Open Access Article
Open Access ArticleChemically modified carbon nanostructures and 2D nanomaterials for fabrics performing under operational tension and extreme environmental conditions
Ioanna K.
Sideri
 * and
Nikos
Tagmatarchis
* and
Nikos
Tagmatarchis
 *
*
Theoretical and Physical Chemistry, Institute National Hellenic Research Foundation, 48 Vassileos Constantinou Avenue, 11635 Athens, Greece. E-mail: tagmatar@eie.gr; isideri@eie.gr
First published on 11th October 2021
Abstract
The extensive research on carbon nanostructures and 2D nanomaterials will come to fruition once these materials steadily join everyday-life applications. Their chemical functionalization unlocks their potential as carriers of customized properties and counterparts to fabric fibers. The scope of the current review covers the chemical modification of carbon nanostructures and 2D nanomaterials for hybrid fabrics with enhanced qualities against critical operational and weather conditions, such as antibacterial, flame retardant, UV resistant, water repellent and high air and water vapor permeability activities.
1. Introduction
Textiles for clothing, an inextricable part of human activity, have been technologically developed tremendously in the last decades in line with the ever-growing demand and increasing living standards. Specifically, the climate crisis has triggered extreme weather conditions, health crises and technological breakthrough, which have forced the clothing industry to seek help from the research community aiming to develop novel garments with versatile applications. In addition to this, the greater challenge is that these garments are supposed not only to meet the operational requirements, but also to provide practical comfort to users. In this context, some of the most important qualities required for textiles performing under operational tension and extreme environmental conditions are the antibacterial properties,1 flame retardant activity,2 high air and water–vapor permeability that ensure breathability,3 water repellency,4 UV protection5 and resistance to wear.6Graphene and its extended family of carbon nanomaterials have emerged as ground-breaking materials with marvelous characteristics that allow them to play a leading role in multiple applications. To name a few, graphene and its related nanocarbons are characterized by high thermal and electrical conductivity owing to their vast sp2 lattice, chemical and mechanical stability, increased Young's modulus and extraordinary electronic properties.7 However, probably the biggest asset is their functionalization capability that accomplishes the tuning of their intrinsic properties as desired and also the acquisition of novel qualities, as well as the ability to hybridize with other materials, thereby enabling their extended applicability.8 Similarly, 2D nanomaterials such as transition metal dichalcogenides (TMDs), MXenes and hexagonal boron nitride (h-BN) constitute pioneer nanomaterials in materials chemistry nowadays, since their emergence a few years ago.9 MXenes are characterized by good electrical conductivity, large surface area and excellent shielding capability and thus have conquered the top among lightweight shielding materials against electromagnetic interference (EMI).10 Mxene coatings can be woven into clothing and other accessories and offer the wearable unique shielding ability and protection from strong microwave radiation.11 As far as 2D TMDs are concerned, the enormous research effort devoted to graphene and carbon nanotubes (CNTs) has paved the way for the handling, treatment and functionalization of these different but also akin nanomaterials. 2D TMDs are generally characterized by mechanical strength, a direct band-gap, astonishing electronic properties and a scarce atomic-scale thickness.12 Molybdenum disulfide (MoS2) is the representative and most-studied of this family of materials owing to its robustness. The chemical modification of MoS2 arose as a direct consequence of graphene functionalization research studies and has allowed its multipurpose exploitation.
Not long after the dominance of nanocarbons and 2D nanomaterials in a series of catalytic and energy-related applications, their hybridization with fabric fibers arrived as a logical outcome towards the realization of multipurpose textiles. Chemical functionalization enables the grafting of carrier agents of the desired properties on the 2D nanomaterial substrates, which are consequently integrated with the textile yarn to produce hybridized versatile fabrics.
While excellent reviews exist regarding smart textiles in general,13 and more specific ones focusing on graphene-based fabrics,14 CNT-based wearable devices and smart textiles15 and MXenes-based coatings for EMI shielding,16 there is a lack of review literature regarding the chemical modification (covalent functionalization or surface physisorption) of carbon nanostructures and 2D nanomaterials as counterparts of fabric fibers towards enhanced operational qualities. In this critical review, we aim to fill this gap and summarize the literature reports covering the chemical functionalization of carbon nanostructured materials and 2D TMDs mainly for fabrics with enhanced antibacterial, flame retardant, air and water permeability, water repellent, UV-resistant and durability properties (Fig. 1). Alongside, a chemistry viewpoint appealing to chemists and materials scientists and extremely useful to industry is provided.
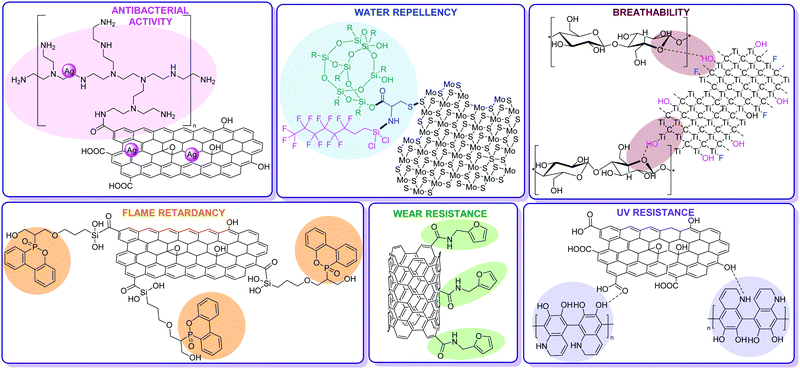 | ||
| Fig. 1 Chemical functionalization of carbon nanostructures and 2D nanomaterials as counterparts of hybridized fabric fibers with enhanced antibacterial,45 flame retardant,63 breathability,79 water repellent,88 UV-resistant96 and durability properties.108 | ||
2. Strategies employed for the hybridization of carbon nanostructures and 2D TMDs with fabric fibers
As the textile industry expands to satisfy the growing demand, so do the different strategies for the modification of the fabric fibers. Initially, these were limited to the traditional dyeing and drying techniques, but the increased research interest devoted to the design and fabrication of multipurpose textiles has come to fruition with novel and efficient strategies for the hybridization of textiles with carbon nanostructures and 2D TMDs. Naturally, solid differences exist between these two families of materials as far as their reactivity is concerned and therefore their hybridization capability with other materials, herein the fabric fibers. Carbon nanostructures possess C atoms that are easily oxidized to O-containing functionalities, which are excellent reactive moieties, enabling various interactions. Furthermore, their sp2 lattice is readily available for the occurrence of common organic reactions on the aromatic C atoms. On the other hand, in the case of 2D TMDs which possess metals and chalcogens such as S, the scene is quite different. Although great research effort has been made towards the chemical derivatization of chalcogens mainly, 2D TMDs still lag behind the carbon nanostructured materials reactivity-wise. As expected, this occurrence mirrors also on the resulting properties of the final composite fabric, since the more reactive the raw material, the easier the acquisition of the desired properties. In any case, the methodologies developed for the hybridization with fabric fibers are divided into two main categories: surface coating techniques and chemical modification via covalent grafting.2.1 Surface coating techniques
Dip-coating constitutes the most common technique employed for the modification of fabrics and is also widely used in industry. The methodology involves the immersion of a textile cloth in a solution containing the coating material and then the removal of the cloth from the tank to drain, followed by a washing and drying process, which can be done either by heating or by employing mechanical force. The significant advantages of this technique are by far the reproducibility, high adhesion and large-scale application. Furthermore, the repetitive dip-coating cycles allow increased loading with analogous effects to the desirable performance.17 Graphene oxide (GO) when diluted in aqueous media is negatively charged owing to its oxygen-containing groups. On the other hand, silk fabric gets positively charged in aqueous acidic solutions due to its abundant amino groups. Therefore, dipping a silk fabric in an acidic solution of GO results in effective coating secured by electrostatic interactions between the oxygen functionalities of GO and the amino-groups of the silk fabric.17 The hybridization of cotton fabric with GO is also achieved via hydrogen bonding18 and further in situ reduction to reduced-GO (RGO) can take place during the dip–dry process.17–19 However, cotton, nylon and polyester fabrics that do not bear amine groups cannot be inherently positively charged and thus strong electrostatic adhesion of GO is prevented, thus the adhesion is governed by weaker yet sufficiently strong hydrogen bonding between the –O/–OH functionalities of GO and the –OH/–O functionalities of cellulose. This setback though is easily overcome with the engagement of pH-sensitive adhesive molecules that are adsorbed by the fabric during dipping and offer positive charges to the fabric, enabling electrostatic interactions with GO and therefore stronger assembly.20,21Padding or pad-dry-cure methodology can perhaps be considered as the industrial approach to dip-coating in the sense that a padding machine equipped with squeezing rollers applies mechanical pressure to the solution-treated textile which is then dried at an elevated temperature. This technique can be applied in both laboratory and industry and is mainly used for the modification of fabrics with carbon nanomaterials functionalized with polymers. Specifically, GO is functionalized with cross-linking polymers, through the sol–gel method and the sol prepared is next applied to the cotton textile via padding and drying cycles utilizing a laboratory double-roll padding machine.22
