops, issues concerning IONP availability, biocompatibility and behaviour in the growth culture medium need to be further investigated. In this context, the objectives of this study were (i) to determine whether magnetite nanoparticles (magnNPs) have any toxic effects on sunflower plants at the exposed concentrations, (ii) to assess the mobility of magnNPs in soil and plants and (iii) to decipher their effects in copper-contaminated soil–plant systems. Ultimately, this study aims to shed light on the environmental risks of IONPs and their remediation potential in Cu-polluted soils, considering that these nanomaterials are already used for industrial and agricultural purposes.
2. Materials and methods
2.1. Iron oxide nanoparticle synthesis and characterization
Fe3O4 nanoparticles (MagnNPs) were prepared as previously described in Demangeat et al. (2018)35 through anaerobic (Jacomex glovebox) coprecipitation of Fe salts (FeCl2·4H2O and FeCl3·6H2O). The final black precipitate of magnNPs was washed with deoxygenated deionized water once and 5 × 10−3 M NaCl solution (2 times) and then stored under anaerobic conditions.The magnNP size was determined using high-resolution transmission electron microscopy (HR-TEM) with a JEOL100CXII instrument (voltage 100 kV) at the THEMIS Analytical Facility at the University of Rennes 1. The magnNP samples were initially suspended in 0.005 mM NaCl (pH = 6), and the suspensions were further diluted to reach a concentration of 1 × 10−3 mg L−1 magnNPs before being carefully placed on 300 mesh Au grids supported with carbon film.
The magnNP surface area was measured using the multipoint N2-Brunauer Emmett Teller (BET) technique with a Coulter (SA 3100) analyser. Prior to the measurements, tubes containing 35 mL solutions (18 g L−1, 0.005 mM NaCl and pH = 6) of magnNPs were centrifuged at 4110g for 30 min. Then, the supernatant was removed, and the wet mineral pastes (concentrated at the bottom of the tubes) were placed in an oven where they were gently dried at 49 °C for 2 days. Once completely dry, the solid pieces were put in a mortar and ground with a pestle to obtain a fine powder.
To determine the pH of the zero point of charge (pHzpc), potentiometric acid–base titrations were conducted on 1 and 2 g L−1 solid solutions at three ionic strengths (1 × 10−2, 5 × 10−2, and 1 × 10−1 M NaCl) using a self-developed titration system with two titrators (794 Basic Titrino – Metrohm).
The Fe(II)/Fe(III) ratio of magnNPs was determined after acidic dissolution of magnNPs (12 h using 0.6 M HCl) under anaerobic conditions. Then, dissolved Fe(II) and Fe(III) concentrations were measured using the 1,10-phenanthroline colorimetric method.33 At the beginning of the experiments, the Fe(II)/Fe(III) ratio was equal to 0.5. The measurement was performed on a 12 g L−1 magnNP suspension in 0.005 mM NaCl, pH = 6.
2.2. Plant growth conditions and treatments
Plants (Helianthus annuus – 1 plant per column) were grown in soil columns (250 mL polypropylene measuring cylinders, VWR). The soil was held over a height of 13 cm (120 g of dry soil) maintained by a circular drilled plate (3.7 cm diameter) covered with a nylon filter (ESI Fig. S1†). Soil solution leakage and regular sampling of leachates were allowed by an aperture created at the cylinder bottom (ESI Fig. S1†). The columns were wrapped with aluminium paper to avoid any exposure of the medium to light and algal development.The soil used in the experiment (ESI Table S1†) was sampled in an inventoried natural observatory (the so-called Zone Atelier Armorique; http://www.za-inee.org/en) in the wetland area of Pleine-Fougères (Brittany, France) in the 0 to 20 cm-deep first horizon. Following its sampling, the soil was homogenized, dried and sieved through a 2.0 mm-diameter sieve. Twenty aliquots of soil (120 g each) were then constituted to obtain five replicates of the four treatments: unmodified soil (control soil), soil containing 1% magnNPs (magnNP-soil), soil polluted with Cu (Cu-soil) and soil polluted with Cu and containing 1% magnNPs (magnNP–Cu soil). Treatments involving Cu contamination were achieved by the dropwise addition of 5.5 M CuSO4 solution over a grid pattern. Because the soil initially contained Cu (20 mg kg−1), the addition of 5.5 M CuSO4 was carried out to provide 480 mg Cu per kg soil and thereby reach 500 mg kg−1 in the Cu-treated soils (versus 20 mg kg−1 in control- and magnNP-soils). This concentration can be encountered in agricultural Cu-polluted soils.29,30 MagnNP solution (12 g L−1) was also dispensed drop by drop above a grid pattern in the NP-treated soils to obtain 1 wt% magnNPs and to ensure a homogeneous distribution. According to Komárek et al. (2013)36 1 wt% NPs provides a consistent amount of material to achieve an optimal removal of trace elements (TEs) in soil.
Because of its high biomass production and its use in phytoremediation for environmental clean-up,37,38Helianthus annuus was chosen for the present experiment. Prior to sowing, 60 seeds were carefully washed in a sodium hypochlorite (NaOCl) bath (3 min) and 5 distilled water baths (12 min each). The seeds were then soaked in distilled water for 45 min under a laminar flow hood. Sunflower seed germination occurred directly in soil columns. To ensure that seedlings were obtained, 3 seeds were sown per column, and immediately after germination, only one plantlet per column was retained for growth. Cyclic growth conditions were ensured in a growth chamber, allowing 16 h light at 22 °C and 8 h in the dark at 18 °C, a photosynthetic photon flux density of 160 μmol m−2 s−1, and a relative humidity of 70%.
A modified Hoagland39 half-strength (×0.5) solution (without iron and micronutrients) or a 2 mM NaCl solution was alternately poured into the soils every 2 days at field capacity. Through this regular sprinkling, the soil was kept moist. Increased volumes of the watering solution were necessary to allow soil solution sampling every 3 to 5 days. Approximately 25 mL of soil solution was collected from each column using a peristaltic pump (set at 1 mL min−1). For each treatment (5 replicated columns), soil solutions collected from two replicates were homogenized at each sampling to allow geochemical analyses. The soil solution from the remaining replicate (for each treatment) was used for pH and Eh measurements.
At the end of the floral initiation (i.e., after 57 days of growth), 4 out of 5 plants per treatment were collected for biological analyses. Pictures were taken, and the fresh weights of the roots, aerial parts and flower buds of the plants were measured for each individual. It is noteworthy that the root weights are not provided in the results, as the whole roots could not be precisely and equally recovered from the soils (some roots were trapped in the soil or were broken). Liquid nitrogen allowed the freezing of plant samples, which were subsequently stored at −80 °C.
For each treatment, a fifth plant was harvested at the early fruit development stage before seed maturity (38 days after the first harvest). These plants provided samples for magnetic susceptibility measurements to study the magnNP translocation efficiency. To perform these geophysical analyses, the aerial parts of the plants were separated into 3 sections that were labelled, from the ground upwards, as follows: “AP1” (lower mid-height stem section), “AP2” (upper mid-height stem section) and “AP3” (flower head). Similarly, root (R1, R2 and R3) and soil (S1, S2 and S3) sections were labelled in the 13 cm-high soil and were numbered from 1 to 3 with increasing depth.