Layer by layer assembly is another versatile surface coating technique that achieves the fabrication of ultrathin nanocomposite films on a given surface and was primarily developed by Decher in 1997.23 This methodology is based on alternating consecutive adsorption of oppositely charged materials with a controlled morphology and structure. Layer by layer assembly is considered simple and environmentally friendly and is largely used for the fabrication of functionalized GO and CNTs on fabric fibers by taking advantage of the charge properties of carboxylic or amino functionalities of the functionalized carbon nanomaterials in an acidic solution, before the dipping and stirring of the fabric in the prepared media repetitively.24,25
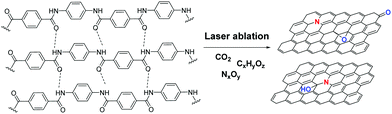
In 2009, dye-printing was utilized for the fabrication of a CNT-modified polyester fabric. In this rather uncommon technique until then, the polyester in the form of yarn was passed through a “dye-bath” that contained aggregated CNTs blended with zwitterionic and anionic surfactants.26 Microwave irradiation ensured the successful dyeing process, followed by heating in a curing oven at 170 °C. Recently, simple dyeing of GO on cotton was achieved in a revolutionary report that claimed more uniform arrangement of GO on cotton compared to the traditional dip-drying methodologies, because the GO's free enthalpy in water is lower, thus allowing GO sheets to align regularly on the cotton surface.27 In a more modern approach, direct writing of laser-induced graphene was realized on a Kevlar textile using a CO2 laser beam. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N bonds in the molecular structure of Kevlar were broken and the remaining carbon atoms were (possibly photo-thermally) re-arranged on the graphene core (Fig. 2).28
O and C–N bonds in the molecular structure of Kevlar were broken and the remaining carbon atoms were (possibly photo-thermally) re-arranged on the graphene core (Fig. 2).28
For the fabrication of MoS2-functionalized polyacrylonitrile (PAN) fabric fibers, the wet-spinning process has been employed. During this procedure, firstly the PAN powder is diluted in a suitable water-miscible solvent and then the MoS2 material dispersed in the same solvent is added to the mixture. The generated uniform wet-spinning solution is finally deaerated and extruded in the form of a fiber in an aqueous solution via a syringe equipped spinning pump.29,30 Similar to the carbon nanostructured materials, the multiple-cycles dip-coating methodology has been utilized for the 2D TMDs as well.31 In certain cases, in order to secure successful coating, adhesive resins may be uniformly blended in the 2D TMD dipping solution.32 However, the simplest coating technique was recently reported for the modification of a pristine polycotton fabric with MoS2 nanosheets.33 In particular, a negative pressure vacuum filtration of MoS2 solutions at various concentrations was performed through a suction filter covered with the fabric sample. The respective MoS2 nanosheet solution was passed through both sides of the fabric multiple times to ensure successful immobilization and finally the MoS2-treated fabric was dried at 60 °C. Finally, drop-casting was also performed for the coating of cotton textile with WS2 quantum dots.34
2.2 Surface chemical functionalization
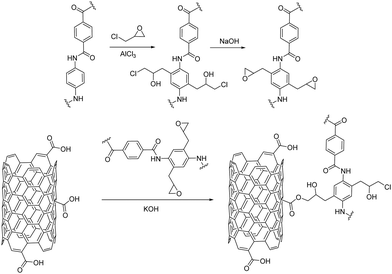
Chemical functionalization of carbon nanostructures and 2D nanomaterials is the key for composite fabrication that unlocks their involvement in versatile applications. The textile industry is one of these applications, where the nanomaterials’ chemical reactivity is exploited for their covalent grafting to the fabric fibers. Compared to the physisorption and coating techniques already described, direct chemical functionalization ensures strong and secure covalent bonding, therefore maximum stability and durability to the hybrid fabric. In other words, fabrics coated via the drop-casting, dip-coating, pad-dry cure methodologies etc., are prone to lose their coating under certain common conditions, for example, when washed with water or other solvents or under strain and over time. Naturally, with regards to fabrics and clothing, this is a serious disadvantage. On the other hand, the coating techniques provide uniformity, allow easy handling and most importantly are scalable and therefore industrially applied. On the contrary, covalent grafting results in strong chemical bonds between the fabric fibers and the respective material and, therefore, durable and washing-resistant fabrics are produced. However, challenges exist in chemical synthesis and more research efforts have to be devoted for its compliance to large-scale industrial fabrication. Nonetheless, great research advancements have been accomplished in the last few years in this domain.In 2009, Kevlar coated CNTs were prepared when Kevlar was added to the acid-assisted purification procedure of CNTs.35 One year later, Sainsbury et al. formed a poly-phenyleneterephthalamide (PPTA) sheath around the CNTs via the covalent attachment of the monomer units of a Kevlar textile, p-phenylenediamine (PDA) and terephthaloyl chloride (TPC) to the nanotube core.36 The acid chlorides on the surface of CNTs, that derive from the corresponding carboxylic acid functionalities, act as linkage groups for the binding of the Kevlar monomers via amide formation through a condensation reaction. Furthermore, hard-core organic chemistry has been applied for the modification of Kevlar fibers. For example, Friedel–Crafts alkylation on the benzene rings of PPTA utilizing aluminum chloride as the catalyst and alkyl chloride as the electrophile has been recently performed, followed by an epoxidation reaction. Then, the epoxide ring-opening reaction via the carboxylate anions of carboxylic acid-modified MWCNTs allows successful covalent immobilization of MWCNTs on Kevlar fibers (Fig. 3).37
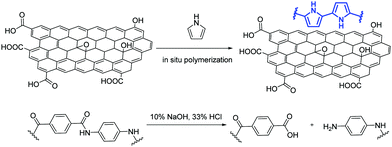
Kevlar fibers were later functionalized with GO and polypyrrole via in situ polymerization and the functionalized Kevlar fibers were then utilized as the substrate for a super-fast, microwave-assisted growth of Fe-CNTs on the fibers’ surface.38 The amidic bonds of the PPTA fibers are firstly hydrolyzed to produce abundant –COOH and –NH2 groups, that facilitate the interaction between polypyrrole and the moieties of GO (Fig. 4). Then, mixing of the functionalized fibers with ferrocene under microwave irradiation enables the growth of Fe-CNTs.38
The same research group later reported NiCo2S4 growth on woven Kevlar fibers functionalized with GO/polyester composites, utilizing the same technique.39
Cross-linking agents can be utilized as bridges for the hybridization, as in the case of CNTs and cellulose fibers, where 1,2,3,4-butanetetracarboxylic acid (BTCA) facilitated their merge.40 Recently, a novel self-cross-linking dispersant with multiple silanol groups was found to be extremely efficient for the covalent linkage of GO and MWCNTs with cellulose fibers (Fig. 5).41
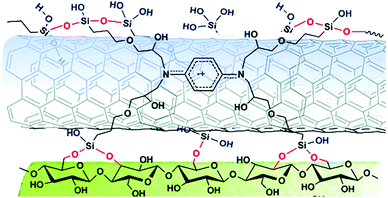 | ||
| Fig. 5 A novel self-cross-linking dispersant as linkage between CNTs and cellulose fibers. Reproduced in part with permission from ref. 41. Copyright © The Royal Society of Chemistry 2018. | ||
Frequently, cellulose fibers are also stabilized on GO via hydrogen bonding interactions between the oxygen functionalities of GO and the –OH groups of cellulose and Ca2+ ions were found to further secure this interaction via the formation of ionic bonds (Fig. 6).42
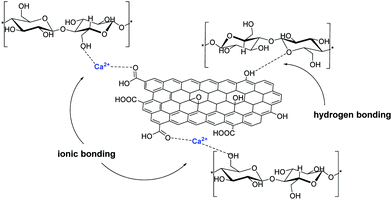 | ||
| Fig. 6 Ca2+ metal ion infiltration increases the bonding interaction between the building blocks of GO and cellulose microfibers in the hybrid material via ionic bonding. | ||
3. Chemical modification towards operational limitations and extreme environmental conditions
3.1 Antibacterial properties
Clothing and textiles, especially those made of natural fibers, can often act as friendly substrates for hosting pathogenic microorganisms. Since these are an actual threat to public global health, antifungal, antimicrobial and antibacterial activity is nowadays a mandatory characteristic for fabrics and textiles deployed for clothing and personal protective equipment. Traditionally, metal and metal oxide nanoparticles (NPs) have been utilized to transfuse antibacterial properties to fabrics.1Graphene oxide is an ideal substrate for the growth of metal NPs, since its oxygen functionalities stabilize the NPs and prevent their aggregation. The antibacterial performance of a graphene/TiO2 composite grafted on a cotton fabric was tested in 2014 by researchers in a quest of a revolutionary electroconductive, self-cleaning and antimicrobial fabric.43 Coating of a GO film on a cotton fabric was achieved by immersion of the latter into an aqueous solution of GO and it has actually been proved that the hydroxyl and carboxylic acid groups of graphene oxide form hydrogen bonds with the hydroxyl groups of cellulose in the fabric.44 In this endeavor, aqueous TiCl3 acted as both a reducing agent for graphene oxide to form RGO and a precursor of TiO2 nucleation on cotton fibers.44 Furthermore, graphene facilitates the decomposition of pathogens and microorganisms by providing an increased contact area between TiO2 NPs and bacteria. In addition to the excellent antibacterial activity, negligible toxicity was also registered for the novel graphene/TiO2 nanomaterial textile.
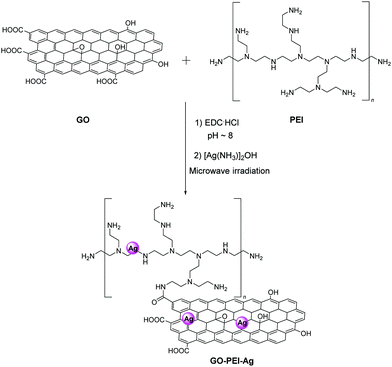
Recently, electrospinning technology was utilized for the production of a nanofiber yarn consisting of polyacrylonitrile (PAN) and GO functionalized with Ag NPs.45 Briefly, GO serves as a suitable substrate for the in situ reduction of diamine silver(I) hydroxide. In order to improve the solubility and stability of the composite, polyethyleneimine (PEI) was added to the blend and secured via amide bonds with the carboxylic acid groups of GO. Fig. 7 depicts the synthetic route for the preparation of the GO–PEI–Ag/PAN fabric that exhibited an antibacterial rate greater than 99.99% against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) that remained as high after multiple washing cycles.45
On the contrary, Noor and coworkers had earlier reported the preparation of a silver-graphene coated polyviscose textile that showcased a 100% antibacterial rate against E. coli, which slightly decreased to 90.1% after a dozen laundering cycles.46 The functionalization procedure involves firstly the treatment of the polyviscose fabric with aqueous NaBH4 as the reducing agent to create binding sites and then the immersion of the as prepared “active” fabric in aqueous AgNO3–GO solution to in situ form the Ag NPs. This simple, two-step pad-dry process allows scalable production with application in the biomedical field and is generic to NP incorporation in medical textiles.46
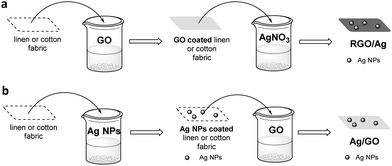
A similar “pad-dry-cure” method was employed for the chemical modification of two cellulose-based fabrics, linen and cotton.47 Depending on the sequence of treatment, two different nanocomposites are produced: Ag/GO coating on the fabric and in situ prepared Ag NPs on GO coated fabric to produce the RGO/Ag nanocomposite (Fig. 8). The interesting aspect of this study is that all fabrics acquired novel qualities, although not to the same degree. In particular, the Ag/GO nanocomposite exhibited excellent UV protection qualities owing to the GO coating, while RGO/Ag developed better antibacterial properties against Gram-positive and Gram-negative strains.
However, metal nanoparticles may penetrate the fabric fibers due to their small size and interface with human tissue, creating problems regarding their toxicity. In this frame, an alternative way to substitute the metal nanoparticles for the modification of graphene and carbon nanomaterials is ion implantation. In this context, GO coated on cotton by microwave irradiation was subsequently bombarded with various concentrations of Fe3+ ions.48 Despite the damage on the GO/cotton substrate, induced by ion bombardment, a considerably higher antibacterial activity is revealed against both E. coli and S. aureus by the modified textile in comparison with the untreated GO/cotton fabric. The antibacterial performance is attributed to the Fe3+ ions and is proportional to the dose employed, as well as to the bombarded GO morphology that provides edges and negatively charged oxygen-containing groups, shaping a sharp, blade-like surface that cuts the cell membrane.49,50
The first evidence of the antimicrobial activity of CNTs was reported in 2007.51 Later on, the antibacterial activity of single-walled CNTs (SWCNTs) was linked to their length; longer SWCNTs aggregate with bacterial cells leading to their death more effectively than shorter ones.52 Soon researchers realized that further functionalization on the CNTs could enhance their antibacterial properties and protect the fabrics. Consequently, a cotton fabric coated with the ZnO/CNT composite material adequately confronted S. aureus and E. coli.53 Such antibacterial activity was exhibited also by nonwovens of polylactide coated with MWCNTs, when modified with Ag NPs via electrochemical deposition.54
The antibacterial activity of MoS2 nanosheets has been studied in the last few years with very interesting results.55 Specifically, owing to their exfoliation-derived sharp edges and corners, it is proposed that the MoS2 nanosheets act as nanoscissors and nanoknives that rip the lipid membrane bilayers of bacteria and extract their vital phospholipids and proteins, thus causing extensive cell damage.56 In addition to this, other studies have showcased the intrinsic ability of MoS2 to generate oxidative stress via both reactive oxygen species dependent and independent pathways, while its extensive 2D plane surface provides a high contact area with bacteria, which eventually provokes cell-membrane stress.55 Inspired by these promising results, researchers recently fabricated MoS2-modified polycotton fabrics that were utilized as an additional layer to personal protective face masks with an excellent antibacterial performance and sun-light-induced photothermal self-disinfection capability.33 The exciting performance is attributed to the rupture of the bacterial membrane upon contact with MoS2, which seems to happen because of a combination of factors: generation of superoxide anions (O2˙−) that causes oxidative stress and high hydrophilicity that aids bacterial absorbance on the active surface.33
3.2 Fire protection/retardant activity
Flame retardant activity constitutes a profound necessity for the fabrication of first responders’ clothing and equipment. Since this important quality applies also to the protection of combustible materials in general, extensive research has been conducted.Even simply exfoliated, non-modified graphene has been found to provide flame retardant properties to fabrics. Coating of a commercial cotton fabric with an aqueous suspension of exfoliated few-layered graphene constitutes a simple, timely, eco-friendly methodology to produce efficient flame retardant and durable multipurpose e-textile fabrics.57 Accordingly, RGO deposited on the silk fabric surface exhibits both fire protection and smoke suppression properties after 9 deposit cycles, compared to the bare silk fabric.58
Functionalization can further enhance the fire-spreading prevention properties of carbon-based nanomaterials utilized in the fabric industry. The convenient synthesis of graphene phosphonic acid and its usage as an effective flame retardant59 was the spark that initiated phosphorous doping on carbon nanomaterials as a weapon against fire. Next, GO was successfully decorated with phosphorous moieties on its oxidized surface, in the presence of a mixture of phosphoric acid and polyphosphoric acid, with NaOH to regulate mildly oxidized pH.60 The phosphorous-doped graphene oxide (PGO) was then used to cover a piece of cloth that did not catch fire within 120 s of flame contact, unlike the reference cloth-only that was totally burned within 25 s. This also revealed a clear advantage of the PGO/cloth against the GO/cloth with respect to flame retardation (Fig. 9). This phenomenon is possibly attributed to thermal condensation of phosphoric acid that produces char as a protective coating to cloth and to the formation of phosphoester, phosphodiester and phosphoanhydride moieties upon dehydration, as a physical heat insulation mechanism.60
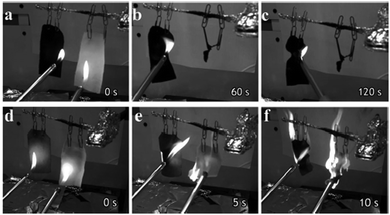 | ||
| Fig. 9 (a–c) Snapshots of the flame treatments of the PGO/cloth (left) and cloth-only (right) in a time span of 0–120 s. (d–f) Snapshots of the flame treatments of the GO/cloth (left) and cloth-only (right) in a time span of 0–10 s. Reprinted with permission from ref. 60. Copyright © 2015 WILEY-VCH. | ||
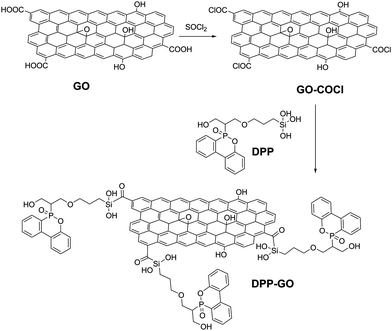
Inspired by this work, graphene was also treated with polyaniline (PANI) and polypyrrole (Ppy) entities in the presence of phosphoric acid, resulting in a plethora of nitrogen-containing and phosphorous-containing functional groups on the graphene surface that efficiently provide fire protection to the cotton cloth.61 In general, molecules rich in phosphorous and nitrogen functionalities have been exploited as reactants for the functionalization of GO by various groups,61,62e.g. a dioxaphosphinyl-acrylamide polymer was secured via hydrogen bonds on a polyacrylamide/GO film fabricated by layer by layer assembly and coated on a cotton fabric with excellent flame retardant properties.62 Fire safety of cotton fabrics was similarly secured with the grafting of a long-chained phosphaphenanthrene (DPP) on GO.63 Successful grafting was achieved according to the synthetic route shown in Fig. 10 and the DPP–GO composite was subsequently coated on a glass fabric to test the fire protection properties. GO is a physical surface barrier to the expansion of the fire and enables the slower combustion of the organophosphorous chain that produces a stiff char surface with great mechanical strength, summarizing a highly efficient system.