2.3. Biological assays
The following biological assays were conducted on dry plant materials. Drying was performed by lyophilization (Christ ALPHA 1-2LDplus). Drying was achieved by placing frozen plant samples into the chamber of the freeze dryer for a primary long drying (0.09 Pa for 72 h), followed by a secondary short drying stage (0.001 Pa for 24 h). Lyophilized samples were immediately sealed and preserved in a cold room. For most analyses, the vegetative lyophilized material was gently ground using an agate mortar and pestle to work on solid powders.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g. Supernatants were recovered in new tubes, and 30 μL of each supernatant was diluted in 270 μL of acetone (80%) in a microplate well. The chlorophyll content was determined by spectrophotometrically reading the absorbance at 470, 645 and 663 nm (spectrophotometer SAFAS FLX-Xenius – EcoChim analytical platform of Rennes 1 University). Based on the equations of Lichtenthaler and Wellburn (1983)40 pigment contents were determined (μg mg−1 DW).
000g. Supernatants were recovered in new tubes, and 30 μL of each supernatant was diluted in 270 μL of acetone (80%) in a microplate well. The chlorophyll content was determined by spectrophotometrically reading the absorbance at 470, 645 and 663 nm (spectrophotometer SAFAS FLX-Xenius – EcoChim analytical platform of Rennes 1 University). Based on the equations of Lichtenthaler and Wellburn (1983)40 pigment contents were determined (μg mg−1 DW).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min, and the supernatants were collected in new tubes. Two aliquots of 200 μL were mixed with TBA+ solution (20.0% trichloroacetic acid (p/v), 0.65% (p/v) TBA and 0.01% (w/v) butyl-hydroxytoluene) and two other aliquots with TBA-solution (20.0% trichloroacetic acid (p/v) and 0.01% (w/v) butyl-hydroxytoluene). Eventually, the samples were heated at 95 °C for 25 min, rapidly cooled to room temperature and immediately centrifuged at 10
000g for 10 min, and the supernatants were collected in new tubes. Two aliquots of 200 μL were mixed with TBA+ solution (20.0% trichloroacetic acid (p/v), 0.65% (p/v) TBA and 0.01% (w/v) butyl-hydroxytoluene) and two other aliquots with TBA-solution (20.0% trichloroacetic acid (p/v) and 0.01% (w/v) butyl-hydroxytoluene). Eventually, the samples were heated at 95 °C for 25 min, rapidly cooled to room temperature and immediately centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. The supernatants were recovered in new tubes, and 300 μL were sampled and placed in a microplate. The absorbance of the supernatants was recorded at 440, 532 and 600 nm spectrophotometrically using a microplate reader (SAFAS FLX-Xenius). The results were finally expressed in terms of malondialdehyde equivalents (MDAeq) per gram of plant DW following the equations of Hodges et al. (1999).42
000g for 10 min. The supernatants were recovered in new tubes, and 300 μL were sampled and placed in a microplate. The absorbance of the supernatants was recorded at 440, 532 and 600 nm spectrophotometrically using a microplate reader (SAFAS FLX-Xenius). The results were finally expressed in terms of malondialdehyde equivalents (MDAeq) per gram of plant DW following the equations of Hodges et al. (1999).42
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 12 min, and the supernatants were collected in new tubes. Soluble protein quantification was performed spectrophotometrically based on Bradford's method (1976).43 Bovine serum albumin (BSA) calibration solutions (0.1 to 1.4 mg L−1) were used to determine the protein contents. Protein extracts were kept at −80 °C until used in POD and SOD enzymatic antioxidant activity assays.
000g for 12 min, and the supernatants were collected in new tubes. Soluble protein quantification was performed spectrophotometrically based on Bradford's method (1976).43 Bovine serum albumin (BSA) calibration solutions (0.1 to 1.4 mg L−1) were used to determine the protein contents. Protein extracts were kept at −80 °C until used in POD and SOD enzymatic antioxidant activity assays.
2.4. Geochemical analyses
Major and trace element concentrations were determined by ICP-MS (Agilent 7700x) using rhenium and rhodium as internal standards. The international geostandard SLRS-5 was used to check the validity and reproducibility of the results.47 Samples were prepared in a clean room in pre-washed digestion vessels (Savillex® Teflon vials) (24 h in nitric acid (1.5 M HNO3) at 45 °C, 24 h in deionized ultrapure water at 45 °C – repeated twice) and were further diluted in prewashed 50 mL tubes (24 h in nitric acid (1.5 M HNO3) at 45 °C, 24 h in deionized ultrapure water at 45 °C). For magnNPs and soil solutions, the samples were first digested for 16 h at 95 °C with subboiled nitric acid (14.6 M HNO3) and heated until complete evaporation of the solvent. The samples were eventually re-solubilized in 0.37 M HNO3 with appropriate dilution(s) considering ICP-MS quantification limits. Lyophilized plant materials (shoots and roots, 50 mg) were digested in subboiled nitric acid (14.6 M HNO3) 5 times, with approximately 8 hours of evaporation on a hot plate (95 °C) between each digestion. When the samples were not completely dissolved after the first digestion, hydrogen peroxide (H2O2) was used for one or two more digestions. In addition, the presence of insoluble organic matter and minerals in root samples often meant that more digestions needed to be performed. Solids obtained after the last evaporation were solubilized in 0.37 M HNO3 with appropriate dilution(s) considering ICP-MS quantification limits.2.5. Magnetic susceptibility measurements
To track and quantify magnNPs in soil and plant matrices, magnetic susceptibility measurements were performed. Magnetic susceptibility has already been employed to evidence magnetite occurrence in various environments.48 In the present study, measurements were conducted with a magnetic susceptibility meter (Kappabridge AGICO KLY3). The magnetic susceptibility is the sum of three main sources. Magnetite is ferrimagnetic and has a large susceptibility range, from 0.2 × 10−3 to 1.1 × 10−3 m3 kg−1. In soil, clay minerals have mainly paramagnetic susceptibilities (∼15 × 10−8 m3 kg−1). Water is diamagnetic, with negative magnetic susceptibility (∼−9 × 10−9 m3 kg−1), while the magnetic susceptibility of dried organic matter, which is also diamagnetic, may vary with the iron content. The magnetic susceptibility of the magnNPs was estimated at 0.57 × 10−3 m3 kg−1, within the range of expected values for magnetite. Although the Kappabridge susceptibility meter is able to detect close to 1–2 μg of magnetite, the low quantity of dried plant material (0.1–1 g) and uncertainties in the correction of the magnetic susceptibility of the uncontaminated matrix contributed to a detection level of magnetite estimated at 40 mg kg−1 DW of plant material.Before the analyses, the different plant parts and soil sections collected from the soil column experiments (95 day-old sunflowers, see Section 2.2) were first dried in an oven and stored in clean plastic containers. To account for the containers' signals and to identify any possible deviation occurring during the analyses, blanks (containers without samples) were used at the beginning and end of the acquisitions and after every three measurements. For each sample (including blanks), magnetic susceptibility measurements were repeated 10 times to ensure the validity and reproducibility of the results. Considering the actual weight of the material, mass susceptibilities (m3 kg−1) were then calculated. From these values, concentrations of magnNPs in the samples were determined based on the blank-corrected magnetic susceptibility of a dry sample of magnNPs (of known weight). The sample measurements were also corrected from the mainly diamagnetic signal of the magnNPs' uncontaminated matrix contributing to the final measurements (either control plants or control soils depending on the sample type).
2.6. Statistical analysis
Unless otherwise noted, each value was presented as the mean ± standard error of the mean (SEM), with a minimum of three replicates. Homoscedasticity and normality were confirmed with Bartlett and Shapiro tests for each assay. Taking p < 0.05 as significant, statistical analyses were conducted using the Tukey test (one-way ANOVA) to assess the significance of the means. In figures and tables, data significantly different are indicated with different letters above bars.3. Results and discussion
3.1. Nanoparticle characterization
MagnNP properties were studied prior to their introduction into the soils. According to HR-TEM analyses (ESI Fig. S2†), magnNPs have well crystallized rounded shapes with a mean diameter of 7 nm. From BET measurements, the specific surface area of the magnNPs was ∼115 m2 g−1. From the potentiometric titration measurements, the magnNPs' pHzpc was determined at 6.2. Using the phenanthroline method, the initial Fe(II)/Fe(III) ratio of magnNPs was determined to be 0.45 under anaerobic conditions which dropped to 0.3 after the addition of the suspension to the soil samples under aerobic conditions.3.2. Physiological and biochemical responses of plants exposed to magnNPs in the context of Cu pollution
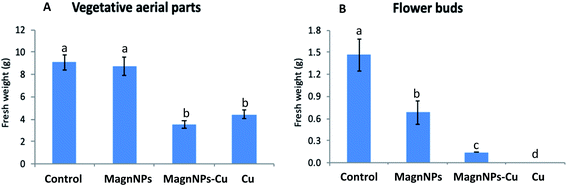
For 57 day-old plants grown in Cu-polluted soils, the mean fresh weight of the vegetative aerial parts (4.4 ± 0.4 g) was significantly decreased (by 55%) compared with the fresh weights of the vegetative aerial parts of the control plants (Fig. 1), and no flower buds were formed. Biomass reduction is a common feature in most plant species exposed to excess Cu.31,49 In fact, Cu toxicity has been reported to reduce the biomass of different plant species, thereby contributing to the retardation of normal growth.50 For sunflower plants grown in magnNP–Cu soils, the mean fresh weight of the vegetative aerial parts (3.6 ± 0.4 g) was also significantly decreased (by 58%) compared with the mean fresh weight of the vegetative aerial parts of the control plants (Fig. 1). This decrease was not significant compared with the mean fresh weights of the vegetative aerial parts of Cu-plants. In addition, although the fresh weights of the roots were not readily quantifiable here (roots were not well separated from the soil and wholly recovered), visual observations provided a sufficient basis to note that root growth was highly impacted by Cu pollution. The inhibitory action of excess Cu on the vegetative growth (aerial parts and roots) of plants likely stemmed from the biochemical impacts induced by Cu, which can also result in reduction in cell division, and toxic effects on photosynthesis, respiration and protein synthesis.51 Although the development of flower buds was severely affected (decrease by 92% of the mean fresh weight), it was not completely inhibited in plants grown in magnNP–Cu soils. Hence, the occurrence of magnNPs could somehow protect flower buds from excess Cu in soils, as suggested by the initiation of flower buds in the 57 day-old magnNP–Cu treated plants.
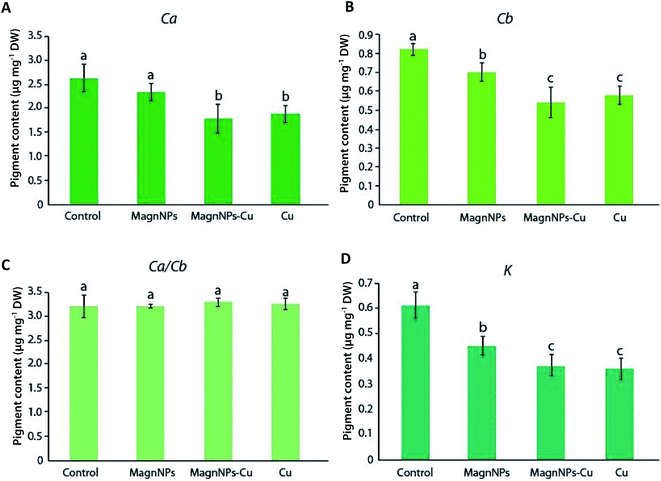
Several studies further showed that IONPs, as well as other NPs (CuO, Ag), can affect the pigment concentrations and the photosynthetic activity of the exposed plants.21,23,53 In the present study, however, no further biological impacts resulting from sunflower exposure to magnNPs alone were directly associated with compositional changes in pigment contents. In particular, the maximum quantum yield of photosynthesis (QY) of 42 day-old plants exposed to magnNPs (QY = 0.831 ± 0.003) was not significantly modified as compared to that of control plants (QY = 0.836 ± 0.002) (Table 1). However, Cu toxicity affected the maximum quantum yield of photosynthesis, which was decreased to a greater extent in Cu plants (QY = 0.770 ± 0.004) than in magnNP–Cu plants (QY = 0.816 ± 0.004) after 42 days (Table 1). This observation suggests that magnNPs reduced Cu phytotoxicity with regard to chloroplast functioning. Lower chlorophyll content, and inactivation of enzymes and other proteins (relating to the photosynthesis process and to the alteration of thylakoid membranes) were reported resulting from Cu toxicity.54,55 In plant chloroplasts, Cu is associated with plastocyanin protein, an essential component of the photosynthetic electron transport chain between cytochrome b6f and photosystem I (PSI).56 Thus, Cu deficiency can reduce PSI electron transport due to a decreased formation of plastocyanin.56 On the other hand, excess Cu has been demonstrated to strongly affect the chloroplast structure with degradation of granum stacks and stroma lamellae.28,31,56 Furthermore, excess Cu usually induces chlorosis and necrosis of leaves, resulting from the inhibition of pigment accumulation and the decrease in chlorophyll integration into photosystems.27,54
| Control | MagnNPs | MagnNPs–Cu | Cu | |
|---|---|---|---|---|
| 34 day-old | 0.829 ± 0.002A | 0.823 ± 0.001A | 0.811 ± 0.006B | 0.757 ± 0.005C |
| 38 day-old | 0.833 ± 0.001A | 0.824 ± 0.002A | 0.812 ± 0.006B | 0.762 ± 0.004C |
| 42 day-old | 0.836 ± 0.002A | 0.831 ± 0.003A | 0.816 ± 0.004B | 0.770 ± 0.004C |
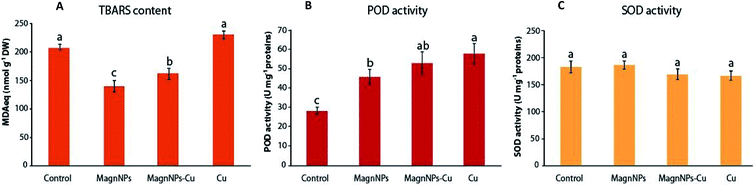
The TBARS content increased from 208 ± 5 MDAeq nmol g−1 DW in plants grown in control soils to 229.4 ± 7.5 MDAeq nmol g−1 DW in Cu-exposed plants. In response to Cu pollution, plant cells likely accumulate ROS, which cause lipid peroxidation of membranes and possibly induce some further metabolic disorders, leading to oxidative stress.29,31 As indicated by increased TBARS content, ROS overproduction was not compensated by SOD and POD enzymatic activities (Fig. 3). In the presence of transition metals such as Fe2+ and Cu2+, H2O2 can break down into damaging OH˙ in the successive Haber–Weiss and Fenton reactions.66 In plants exposed to excess Cu, the conversion of H2O2 into OH leads to lipid peroxidation, perturbation of the mitochondrial and photosynthetic electron transport chains, and substantial damage to DNA and protein expression.67 Despite the excess Cu in the soil, a decreased lipid peroxidation (161.3 ± 9.9 MDAeq nmol g−1 DW) was observed in plants exposed to magnNPs–Cu compared to Cu-exposed plants. Along with the decreased TBARS content, POD was increased (52.7 ± 5.9 U mg−1 protein) – close to that measured in Cu-polluted plants (57.5 ± 5.2 U mg−1 protein) – and it is possible that magnNPs also influenced the activity of other antioxidant enzymes and systems.