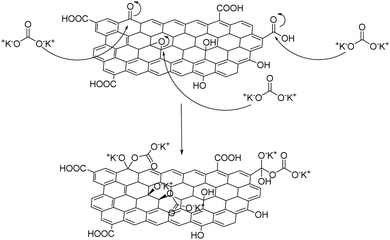
Nonetheless, limitations exist regarding phosphorous-based flame retardants, mainly because of their poor mechanical strength, potential toxicity and troubled handling after fire contact.64 Alternatively, the graphene oxide surface can be functionalized with a simple inorganic salt such as K2CO3 forming a “salt-functionalized” GO,65 according to the mechanism shown in Fig. 11, which is then coated on cotton via dip-coating. Dehydration that takes place upon ignition releases gaseous CO and H2O, leaving behind a char cotton surface that eventually ceases fire.
Similarly, the inherent flame resistant and anisotropic properties of the CNTs are improved with chemical modification as tested after hybridization with fabric fibers.66 Vinylophosphonic acid was utilized as a bridge between oxidized CNTs and cellulose fibers in a benzophenone-catalyzed UV-induced graft polymerization.67 Polyurethane fibers were successfully protected against fire via modification with a mixture of aluminum hydroxide and multi-walled CNTs (MWCNTs) by oxyfluorination treatment.68 Recently, a novel CNT/natural lignin/K2CO3 composite material was investigated as a protective flame retardant coating to cotton roving.69 This “magic blend” exploits lignin's natural char forming capability, which is advanced by the CNTs and potassium carbonate's ability to dilute the flammable gases, resulting in a doubled limiting oxygen index (LOI) for the modified fabric compared to the commercial one.
While MoS2 is characterized by gaseous impermeability, mechanical strength and non-flammability, which render it an ideal candidate for the fire protection of textiles, cobalt borate is known for its catalytic properties and thermal stability. Hence, scientists reasonably thought to merge these nanomaterials by decorating chemically exfoliated MoS2 with Co3(BO3)2 nanosheets in order to improve PAN fibers’ flame retardant performance (Fig. 12).29 In brief, Co anions effectively catalyze the oxidation of CO and facilitate the charring process. Also, Co3(BO3)2 enhances surface interactions between the sandwich-like Co3(BO3)2/MoS2 composite and PAN polymeric fibers.
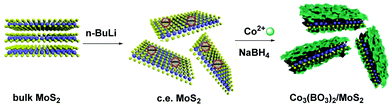 | ||
| Fig. 12 Preparation of Co3(BO3)2/MoS2 hybrids. Reproduced in part with permission from ref. 29. Copyright © 2020 Elsevier Ltd. | ||
Defect-rich MoS2 was decorated with thiol-functionalized SiO2 nanospheres and then utilized to prepare modified PAN fibers with high fire resistance.30 Another approach is the biomimetic construction of a MoS2-based coating on PAN using tannic acid-modified cellulose fibers and MoS2 nanosheets, bridged through Fe3+-catechol chelating and borate cross-linking to tannic acid.31 The char layers created in the case of fire produce high amounts of CO2 inflammable gas, thus protecting the fabric from catching fire. Finally, an interesting endeavor includes the modification of MoS2 nanosheets with allyl mercaptan and the subsequent addition of phosphaphenanthrene oxide to the carbon–carbon double bond. The modified MoS2 has shown great flame resistance and smoke suppression in polyurethane foam but is yet to be tested on yarn or fabric.70
3.3 High air permeability and low resistance to water vapor permeability
Fabrication of breathable textiles that remove heat and body moisture, ensure body temperature regulation and provide comfort to users, remains an ongoing challenge in the personal protective equipment clothing industry. The main bottleneck is that although inherently breathable fabrics exist, their high porosity allows also the penetration of environmental and other toxicants, making their usage prohibitive for first responders.In 2012 Nair et al. proved that GO membranes can be highly permeable to water and in the presence of water or humidity, an intercalation mechanism takes place.71 This work inspired the research community to study GO in various related applications, one of which is its breathability. Soon enough, it was found that GO membranes allow outward perspiration and at the same time block the penetration of toxicants such as trichloroethylene and benzene and thus can be used as layers on personal protective fabrics.72 In addition, a GO-based membrane has been developed as a counterpart of a protective garment against chemical warfare agents, yet with high air permeability.73 Another important finding covers the reversibility of the wetting behavior of textiles upon chemical functionalization. Polyethylene terephthalate (PET) is rather hydrophobic in the form of a coating or membrane, but when modified with polypyrrole functionalized GO in a textile form, becomes actually hydrophilic with remarkable capability for absorbing moisture.74 On the contrary, reduction of GO to RGO transforms the chemically-modified fabric back to hydrophobic.