3.3. Fate of magnNPs in soil–plant systems: implications for Cu-polluted soils and plants
Uptake of magnNPs by the roots. Based on magnetic susceptibility measurements, magnNPs were detected in the tissues (particularly in the roots) of magnNP- and magnNP–Cu-treated plants, with increasing concentrations over time (Tables 2 and 3, and ESI Tables S2 and S3†). When IONPs reach the soil–root interface, the first step of uptake consists of the adsorption of IONPs onto the root surface, as suggested by SEM-EDS analyses (ESI Fig. S3†). This adsorption is strongly based on the IONP surface charge14 and generally, root surfaces are negatively charged, meaning that positively charged NPs are more likely to adsorb and accumulate on the root surface. Then, at the root cellular level, magnNPs are expected to interact with the plant cell wall before penetration into the cell and subsequent intracellular transportation.68 Indeed, the entry of aggregated magnNPs is restricted to the pore size of the cell walls, which ranges between 5 and 20 nm in diameter12,69 although new pores or wounds can form due to NP deposition.12,57 In fact, several uptake mechanisms are still under consideration to explain NP translocation, such as the symplastic, apoplastic and endocytic pathways, which are considered significant alternatives to allow the passage of magnNPs through plant physiological barriers.14,21,70
| Roots | Stem | Leaves | Flowers | |
|---|---|---|---|---|
| MagnNPs | 1400 ± 310A | <QL | <QL | <QL |
| MagnNPs–Cu | 920 ± 560A | <QL | <QL | <QL |
| MagnNPs | MagnNPs–Cu | ||
|---|---|---|---|
| Aerial parts | AP3 | <QL | <QL |
| AP2 | <QL | <QL | |
| AP1 | 40 ± 10A | <QL | |
| Roots | R1 | 2260 ± 670B | 1610 ± 500BC |
| R2 | 2810 ± 510B | 1090 ± 490C | |
| R3 | 2520 ± 560B | 660 ± 431C | |
| Soil | S1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 ± 200D 060 ± 200D |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 550E 500 ± 550E |
| S2 | 9360 ± 320D | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 ± 410E 680 ± 410E |
|
| S3 | 9970 ± 210D | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 ± 430E 130 ± 430E |
According to the measurements of magnetic susceptibility (Tables 2 and 3), the amount of magnNPs was higher in the roots of magnNP-treated plants than in those of magnNP–Cu plants, highlighting that Cu likely affected the uptake process in root tissues. Direct physiological and biochemical impacts of Cu on plants may be responsible for the observed decreased magnNP uptake. As noted earlier (Section 3.1.1), the root biomass was severely decreased in sunflowers exposed to Cu- and magnNP–Cu-soils. Previous studies showed that under excess Cu, the primary root growth is indeed decreased, along with an increased density of short lateral roots.29,30,71 Therefore, the access of Cu-affected plants to magnNPs in the deeper part of the column was probably reduced. Furthermore, lignin deposition has been reported in Cu-affected plant roots, leading to an impact on the uptake of nutrients and therefore possibly on that of magnNPs in plant roots.71 Lastly, as previously observed in this study (Fig. 3), an increased lipid peroxidation is known to significantly affect the fluidity and permeability of the membranes and consequently the acquisition kinetics of nutrients (and thus also possibly that of magnNPs in this work).21
Translocation of magnNPs to the aerial parts of sunflowers. According to magnetic susceptibility measurements, magnNPs did not accumulate in the upper levels of the plant aerial parts after 57 days of growth (Table 2). However, positive signals evidenced the possible occurrence of a small amount of magnNPs in the close-to-ground section of the aerial parts after 95 days (ESI Table S3†). The amount detected (40 mg kg−1) matched the quantification limit of the method (Table 3). According to previous studies, the access of magnNPs to the aerial parts of the plants could result from a movement into the xylem vessels through the transpiration stream.72 Magnetic susceptibility measurements performed in the aerial parts of magnNP–Cu plants (even in the close-to-ground parts) clearly indicated that no translocation occurred in Cu-contaminated soils. This result can be explained by the physiological impacts induced by the excess Cu on plants, such as decreased root growth – which is consistent with the lower magnNP concentrations observed in the roots of magnNP–Cu plants – and/or by a lower potential of magnNPs to translocate into the vascular system of sunflower plants.
Mobility of magnNPs in the soils. As observed from the magnetic susceptibility measurements performed on soil samples (Table 3), the final magnNP concentration was close to the initial introduced concentration in the soils (10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 is worth 1% of the soil dry weight). The homogeneous distribution of the magnNPs from the bottom to the top of the columns highlights that leaching had few effects on magnNP mobility. According to Table 4, the amount of Fe leached after 37 days only represents 2 mg (i.e., 0.2% of the magnNPs when considering that all the measured Fe originates from the magnNPs). In fact, the aggregation of magnNPs to μm-sized aggregates was evidenced in a previous study35 and such propensity to aggregate may result in decreased magnNP mobility in porous media.73 In addition, most magnNPs were probably bound to clays and organic matter in the soil because of their high affinity for each other.73 In most fine-textured soils, the strong binding of trace elements and nanoscale compounds with clays (and, to a larger extent, soil colloids displaying high specific surface area) has been shown to limit the leaching of these elements.74–76
000 mg kg−1 is worth 1% of the soil dry weight). The homogeneous distribution of the magnNPs from the bottom to the top of the columns highlights that leaching had few effects on magnNP mobility. According to Table 4, the amount of Fe leached after 37 days only represents 2 mg (i.e., 0.2% of the magnNPs when considering that all the measured Fe originates from the magnNPs). In fact, the aggregation of magnNPs to μm-sized aggregates was evidenced in a previous study35 and such propensity to aggregate may result in decreased magnNP mobility in porous media.73 In addition, most magnNPs were probably bound to clays and organic matter in the soil because of their high affinity for each other.73 In most fine-textured soils, the strong binding of trace elements and nanoscale compounds with clays (and, to a larger extent, soil colloids displaying high specific surface area) has been shown to limit the leaching of these elements.74–76
| Total Fe (mg) expected in 120 g soils1 | Total Fe from NPs (mg) in 120 g soilsa | Fe (μg) in released soil solutionb | Fe2+ (μg) in released soil solutionc | Total Cu (mg) in 120 g soilsd | Cu (μg) in released soil solution | |
|---|---|---|---|---|---|---|
| a Theoretical calculations based on the molar mass of Fe and O (and the initial Fe(II)/Fe(III) ratio of magnNPs). b Fe (μg) amount (ionic and colloidal forms) calculated from ICP-MS measurements. c Fe2+ (μg) amount (truly dissolved) calculated from spectroscopic measurements (1,10-phenanthroline method).32 d Theoretical calculation of the initial Cu in the soil (based on the Institut en Santé Agro-Environnement (Combourg, France) analyses and Cu molar mass) and addition of CuSO4. | ||||||
| Control | 3385 | — | 598 ± 78A | 339 ± 37A | 2.3 | 14.4 ± 1.0E |
| MagnNPs | 4254 | 869 | 2093 ± 655B | 623 ± 48B | 2.3 | 16.9 ± 1.5E |
| MagnNPs–Cu | 4254 | 869 | 549 ± 43C | 185 ± 8C | 60 | 1054.5 ± 104.4F |
| Cu | 3385 | — | 496 ± 16D | 269 ± 4D | 60 | 1618.8 ± 81.5G |
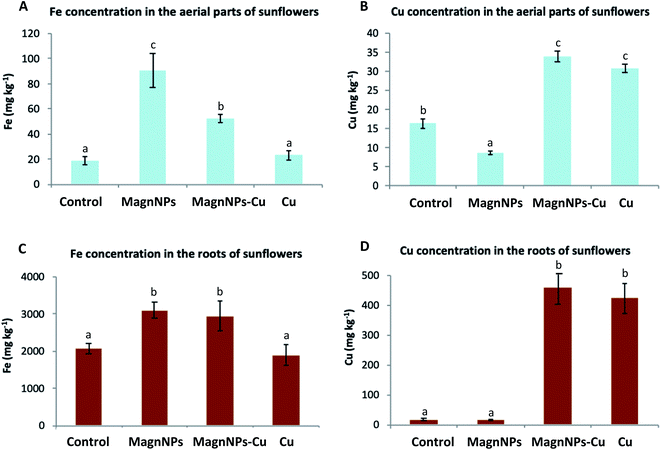
Fe assimilation by sunflowers and the fate of magnNPs. From the ICP-MS analyses, higher Fe contents were measured in magnNP (90.4 ± 9.1 mg kg−1) and magnNP–Cu (52.3 ± 5.8 mg kg−1) plant tissues compared with the Fe content measured in control (18.8 ± 4.1 mg kg−1) and Cu-treated plants (23.3 ± 3.7 mg kg−1) (Fig. 4). This observation suggests that magnNPs provided plants with a bioavailable pool of Fe or somehow enhanced Fe uptake. It also suggests that the presence of Cu limits Fe uptake and/or assimilation by plants. In fact, root exudates (organic acids, sugars, fatty acids, amino acids, and proteins) or microbial activity (i.e., iron-reducing bacteria) may foster the dissolution of magnNPs and Fe release into more bioavailable forms (such as those induced by the action of phytosiderophores).77,78 As observed in Table 4, the amount of Fe released in the magnNP soil solution (2093 ± 655 μg) was higher than that in the soil solutions of the control soil (598 ± 78 μg), Cu soil (496 ± 16 μg) and magnNP–Cu soil (549 ± 43 μg). In addition, more Fe2+ was detected in the magnNP soil solution than in the other soil solutions (Table 4), hence providing a greater source of bioavailable Fe to magnNP-exposed plants. This observation is consistent with the Eh measurements. The range of redox potentials measured in the magnNP soil solutions was higher and reached lower values (from 300 to 650 mV) compared with those measured in the other soil solutions: control (400 to 650 mV), magnNP–Cu (450 to 600 mV) and Cu (450 to 650 mV) soil solutions. These results highlight the role of biotic reduction in driving Fe release, which was a dominant process in magnNP-soil. In Cu and magnNP–Cu soils, it is possible that excess Cu affected the soil microbial activity (i.e., the microbial respiration rate, C mineralization and the ensuing pH and Eh)79 thereby decreasing Fe bioreduction and leaching. In this study, the physiological impacts induced by excess Cu (Section 3.1) could also explain the decreased Fe biotic reduction observed in Cu-contaminated soil systems.
On the other hand, the total iron content measured in the aerial parts of magnNP and magnNP–Cu plants was different (Fig. 4), although similar Fe contents were initially introduced in the magnNP and magnNP–Cu systems. This observation suggests that Cu plays an important role in regulating Fe translocation from the roots to the aerial parts80 both for ionic and nanoparticulate forms. The results obtained by ICP-MS for total Fe are consistent with the magnetic susceptibility measurements (Table 4). These results are also in agreement with the biochemical and physiological impacts induced by Cu on the plants, which may result in a weaker ability to mobilize Fe from the roots to the aerial parts.
Cu dynamics in soil and plants with and without magnNPs: remediation perspectives. With the input of Cu in the soils, the amount of Cu was increased in plants (Fig. 4) and in the leaching solutions (Table 4). Remarkably, the leaching of Cu was decreased by 37% in Cu-contaminated soil enriched with magnNPs (1054.5 ± 104.4 μg) relative to magnNP-free soil (1618.8 ± 81.5 μg). In fact, IONPs display a high affinity for Cu under relevant environmental conditions;31,32 therefore, magnNPs likely scavenge Cu in the soil. As a consequence, magnNPs were expected to decrease Cu bioavailability and/or assimilation by the plants, which did not occur (Fig. 4). Cu adsorbed onto magnNPs was still available for root uptake. On the other hand, because Cu adsorption onto the magnNP surface is even more favoured with increasing Cu concentrations,33 lower leaching of Cu occurred in magnNP–Cu soil relative to magnNP-soil. Eventually, since plants from magnNP–Cu columns were affected by Cu toxicity, it is likely that the concentration of Cu was too high to allow magnNPs to scavenge enough Cu to prevent any excess Cu in the plant tissues and subsequent Cu toxicity.