An even greater challenge, though, is the design of a revolutionary fabric that can reversibly switch from a breathable to protective state when needed. In this frame, CNT membranes with moisture conductive pores, grown on Si, were designed to adapt to the environmental conditions accordingly.75 Water permeability was actually higher than that of the commercial breathable fabrics and protection from biological threats was secured by size exclusion, summarizing a selective penetration mechanism. Furthermore, CNTs functionalized with poly(allylamine hydrochloride) with the aid of layer by layer assembly and deposited on cellulose fibers, contribute to the fabrication of a soft and breathable coating.76 On the other hand, CNTs functionalized with hydrophilic polyurethane form a water vapor permeable coating that provides also great UV resistance to cotton fabric.77
A recent study marries water vapor permeability and strain sensing on the production of breathable e-textiles with a fast sensing response.78 A wool fabric dyed with GO and then reduced with the aid of L-ascorbic acid to produce RGO exhibits outstanding moisture resilience, yet excellent air and heat permeability and unchanged mechanical strength after multiple washing and usage cycles. This unique performance is attributed to the hydrophobicity of RGO and the rapid moisture diffusion potential of the wool textile.78
Besides graphene, other 2D nanomaterials such as MXenes have also joined the venture towards breathable and comfortable wearables. In this frame, 2D Ti3C2Tx nanosheets grafted on cellulose non-woven fibers via hydrogen bonds, apart from the satisfying breathability and humidity response, provide also metal-like conductivity that can effectively kill bacteria in case of an infected wound.79
Finally, 2D boron nitride nanosheets, when arranged uniformly on textile fibers, offer not only thermal conductivity and moisture permeability, but also extreme hydrophobicity, thus the modified fabric can be utilized for personal cooling.80
3.4 Water repellency
Design of water-repellent coatings is nowadays an attractive research topic towards the fabrication of clean and durable fabrics. Protection from extreme environmental conditions, as well as long-term corrosion resistance, are mandatory for personal protective equipment garments.The otherwise super water-absorptive cotton fabric acquires highly hydrophobic properties when coated with CNTs.81 When water droplets gather on the lotus-leaf-like-surface of the CNT/cotton fabric, they merge together forming water beads and slip easily off its surface. Then, SWCNTs coated on RGO formed a SWCNT/RGO composite material that was utilized for the fabrication of a glove that was intended as a device to detect motion signals. The composite material exhibited also excellent water repellent properties even after ten washings, owing to the synergistic hydrophobicity of SWCNT and RGO.81 Furthermore, nonwovens of commercially available polylactide were produced in a laboratory through electrospinning and were subsequently treated with MWCNTs and functionalized with methyltrichlorosilane.82 The final modified textile turned to be highly hydrophobic mirrored by its water contact angle of around 165°.
Of course, a popular way to add hydrophobicity to GO is by taking advantage of its multiple oxygen functionalities that enable the grafting of aliphatic, long-chained molecules, via robust covalent bonds.83 The next step is the incorporation of the hydrophobic composite in textiles. In this direction, Zahouily and coworkers designed a simple and scalable methodology for the coating of octadecylamine (ODA)-functionalized GO on PET fabrics (Fig. 13).84 The PET@G-ODA showcased remarkable water repellency compared to the reference PET fabric, with water contact angle up to 148° with just 7 wt% loading of G-ODA on the fabric.
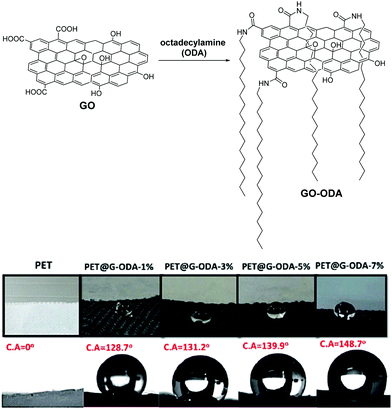 | ||
| Fig. 13 Chemically functionalized GO for waterproof fabrics and water contact angles (C. A.) in increasing GO-ODA loadings on PET fabric. Reproduced in part with permission from ref. 84. Copyright © 2020 The Royal Society of Chemistry. | ||
MXenes are generally characterized by high hydrophilicity owing to their oxygen and fluorine terminal groups. The first work to report a hydrophobic MXene coating foam was reported in 2019, where Ti3AlC2 was treated with hydrazine which effectively quenched the –OH hydrophilic moieties.85 Remarkable water resistant activity was then registered to MXene sheets in situ polymerized with polypyrrole (PPy), coated with silicone and grafted on PET textiles by a scalable dip-coating process.86 PPy presence facilitated the interaction with the PET fabric fibers via hydrogen bonding and van der Waals forces and at the same time enhanced the inherent EMI shielding ability of MXenes. On the other hand, silicone coating converts the MXene surface from hydrophilic (water contact angle of 88°) to hydrophobic (water contact angle of 126°), while allowing sufficient air permeability.
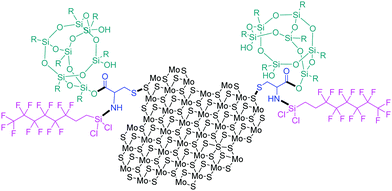
A superhydrophobic spray coating consisting of MoS2 nanoparticles and polyurethane was formulated and sprayed on fabric, stainless steel and paper and exhibited a water contact angle of 157° and an excellent water resistance up to 6000 cycles.87 Moreover, when modified with hydrophobic perfluorooctyl trichlorosilane, the water contact angle increased to 167°. MoS2 is responsible for the reduced adhesion and friction, offering abrasion resistance and maximized durability to the novel coating, while perfluorooctyl silane is known for its hydrophobicity.87 Recently, the aforementioned silane was utilized along with polysilsesquioxane in order to covalently functionalize n-butyl lithium-exfoliated MoS2 through a cysteine bridge.88 Cysteine binds on sulphur by a disulphide bond and then nucleophilic substitution and esterification take place to immobilize perfluorooctyl silane and polysilsesquioxane, respectively, to the MoS2 substrate (Fig. 14). Si–O–Si moieties add to the hydrophobicity and self-cleaning ability, while MoS2 offers its lubrication characteristics to the prepared coating.88
3.5 Protection against UV radiation
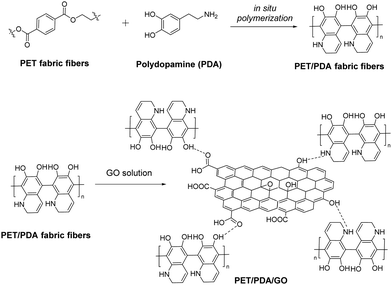
Ultraviolet (UV) radiation, the electromagnetic radiation with wavelength ranging between visible light and X-rays (100–400 nm), has proved to be highly dangerous to human skin and corrosive to fabrics and materials utilized in every-day life, since the depletion of the stratospheric ozone layer.89 In order to shield coatings and textiles and therefore protect the human body from the damaging effects of UV radiation, a great deal of research effort has been devoted to the design of UV-blocking composites.Graphene was studied for the first time in 2014 as a potential UV blocker to protect cotton fabric from external UV radiation.90 Notably, polyurethane-functionalized graphene nanoplates (GNP) were coated to cotton via the pad-dry method and the hybrid fabric was characterized and tested thoroughly for its UV-blocking properties, exhibiting an extraordinary ultraviolet protection factor (UPF) greater than 100 at a low loading, ranging between 0.05 and 0.4% w/w.90 Furthermore, GO treated onto silk fabric provides satisfying protection against UV radiation without destroying the micro-structure of the silk fibroin, while exhibiting antibacterial properties and maintaining decent air permeability.91 On the other hand, RGO deposited on cotton by a hydrothermal method showcased a 442 UPF value that was reduced to 422 after laundering, summarizing a UV-protective and durable fabric.92 This study also revealed the superiority of RGO compared to GO with respect to the UV-blocking properties. Robust UV-blocking performance was later registered to a chitosan/graphene nanocomposite grafted on cotton fabric, mirrored to a UPF value of 465.8 which is more than 60 times greater than that of a pristine cotton fabric.93 A chitosan/GO nanocomposite fabricated on cotton by layer by layer assembly is also a successful UV blocker with an analogous UPF value.94 In both cases, the enhanced UV absorbance is attributed to the planar structure of graphene nanosheets and their presence in the fabric composite.93 Similar UV-protection activity was exhibited by a polyaniline-functionalized GO nanosheet coated on cotton fabric, that remained unchanged after multiple laundering cycles.95 Alternatively, RGO fabricated on a polydopamine-templated PET fabric via electrostatic interactions and hydrogen bonds between the amine functionalities of polydopamine and the oxygen-functionalities of GO demonstrates a UPF value greater than 500 even after repeated washings (Fig. 15).96
Silver nanoparticles, known for their UV absorbing qualities, were in situ prepared and incorporated on RGO coated on knit polyester fabric and found to reduce the UV transmittance of the fabric sample up to 73% compared to the bare polyester.97 The UV blocking ability of both GO and RGO was confirmed, with the blocking ability of RGO exceeding that of GO and being further ameliorated in the presence of small amounts of silver NPs.