4. Conclusions
In conclusion, even at the high concentrations used in water/soil remediation, magnNPs are expected to have only a slight impact on the physiological and biochemical responses of sunflowers while enhancing the plant protection system against ROS. In contrast, the contamination of soils with Cu (500 mg kg−1) considerably reduced plant growth, decreased pigment contents and induced oxidative damage to sunflowers. The occurrence of magnNPs in Cu-polluted soils did not prevent other damaging effects induced by excess Cu, despite the positive effects suggested by a decrease in lipid peroxidation. Besides, since the concentration of Cu was comparable in magnNP–Cu and Cu-exposed plants (without magnNPs), it was inferred that magnNPs did not decrease Cu bioavailability and assimilation by sunflowers. A significant increase in Fe in the tissues of plants exposed to magnNPs was observed relative to control plants, while Fe solubilization and assimilation by the plants were reduced in magnNP–Cu- and Cu-polluted soils. A decrease in magnNP uptake in magnNP–Cu plants was also evidenced by magnetic susceptibility measurements. The impacts of Cu on plant physiology and the associated biotic processes in the soil likely affected the magnNP uptake capacity of the plants as well as the regulation of Fe uptake and assimilation. Although magnNPs were not efficient in preventing the phytotoxic effect of high Cu concentrations in soils, magnNPs were able to scavenge Cu and decrease Cu mobility in the soil without hampering Cu bioavailability. These last properties are promising assets for remediation purposes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful to the University of Rennes 1 for enabling the PhD thesis to be conducted. The study was funded by the University Rennes 1 through the ‘Défis Scientifiques Emergents’ program awarded to Dr Aline Dia, and the CNRS EC2CO and the Mission for Cross-cutting and Interdisciplinary Initiatives programs through ‘SURFNANO’ and ‘ALIEN’ projects, respectively, both awarded to Dr Mathieu Pédrot. We also thank the Author Services of Springer Nature for post-editing the English style. The authors are grateful to V. Dorcet for the assistance in TEM experiments performed on THEMIS platform (ScanMAT, UMS 2011 University of Rennes 1-CNRS; CPER-FEDER 2007–2014)References
- Y. Wang and B. Nowack, Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions, Environ. Pollut., 2018, 235, 589–601 CrossRef CAS.
- L. Wang, J. Li, Q. Jiang and L. Zhao, Water-soluble Fe3O4 nanoparticles with high solubility for removal of heavy-metal ions from waste water, Dalton Trans., 2012, 41, 4544–4551 RSC.
- R. Podila and J. M. Brown, Toxicity of engineered nanomaterials: a physic-chemical perspective, J. Biochem. Mol. Toxicol., 2013, 27(1), 50–55 CrossRef CAS.
- W. H. Suh, K. Suslick, G. D. Stucky and Y.-H. Suh, Nanotechnology, nanotoxicology, and neuroscience, Prog. Neurobiol., 2009, 87, 133–170 CrossRef CAS.
- L. R. Khot, S. Sankaran, J. M. Maja, R. Ehsani and E. W. Schuster, Applications of nanomaterials in agricultural production and crop protection: a review, Crop Prot., 2012, 35, 64–70 CrossRef CAS.
- M. Kah, S. Beulke, K. Tiede and T. Hofmann, Nanopesticides: state of knowledge, environmental fate, and exposure modeling, Crit. Rev. Environ. Sci. Technol., 2013, 43(16), 1823–1867 CrossRef CAS.
- P. Solanki, A. Bhargava, H. Chhipa, N. Jain and J. Panwar, in Nanotechnologies in Food and Agriculture, ed. M. Rai, C. Ribeiro, L. Mattoso and N. Duran, Springer International, 2015, ch. 4, pp. 81–101 Search PubMed.
- G. Batley, J. K. Kirbyand and M. J. McLaughlin, Fate and Risks of nanomaterials in aquatic and terrestrial environments, Acc. Chem. Res., 2013, 46(3), 854–862 CrossRef CAS.
- M. A. Ahmed, S. M. Ali, S. I. El-Dek and A. Galal, Magnetite-hematite nanoparticles prepared by green methods for heavy metal ions removal from water, J. Mater. Sci. Eng. B, 2013, 178, 744–751 CrossRef CAS.
- P. N. Dave and L. V. Chopda, Application of iron oxide nanoparticles for the removal of heavy metals, J. Nanotechnol., 2014, 2014, 398569 Search PubMed.
- L. Goswani, K.-H. Kim, A. Deep, P. Das, S. S. Bhattacharya, S. Kumar and A. A. Adelodun, Engineered nanoparticles: nature, behavior and effects on the environment, J. Environ. Manage., 2017, 196, 297–315 CrossRef.
- E. Navarro, A. Baun, R. Behra, N. B. Hartman, J. Filser, A.-J. Miao, A. Qigg, P. H. Santschi and L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants and fungi, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS.
- V. K. Sharma, J. Filip, J. Zboril and R. S. Varma, Natural inorganic nanoparticles – formation, fate and toxicity in the environment, Chem. Soc. Rev., 2015, 44(23), 8410–8423 RSC.
- J. Lv, P. Christie and S. Zhang, Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges, Environ. Sci.: Nano, 2019, 6, 41–59 RSC.
- T. Hiemstra and W. H. Van Riemsdijk, On the relationship between charge distribution, surface hydration and the structure of the interface of metal hydroxides, J. Colloid Interface Sci., 2006, 301(1), 1–18 CrossRef CAS.
- S. H. Joo and D. Zhao, Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: a review, J. Hazard. Mater., 2017, 322, 29–47 CrossRef CAS.
- G. R. Aïken, H. Hsu-Kim and J. N. Ryan, Influence of dissolved organic matter on the environmental fate of metals, nanoparticles and colloids, Environ. Sci. Technol., 2011, 45, 3196–3201 CrossRef.
- H. Zhu, J. Han, J. Q. Xiao and Y. Jin, Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants, J. Environ. Monit., 2008, 10, 713–717 RSC.
- J. Li, P. R. Chang, J. Huang, Y. Wang, H. Yuan and H. Ren, Physiological effects of magnetic iron oxide nanoparticles towards watermelon, J. Nanosci. Nanotechnol., 2013, 13, 5516–5567 Search PubMed.
- J. Li, J. Hu, C. Ma, Y. Wang, C. Wu, J. Huang and B. Xing, Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe3O4) nanoparticles in corn (Zea mays L.), Chemosphere, 2016, 159, 326–334 CrossRef CAS.
- D. K. Tripathi, Shweta, S. Singh, S. Singh, R. Pandey, V. P. Singh, N. C. Sharma, S. M. Prasad, N. K. Dubey and D. K. Chauhan, An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity, Plant Physiol. Biochem., 2017, 110, 2–12 CrossRef CAS.
- R. Barrena, E. Casals, J. Colon, X. Font, A. Sanchez and V. Puntes, Evaluation of the ecotoxicity of model nanoparticles, Chemosphere, 2009, 75, 850–857 CrossRef CAS.
- F. Perreault, M. Samadaniand and D. Dewez, Effect of soluble copper released from copper oxide nanoparticles solubilization on growth and photosynthetic processes of Lemnagibba L, Nanotoxicology, 2013, 8(4), 374–382 CrossRef.
- A. Rastogi, M. Zivcak, O. Sytar, H. M. Kalaji, X. He, S. Mbarki and M. Brestic, Impact of metal and metal oxide nanoparticles on plant: a critical review, Front. Chem., 2017, 5, 78–94 CrossRef.
- M. Tatari, R. F. Ghazvini, N. Etemadi, A. M. Ahadi and A. Mousavi, Analysis of Antioxidant Enzymes Activity, Lipid Peroxidation and Proline Content of Agropyron desertorum Under Drought Stress, South Western Journal of Horticulture, Biology and Environment, 2012, 3(1), 9–24 Search PubMed.
- C. Huth, L. M. Mertz-Henning, S. J. Lopes, L. A. Tabaldi, L. V. Rossato, F. C. Krzyzanowski and F. A. Henning, Susceptibility to weathering damage and oxidative stress on soybean seeds with different lignin contents in the seed coat, J. Seed Sci., 2016, 38(4), 296–304 CrossRef.
- M. Adrees, S. Ali, A. Rizwan, M. Ibrahim, F. Abbas, M. Farid, M. Zia-ur-Rehman, M. Kashif Irshad and S. Aslam Bharwana, The effect of excess copper on growth and physiology of important food crops: a review, Environ. Sci. Pollut. Res., 2015, 22, 8148–8162 CrossRef CAS.
- A. Michaud, M. Bravin, M. Galleguillos and P. Hinsinger, Copper uptake and phytotoxicity as assessed in situ for durum wheat (Triticumturgidum, durum L.) cultivated in Cu-contaminated, former vineyard soils, Plant Soil, 2007, 298(1–2), 99–111 CrossRef CAS.
- E. Besnard, C. Chenu and M. Robert, Influence of organic amendments on copper distribution among particle-size and density fractions in Champagne vineyard soils. Environmental Pollution 9 (2001). Copper in plants, Braz. J. Plant Physiol., 2005, 17(1), 145–156 CrossRef.
- K. A. Mackie, T. Müller and E. Kandeler, Remediation of copper in vineyards – a mini review, Environ. Pollut., 2012, 167, 16–26 CrossRef CAS.
- I. Yruela, Copper in Plants, Braz. J. Plant Physiol., 2005, 17(1), 145–156 CrossRef CAS.
- J.-F. Liu, Z.-S. Zhao and G.-B. Jiang, Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High efficient Removal of Heavy Metals in water, Environ. Sci. Technol., 2008, 42, 6949–6954 CrossRef CAS.
- E. Demangeat, M. Pédrot, A. Dia, M. Bouhnik-Le-Coz, M. Davranche and F. Cabello-Hurtado, Surface modifications at the oxide/water interface: implications for Cu binding, solution chemistry and stability of iron oxide nanoparticles, Environ. Pollut., 2020, 257, 113626 CrossRef CAS.
- B. Rutkowska, W. Szulc and K. Bomze, Effects of soil properties on copper speciation in soil solution, J. Elem., 2013, 18, 695–703 Search PubMed.
- E. Demangeat, M. Pédrot, A. Dia, M. Bouhnik-Le-Coz, F. Grasset, K. Hanna, M. Kamagate and F. Cabello-Hurtado, Colloidal and chemical stabilities of iron oxide nanoparticles in aqueous solutions: the interplay of structural, chemical and environmental drivers, Environ. Sci.: Nano, 2018, 5, 992–1001 RSC.
- M. Komárek, A. Vaněk and V. Ettler, Chemical stabilization of metals and arsenic in contaminated soils using oxides – a review, Environ. Pollut., 2013, 172, 9–22 CrossRef.
- J. Lin, W. Jiang and D. Liu, Accumulation of copper by roots, hypocotyls, cotyledons and leaves of sunflower (Helianthus annuus L.), Bioresour. Technol., 2003, 86, 151–155 CrossRef CAS.
- P. Soudek, S. Petrova, D. Benesova and T. Vanek, Phytoextraction of toxic metals by sunflower and corn plants, J. Food, Agric. Environ., 2010, 8(3 and 4), 383–390 CAS.
- D. R. Hoagland and D. I. Arnon, The water-culture method for growing plants without soil, Circ. - Calif. Agric. Exp. Stn., 1950, 347, 1–31 Search PubMed.
- H. K. Lichtenthaler and A. R. Wellburn, Determination of total carotenoids and chlorophyll a and b of leaf extract in different solvents, Biochem. Soc. Trans., 1983, 11, 591–592 CrossRef CAS.
- K. Rohacek and M. Bartak, Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications, Photosynthetica, 1999, 37, 339–363 CrossRef CAS.
- D. M. Hodges, J. M. DeLong, C. F. Forney and R. K. Prange, Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds, Planta, 1999, 207, 604–611 CrossRef CAS.
- M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- J. Putter, in Methods of Enzymatic Analysis, ed. H. U. Bergmeyer, Verlag Chemie, Weinham, 2nd edn, 1974, vol. 2, pp. 685–690 Search PubMed.
- I. Cakmak and H. Marschner, Magnesium deficiency and high light intensity enhance activities of super oxide dismutase, ascorbate peroxidase and gluthatione reductase in bean leaves, Plant Physiol., 1992, 98, 1222–1227 CrossRef CAS.
- C. N. Giannopolis and S. K. Ries, Superoxide Dismutases: I. Occurrence in higher plants, Plant Physiol., 1977, 59(2), 309–314 CrossRef.
- D. Yéghicheyan, C. Bossy, M. Bouhnik Le Coz, C. Douchet, G. Granier, A. Heimburger, F. Lacan, A. Lanzanova, T. C. C. Rousseau, J. L. Seidel, M. Tharaud, F. Candaudap, J. Chmeleff, C. Cloquet, S. Delpoux, M. Labatut, R. Losno, C. Pradoux, Y. Sivry and J. E. Sonke, A compilation of silicon, rare earth element and twenty-one other trace element concentrations in the natural river water reference material slrs-5 (nrc-cnrc), Geostand. Geoanal. Res., 2013, 37(4), 449 CrossRef.
- D. P. Maxbauer, J. M. Feinberg, D. L. Fox and E. A. Nater, Response of pedogenic magnetite to changing vegetation in soils developed under uniform climate, topography, and parent material, Sci. Rep., 2017, 7, 17575–17785 CrossRef.
- J. Lin, W. Jiang and D. Liu, Accumulation of copper by roots, hypocotyls cotyledons and leaves of sunflower (Helianthus annuus L.), Bioresour. Technol., 2003, 86, 151–155 CrossRef CAS.
- R. Manivasagaperumal, P. Vijayarengengan, S. Balamurugan and G. Thiyagarajan, Effect of copper on growth, dry matter yield and nutrient content of Vigna Radiata (L.), Wilczek. J. Phytol., 2011, 3(3), 53–62 CAS.
- R. Esteban, O. Barruia, U. Artetxe, B. Fernandez-Marin, A. Hernandez and J. I. Garcia-Plazaola, Internal and external factors affecting photosynthetic pigment composition in plants: a meta-analytical approach, New Phytol., 2015, 206, 268–280 CrossRef CAS.
- P. K. Yudina, L. A. Ivanova, D. A. Rhondina, N. V. Zolotareva and L. A. Ivanov, Variation of leaf traits and pigment content in three species of steppe plants depending on climate aridity, Russ. J. Plant Physiol., 2017, 64(3), 410–422 CrossRef CAS.
- H. Qian, X. Peng, X. Han, J. Ren, L. Sun and Z. Fu, Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana, J. Environ. Sci., 2013, 25, 1947–1956 CrossRef CAS.
- S. Dey, P. B. Mazumder and S. B. Paul, Effect of copper on growth and chlorophyll content in tea plants (Camellia sinensis (L.) O. Kuntze), International Journal of Research in Applied, Natural and Social Sciences, 2014, 2(5), 223–230 Search PubMed.