Also, CNTs were found to provide some UV protection at high concentrations that is reinforced when functionalized with the polypyrrole–ZnO nanocomposite.98 Cotton textile treated with 1,2,3,4-butane tetracarboxylic acid (BTCA) as the cross-linking agent is efficiently shielded against damaging UV radiation by a polypyrrole–ZnO/CNT nanocomposite.98 The decrease observed in the UV transmittance is proportional to the ZnO ratio in the modified fabric. MWCNTs/PAN composite nanofibers with various thicknesses and MWCNT weight contents were prepared by electrospinning technology and found to exhibit remarkable UV and microwave irradiation resistance.99 The study showcased the UV-shielding ability of MWCNTs, since the PAN nanofibers without MWCNT content did not adsorb the UV radiation and actually reflected most of the radiation applied. Then, with increasing MWCNT contents, the UV transmittance experienced a significant decrease which is attributed to the development of electron transferring channels throughout the PAN matrix. A UPF value of 677.2 was registered for the composite fibers with a 10 wt% MWCNT content due to the excellent dense connection network of the hybrid material.99
Finally, a rare report describes promising enhancement of the UV-blocking properties of a polymer matrix upon modification with hydroxyl-functionalized MoS2 nanosheets.100 Specifically, the MoS2-based nanofiller offers enhanced UV-absorbance mainly in the UV-B and UV-C regions, directly proportional to the MoS2 loading in the composite material. Even though interesting, the incorporation of functionalized MoS2 on polymeric fabric fiber and its effect on the UV transmittance are yet to be comprehensively assessed.
3.6 Increased resistance to wear
Wear resistance, the material's ability to resist damage and loss upon mechanical action, is one of the most important requirements in the fabrication and production process of fabrics performing under operational tension and extreme environmental conditions. Furthermore, the acquisition of the previously discussed qualities is invalid without the security of the appropriate durability and wear resistance of the novel fabrics.Previous reports highlight the use of graphene to improve the electrical and mechanical properties of a graphene–Kevlar nanofiber composite.101 Then, to further enhance the durability of the fabrics, polymers were brought to the spotlight. Poor abrasion resistance is a common problem encountered in coated cotton textiles and causes loss of the protective coating due to friction and external scratching. A potential solution to this matter was provided by the binding capability of water-soluble polyurethane (WPU) in a sandwich-like graphene/tourmaline/WPU composite coated on cotton.102 WPU serves as both a grafting agent due to its isocyanate groups binding to carboxylic acid groups of cotton fibers and as a cross-linking agent building a network macromolecule responsible for a high abrasion resistance. A WPU/graphene nanocomposite provides also heat and bending resistance to a para-aramid knit manufactured by dip-coating.103 Similarly, a polyurethane/graphene nanocomposite provides protection to a nylon textile in all weather and over time, as well as decent crocking performance and tearing strength, enabling serviceability.104 Polydimethylsiloxane on the other hand was utilized as an encapsulation polymer to shield graphene nanosheets deposited on cotton fibers against stretching deformation.105 Crack formation is successfully delayed in a series of both natural (cotton, cellulose) and synthetic fabrics (acrylic, polyester, nylon) when a graphene coating is enriched with a polystyrene elastomer.106
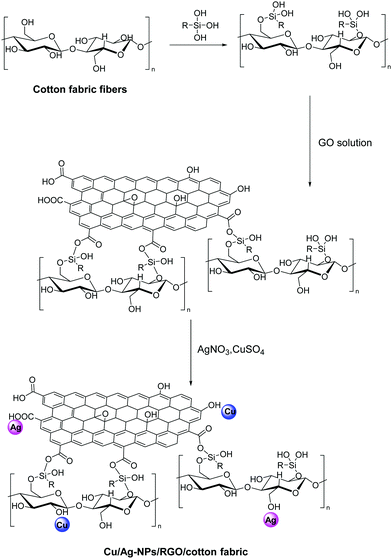
Enhanced durability of the modified fabric is also ensured when strong bonding exists between the fabric, the 2D nanomaterial and the additive, that is reflected in stability after repeated washing cycles. This is achieved in a Cu/Ag-NPs/RGO/cotton fabric via the effective immobilization of Cu and Ag NPs on graphene with the aid of 3-glycidyloxypropyl trimethoxysilane as a coupling agent between cotton and GO (Fig. 16).107
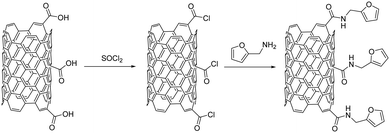
Recently, interesting studies have been reported on the wear behavior and tribological properties of furfuryl amine-functionalized MWCNTs grafted on carbon fibers and excellent wear resistance under various sliding conditions was registered for the hybrid composite (Fig. 17).108 The functionalized MWCNTs offer the polymeric matrix increased wear resistance because of the furfuryl additive acting as an interfacial adhesion factor. At the same time, the unique topological hollow structure of MWCNTs is responsible for the positive effect on the tribological properties of the modified fabric.108
Furthermore, the study on the tribological properties of a hybrid PTFE/Kevlar fabric composite filled with nano-SiC and submicron-WS2 fillers emphasizes the mechanical strength and tearing resistance added by WS2 to the composite textile.32 However, more research efforts have to be devoted to 2D TMDs as textile additives with promising qualities towards the realization of durable and wear resistant fabrics.
4. Conclusions and perspectives
Technical textile requirements for personal protective clothing have multiplied in the last few years due to the climate crisis, technological evolution, human interference and the increased living standards. From antimicrobial properties and fire protection, to water repellency and UV radiation resistance, to breathability and durability, the fabric industry faces a huge challenge to meet these excessive requirements. Therefore, researchers have brought along nanomaterials with proven versatility, e.g. carbon nanostructures and 2D TMDCs, as allies in this tricky endeavor. Chemical functionalization of these nanostructures enables not only the acquisition of the desired properties but also the successful grafting to the fabric fibers. Herein, the methodologies developed for the hybridization of nanocarbons and TMDs with the various textile substrates have been reviewed. Furthermore, there is an extensive report on the chemical modification of these nanomaterials as counterparts of the fabric fibers towards the most critical operational limitations and environmental conditions.While there is a decent amount of research focused on incorporating antibacterial properties into fabrics, this is limited to the usage of metal nanoparticles, which although effective have been accused of toxicity.109 In parallel, great progress has been registered for the flame retardancy of the fabrics, where not only modified carbon nanostructures (graphene and CNTs) but also functionalized MoS2 have exhibited key performance against fire. On the other hand, in other research areas which are rather recent, such as the air and water permeability of the fabrics, there is a long way to be covered. All in all, unlike graphene and CNTs, 2D TMDs are still in the beginning of their adventure as textile counterparts but it seems that they hold great promise. Nonetheless, surface functionalization of 2D nanomaterials still faces quite a few challenges. These range between technical issues, such as their handling and the large-scale/industrial application, and scientific challenges since a deeper understanding of the structure–properties relationship is of vital importance for future advances in 2D nanomaterial development and hybridization with fabric fibers. Future research should focus on assembling the single layers of 2D nanomaterials into industrial materials, without sacrificing their original performance. In any case, we firmly believe that the chemical functionalization of these nanomaterials, which has been tremendously developed in the past few years, is the key and actually constitutes a problem-solver to the fabric industry in its quest to fulfill the current technical expectations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T2EΔK-01316) is acknowledged.Notes and references
- D. S. Morais, R. M. Guedes and M. A. Lopes, Materials, 2016, 9, 498 CrossRef PubMed.
- S. T. Lazar, T. J. Kolibaba and J. C. Grunlan, Nat. Rev. Mater., 2020, 5, 259 CrossRef CAS.
- Q. Liu, J. Huang, J. Zhang, Y. Hong, Y. Wan, Q. Wang, M. Gong, Z. Wu and C. F. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 2026 CrossRef CAS PubMed.
- B. Mahltig and A. Fischer, J. Polym. Sci. B, Polym. Phys., 2010, 48, 1562 CrossRef CAS.
- N. R. Dhineshbabu and S. Bose, ACS Omega, 2018, 3, 7454 CrossRef CAS PubMed.
- H. Zhou, H. Wang, H. Niu and T. Lin, Adv. Mater. Interfaces, 2018, 5, 1800461 CrossRef.
- Z. Li, Z. Liu, H. Sun and C. Gao, Chem. Rev., 2015, 115, 7046 CrossRef CAS PubMed.