- G. Ouzounidou, Copper induced changes on growth, metal content and photosynthetic function of Alyssum montanum L. plants, Environ. Exp. Bot., 1994, 34(2), 165–172 CrossRef CAS.
- T. Baszynsk, A. Tukendorf, M. Ruszkowska, E. Skorzynska and W. Maksymiec, Characteristics of the photosynthetic apparatus of copper non tolerant spinach exposed to excess copper, J. Plant Physiol., 1988, 132, 708–713 CrossRef.
- Z. Siddique, K. P. Akhtar, A. Hameed, N. Sarwar, I. U. Haq and S. A. Khan, Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus, J. Plant Interact., 2014, 9(1), 702–711 CrossRef.
- M. F. Iannone, M. D. Groppa, M. E. de Sousa, M. Beatriz Fernandez van Raapand and M. P. Benavides, Impact of Magnetite Iron Oxide nanoparticles on wheat (Triticum aestivum L.) development: evaluation of oxidative damage, Environ. Exp. Bot., 2016, 131, 77–88 CrossRef CAS.
- M. G. M. Palmqvist, G. A. Seisenbaeva, P. Svedlindh and V. G. Kessler, Maghemite Nanoparticles Act as Nanoenzymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus, Nanoscale Res. Lett., 2017, 12, 631–640 CrossRef.
- A. Praveen, E. Khan, S. Ngiimei D, M. Perwez, M. Sadar and M. Gupta, Iron Oxide Nanoparticles as Nano-adsorbents: A Possible Way to Reduce Arsenic Phytotoxicity in Indian Mustard Plant (Brassica juncea L.), J. Plant Growth Regul., 2018, 37(2), 612–624 CrossRef CAS.
- T. Abedi and H. Pakniyat, Antioxidant Enzyme Changes in Response to Drought Stress in Ten Cultivars of Oilseed Rape (Brassica napus L.), Czech J. Genet. Plant Breed., 2010, 46(1), 27–34 CrossRef CAS.
- R. G. Alscher, N. Erturk and L. S. Heath, Role of superoxide dismutases (SODs) in controlling oxidative stress, J. Exp. Bot., 2002, 5, 1331–1341 CrossRef.
- S. Gao, C. Ouyang, S. Wang, Y. Xu, L. Tang and F. Chen, Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-liase activities in Jatropha curcas L. seedlings, Plant, Soil Environ., 2008, 54(9), 374–381 CrossRef CAS.
- J. Patykowski and J. Kolodziejek, Changes in Antioxidant Enzyme Activities of European Mistletoe (Viscum album L. subsp. Album) Leaves as a Response to Environmental Stress Caused by Pollution of the Atmosphere by Nitrogen Dioxide, Pol. J. Environ. Stud., 2016, 25(2), 725–732 CrossRef CAS.
- N. Liu, Z. Lin, L. Guan, G. Gaughan and G. Lin, Antioxidant Enzymes Regulate Reactive Oxygen Species during Pod Elongation in Pisum sativum and Brassica chinensis, PLoS One, 2014, 9(2), 1–9 Search PubMed.
- E. Birben, U. M. Sahiner, C. Sackesen, S. Erzurum and O. Kalayci, Oxidative Stress and Antioxidant Defense, World Allergy Organ. J., 2012, 5(1), 9–19 CrossRef CAS.
- W. Wu, X. Wan, F. Shah, S. Fahad and J. Huang, The Role of Antioxidant Enzymes in Adaptive Responses to Sheath Blight Infestation under Different Fertilization Rates and Hill Densities, Sci. World J., 2014, 2014, 502134 Search PubMed.
- N. A. Anjum, M. A. Merios Rodrigo, A. Moulick, Z. Heger, P. Kopel, O. Zitka, V. Adam, A. S. Lukatkin, A. C. Duarte, E. Pereira and R. Kizek, Transport phenomena of nanoparticles in plants and animals/humans, Environ. Res., 2016, 151, 233–243 CrossRef CAS.
- M. Amde, J.-F. Liu, Z.-Q. Tan and D. Bekana, Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review, Environ. Pollut., 2017, 230, 250–267 CrossRef CAS.
- P. Miralles, T. L. Church and A. T. Harris, Toxicity, Uptake, and Translocation of Engineered Nanomaterials in Vascular plants, Environ. Sci. Technol., 2012, 46, 9224–9239 CrossRef CAS.
- H. Lequeux, C. Hermans, S. Lutts and N. Verbruggen, Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignine accumulation and mineral profile, Plant Physiol. Biochem., 2010, 48, 673–682 CrossRef CAS.
- Z. Cifuentes, L. Custardoy, J. M. de la fuente, C. Marquina, M. R. Ibarra, D. Rubiales and A. Peres-de-Luque, Absorption and translocation to the aerial part of magnetic carbon–coated nanoparticles through the root of different crop plants, J. Nanobiotechnol., 2010, 8, 8–26 CrossRef.
- M. Al-Sid-Cheikh, M. Pédrot, A. Dia, M. Davranche, L. Jeanneau, P. Petitjean, M. Bouhnik-Le Coz, M.-A. Cormier and F. Grasset, Trace element and organic matter mobility impacted by Fe3O4-nanoparticle surface coating within wetland soil, Environ. Sci.: Nano, 2019, 6, 3049–3059 RSC.
- B. K. G. Theng and G. Yuan, Nanoparticles in the soil environment, Elements, 2008, 4, 395–399 CrossRef CAS.
- F. Reith and G. Cornelis, Effect of soil properties on gold- and platinum nanoparticle mobility, Chem. Geol., 2017, 466, 446–453 CrossRef CAS.
- O. Sagee, I. H. Dror and B. Berkowitz, Transport of silver nanoparticles (AgNPs) in soil, Chemosphere, 2012, 88, 670–675 CrossRef CAS.
- S. Bastani, R. Hajiboland, M. Khatamian and M. Saket-Oskoui, Nano iron (Fe) is an effective source of Fe for Tobacco plants grown under low Fe supply, J. Soil Sci. Plant Nutr., 2018, 18(2), 524–541 CAS.
- J. Yuan, Y. Chen, H. Li, J. Lu, H. Zhao, M. Liu, G. S. Nechitaylo and N. N. Guschchenko, New insights into the cellular responses to iron nanoparticles in Capsicum annuum, Sci. Rep., 2018, 8(1), 3228 CrossRef.
- K. M. Keiblinger, M. Schneider, M. Gorfer, M. Paumann, E. Deltedesco, H. Berger, L. Jöchlinger, A. Mentler, S. Zechmeister-Boltenstern, G. Soja and F. Zehetner, Assessment of Cu application in two contrasting soils-effects on soil microbial activity and the fungal activity structure, Ecotoxicology, 2018, 27, 217–233 CrossRef CAS.
- P. Karimi, R. A. Khavari-Nejad, V. Niknam, F. Ghahremaninejad and F. Najafi, The effects of excess copper on antioxydative enzymes, lipid peroxidation, proline, chlorophyll, and concentration of Mn, Fe and Cu in Astragalus neo-mobayenii, Sci. World J., 2012, 2012, 615670 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00825g |
| This journal is © The Royal Society of Chemistry 2021 |