- A. Stergiou, R. Canton-Vitoria, M. N. Psarrou, S. P. Economopoulos and N. Tagmatarchis, Prog. Mater. Sci., 2020, 114, 100683 CrossRef CAS; S. P. Economopoulos and N. Tagmatarchis, Chem. – Eur. J., 2013, 19, 12930 CrossRef PubMed; N. Karousis, N. Tagmatarchis and D. Tasis, Chem. Rev., 2010, 110, 5366 CrossRef PubMed; D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef PubMed.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- H. Xu, X. Yin, X. Li, M. Li, S. Liang, L. Zhang and L. Cheng, ACS Appl. Mater. Interfaces, 2019, 11, 10198 CrossRef CAS PubMed; R. Sun, H.-B. Zhang, J. Liu, X. Xie, R. Yang, Y. Li, S. Hong and Z.-Z. Yu, Adv. Funct. Mater., 2017, 27, 1702807 CrossRef; L. Wang, L. Chen, P. Song, C. Liang, Y. Lu, H. Qiu, Y. Zhang, J. Kong and J. Gu, Composites, Part B, 2019, 171, 111 CrossRef; F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. M. Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137 CrossRef PubMed.
- W.-T. Cao, F.-F. Chen, Y.-J. Zhu, Y.-G. Zhang, Y.-Y. Jiang, M.-G. Ma and F. Chen, ACS Nano, 2018, 12, 4583 CrossRef CAS PubMed.
- A. A. Tedstone, D. J. Lewis and P. O’Brien, Chem. Mater., 2016, 28, 1965 CrossRef CAS.
- G. Chen, Y. Li, M. Bick and J. Chen, Chem. Rev., 2020, 120, 3668 CrossRef CAS PubMed.
- R. J. Molina, RSC Adv., 2016, 6, 68261 RSC; S. Bhattacharjee, R. Joshi, A. A. Chughtai and C. R. Macintyre, Adv. Mater. Interfaces, 2019, 6, 1900622 CrossRef CAS PubMed; H. J. Salavagione, M. A. Gómez-Fatou, P. S. Shuttleworth and G. J. Ellis, Front. Mater., 2018, 5, 18 CrossRef.
- J. Di, X. Zhang, Z. Yong, Y. Zhang, D. Li, R. Li and Q. Li, Adv. Mater., 2016, 28, 10529 CrossRef CAS PubMed; J. Xiong, J. Chen and P. S. Lee, Adv. Mater., 2020, 2002640 Search PubMed.
- Z. Wang, Z. Cheng, C. Fang, X. Hou and L. Xie, Composites, Part A, 2020, 136, 105956 CrossRef CAS; A. Iqbal, P. Sambyal and C. M. Koo, Adv. Funct. Mater., 2020, 30, 2000883 CrossRef.
- J. Cao and C. Wang, Appl. Surf. Sci., 2017, 405, 380 CrossRef CAS.
- G. Cai, Z. Xu, M. Yang, B. Tang and X. Wang, Appl. Surf. Sci., 2017, 393, 441 CrossRef CAS.
- J. Ren, C. Wang, X. Zhang, T. Carey, K. Chen, Y. Yin and F. Torrisi, Carbon, 2017, 111, 622 CrossRef CAS.
- M. Hasani and M. Montazer, J. Appl. Polym. Sci., 2017, 134, 45493 CrossRef.
- Y. J. Yun, W. G. Hong, W. J. Kim, Y. Jun and B. H. Kim, Adv. Mater., 2013, 25, 5701 CrossRef CAS PubMed.
- D. Kowalczyk, W. Fortuniak, U. Mizerska, I. Kaminska, T. Makowsk, S. Brzezinski and E. Piorkowska, Cellulose, 2017, 24, 4057 CrossRef CAS.
- G. Decher, Science, 1997, 277, 1232 CrossRef CAS.
- L. Zheng, X. Su, X. Lai, W. Chen, H. Li and X. Zeng, Mater. Lett., 2019, 253, 230 CrossRef CAS.
- F. Ke, F. Song, H. Zhang, J. Xu, H. Wang and Y. Chen, Composites, Part B, 2020, 200, 108253 CrossRef CAS.
- B. Fugetsu, E. Akiba, M. Hachiya and M. Endo, Carbon, 2009, 47, 527 CrossRef CAS.
- J. Zhou, Q. Luo, P. Gao and H. Ma, RSC Adv., 2020, 10, 11982 RSC.
- H. Wang, H. Wang, Y. Wang, X. Su, C. Wang, M. Zhang, M. Jian, K. Xia, X. Liang, H. Lu, S. Li and Y. Zhang, ACS Nano, 2020, 14, 3219 CrossRef CAS PubMed.
- H. Peng, D. Wang, L. Zhang, M. Li, M. Liu, C. Wang and S. Fu, Composites, Part B, 2020, 201, 108298 CrossRef CAS.
- H. Peng, D. Wang, M. Li, L. Zhang, M. Liu and S. Fu, Composites, Part B, 2019, 174, 107037 CrossRef.
- H. Peng, D. Wang and S. Fu, Composites, Part B, 2021, 215, 108742 CrossRef CAS.
- H. Li, F. Zeng, Z. Yin, D. Jiang and Y. Huo, Polym. Compos., 2016, 37, 2218 CrossRef CAS.
- P. Kumar, S. Roy, A. Sarkar and A. Jaiswal, ACS Appl. Mater. Interfaces, 2021, 13, 12912 CrossRef CAS PubMed.
- Abid, P. Sehrawat, C. M. Julien and S. S. Islam, ACS Appl. Mater. Interfaces, 2020, 12, 39730 CrossRef CAS PubMed.
- I. O’Connor, H. Hayden, S. O’Connor, J. N. Coleman and Y. K. Gun’ko, J. Phys. Chem. C, 2009, 113, 20184 CrossRef.
- T. Sainsbury, K. Erickson, D. Okawa, C. S. Zonte, J. M. J. Frechet and A. Zettl, Chem. Mater., 2010, 22, 2164 CrossRef CAS.
- X. Yang, Q. Tu, X. Shen, P. Zhu, Y. Li and S. Zhang, Polymers, 2019, 11, 374 CrossRef PubMed.
- A. Hazarika, B. K. Deka, D. Y. Kim, Y.-B. Park and H. W. Park, Comput. Sci. Technol., 2016, 129, 137 CrossRef CAS.
- A. Hazarika, B. K. Deka, D. Y. Kim, H. D. Roh, Y.-B. Park and H. W. Park, ACS Appl. Mater. Interfaces, 2017, 9, 36311 CrossRef CAS PubMed.
- F. Alimohammadi, M. P. Gashti and A. Shamei, Prog. Org. Coat., 2012, 74, 470 CrossRef CAS.
- J. Cui and S. Zhou, J. Mater. Chem. C, 2018, 6, 12273 RSC.
- Y. Li, H. Zhu, S. Zhu, J. Wan, Z. Liu, O. Vaaland, S. Lacey, Z. Fang, H. Dai, T. Li and L. Hu, NPG Asia Mater., 2015, 7, 150 CrossRef.
- L. Karimi, M. E. Yazdanshenas, R. Khajavi, A. Rashidi and M. Mirjalili, Cellulose, 2014, 21, 3813 CrossRef CAS.
- K. Krishnamoorthy, U. Navaneethaiyer and R. Mohan, Appl. Nanosci., 2012, 2, 119 CrossRef CAS.
- W. Yu, X. Li, J. He, Y. Chen, L. Qi, P. Yuan, K. Ou, F. Liu, Y. Zhou and X. Qin, J. Colloid Interface Sci., 2021, 584, 164 CrossRef CAS PubMed.
- N. Noor, S. Mutalik, M. W. Younas, C. Y. Chan, S. Thakur, F. Wang, M. Z. Yao, Q. Mou and P. H.-M. Leung, Polymers, 2019, 11, 2000 CrossRef CAS PubMed.
- A. Farouk, S. El-Sayed Saeed, S. Sharafa and M. M. Abd El-Hady, RSC Adv., 2020, 10, 41600 RSC.
- J. Hu, J. Liu, L. Gan and M. Long, ACS Sustainable Chem. Eng., 2019, 7, 7686 CrossRef CAS.
- W. Hu, C. Peng, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 4, 4317 CrossRef CAS PubMed.
- O. Akhavan and E. Ghaderi, ACS Nano, 2010, 4, 5731 CrossRef CAS PubMed.
- S. Kang, M. Pinault, L. D. Pfefferle and M. Elimelech, Langmuir, 2007, 23, 8670 CrossRef CAS PubMed.
- C. Yang, J. Mamouni, Y. Tang and L. Yang, Langmuir, 2010, 26, 16013 CrossRef CAS PubMed.
- K. B. Yazhini and H. G. Prabu, Appl. Biochem. Biotechnol., 2015, 175, 85 CrossRef CAS PubMed.
- T. Makowskia, M. Svyntkivska, E. Piorkowska and D. Kregiel, Appl. Surf. Sci., 2020, 527, 146898 CrossRef.
- X. Yang, J. Li, T. Liang, C. Ma, Y. Zhang, H. Chen, N. Hanagata, H. Su and M. Xu, Nanoscale, 2014, 6, 10126 RSC; S. Roy, A. Mondal, V. Yadav, A. Sarkar, R. Banerjee, P. Sanpui and A. Jaiswal, ACS Appl. Bio Mater., 2019, 2, 2738 CrossRef CAS.
- R. Wu, X. Ou, R. Tian, J. Zhang, H. Jin, M. Dong, J. Li and L. Liu, Nanoscale, 2018, 10, 20162 RSC.
- H. Ba, L. Truong-Phuoc, V. Papaefthimiou, C. Sutter, S. Pronkin, A. Bahouka, Y. Lafue, L. Nguyen-Dinh, G. Giambastiani and C. Pham-Hu, ACS Appl. Nano Mater., 2020, 3, 9771 CrossRef CAS.
- Y. Ji, G. Chen and T. Xing, Appl. Surf. Sci., 2019, 474, 203 CrossRef CAS.
- M.-J. Kim, I.-Y. Jeon, J.-M. Seo, L. Dai and J.-B. Baek, ACS Nano, 2014, 8, 2820 CrossRef CAS PubMed.
- S. Some, I. Shackery, S. J. Kim and S. C. Jun, Chem. – Eur. J., 2015, 21, 15480 CrossRef CAS PubMed.
- D. A. Pethsangave, R. V. Khose, P. H. Wadekar and S. Some, ACS Sustainable Chem. Eng., 2019, 7, 11745 CrossRef CAS.
- G. Huang, J. Yang, J. Gao and X. Wang, Ind. Eng. Chem. Res., 2012, 51, 12355 CrossRef CAS.
- W. Chen, Y. Liu, P. Liu, C. Xu, Y. Liu and Q. Wang, Sci. Rep., 2017, 7, 8759 CrossRef PubMed.
- I. V. D. Veen and J. D. Boer, Chemosphere, 2012, 88, 1119 CrossRef PubMed.
- K. S. Chavali, D. A. Pethsangave1, K. C. Patankar, R. V. Khose1, P. H. Wadekar, S. Maiti, R. V. Adivarekar and S. Some, J. Mater. Sci., 2020, 55, 14197 CrossRef CAS.
- Q. Wu, W. Zhu, C. Zhang, Z. Liang and B. Wang, Carbon, 2010, 48, 1799 CrossRef CAS.
- M. Parvinzadeh Gashti and A. Almasian, Composites, Part B, 2013, 45, 282 CrossRef CAS.
- J. Sun Im, B. C. Bai, T. Bae, S. J. In and Y. S. Lee, Mater. Chem. Phys., 2011, 126, 685 CrossRef.
- D. Łukawski, W. Grzeskowiak, A. Lekawa-Raus, M. Widelicka, F. Lisiecki and A. Dudkowiak, Cellulose, 2020, 27, 7271 CrossRef.
- M. Zhi, Q. Liu, Y. Zhao, S. Gao, Z. Zhang and Y. He, ACS Omega, 2020, 5, 2734 CrossRef CAS PubMed.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442 CrossRef CAS PubMed.
- R. S. Steinberg, M. Cruz, N. G. A. Mahfouz, Y. Qiu and R. H. Hurt, ACS Nano, 2017, 11, 5670 CrossRef PubMed.
- C. Peng, Z. Iqbal, K. K. Sirkar and G. W. Peterson, ACS Appl. Mater. Interfaces, 2020, 12, 11094 CrossRef CAS PubMed.
- A. Berendjchia, R. Khajavib, A. A. Yousefic and M. E. Yazdanshenas, Appl. Surf. Sci., 2016, 367, 36 CrossRef.
- N. Bui, E. R. Meshot, S. Kim, J. Peña, P. W. Gibson, K. J. Wu and F. Fornasiero, Adv. Mater., 2016, 28, 5871 CrossRef CAS PubMed.
- C. Lan, C. Li, J. Hu, S. Yang, Y. Qiu and Y. Ma, Adv. Mater. Interfaces, 2018, 1800116 CrossRef.
- S. Mondal and J. L. Hu, J. Appl. Polym. Sci., 2007, 103, 3370 CrossRef CAS.
- L. Xu, Z. Liu, H. Zhai, X. Chen, R. Sun, S. Lyu, Y. Fan, Y. Yi, Z. Chen, L. Jin, J. Zhang, Y. Li and T. T. Ye, ACS Appl. Mater. Interfaces, 2020, 12, 13265 CrossRef CAS PubMed.
- X. Zhao, L.-Y. Wang, C.-Y. Tang, X.-J. Zha, Y. Liu, B.-H. Su, K. Ke, R.-Y. Bao, M.-B. Yang and W. Yang, ACS Nano, 2020, 14, 8793 CrossRef CAS PubMed.
- X. Yu, Y. Li, X. Wang, Y. Si, J. Yu and B. Ding, ACS Appl. Mater. Interfaces, 2020, 12, 32078 CrossRef CAS PubMed.
- Y. Liu, X. Wang, K. Qia and J. H. Xin, J. Mater. Chem., 2008, 18, 3454 RSC.
- S. J. Kim, W. Song, Y. Yi, B. K. Min, S. Mondal, K.-S. An and C.-G. Choi, ACS Appl. Mater. Interfaces, 2018, 10(4), 3921 CrossRef CAS PubMed.
- L. Xu, J. Teng, L. Li, H.-D. Huang, J.-Z. Xu, Y. Li, P.-G. Ren, G.-J. Zhong and Z.-M. Li, ACS Omega, 2019, 4, 509 CrossRef CAS PubMed.
- G. Achagri, Y. Essamlali, O. Amadine, M. Majdou, A. Chakira and M. Zahouily, RSC Adv., 2020, 10, 24941 RSC.
- J. Liu, H.-B. Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z.-Z. Yu, Adv. Mater., 2017, 29, 1702367 CrossRef PubMed.
- Q.-W. Wang, H.-B. Zhang, J. Liu, S. Zhao, X. Xie, L. Liu, R. Yang, N. Koratkar and Z.-Z. Yu, Adv. Funct. Mater., 2019, 29, 1806819 CrossRef.
- Y. Tang, J. Yang, L. Yin, B. Chen, H. Tang, C. Liu and C. Li, Colloids Surf., A, 2014, 459, 261 CrossRef CAS.
- E. Koh and Y. T. Lee, Prog. Org. Coat., 2021, 153, 106161 CrossRef CAS.
- R. M. Lucas, S. Yazar, A. R. Young, M. Norval, F. R. de Gruijl, Y. Takizawa, L. E. Rhodes, C. A. Sinclair and R. E. Neale, Photochem. Photobiol. Sci., 2019, 18, 641 CrossRef CAS PubMed.
- L. Qu, M. Tian, X. Hu, Y. Wang, S. Zhu, X. Guo, G. Han, X. Zhang, K. Sun and X. Tang, Carbon, 2014, 80, 565 CrossRef CAS.
- S.-D. Wang, K. Wang, Q. Ma and C.-X. Qu, Mater. Today Commun., 2020, 23, 100893 CrossRef CAS.
- V. Pandiyarasana, J. Archanaa, A. Pavithrac, V. Ashwinc, M. Navaneethana, Y. Hayakawaa and H. Ikeda, Mater. Lett., 2017, 188, 123 CrossRef.
- M. Tian, X. Tang, L. Qu, S. Zhu, X. Guo and G. Han, Mater. Lett., 2015, 145, 340 CrossRef CAS.
- M. Tian, X. Hu, L. Qu, M. Du, S. Zhu, Y. Sun and G. Han, Appl. Surf. Sci., 2016, 377, 141 CrossRef CAS.
- X. Tang, M. Tian, L. Qua, S. Zhu, X. Guo, G. Han, K. Sun, X. Hua, Y. Wang and X. Xu, Synth. Met., 2015, 202, 82 CrossRef CAS.
- D. Cheng, X. Bai, J. Pan, J. Ran, J. Wu, S. Bi, G. Cai and X. Wang, Mater. Chem. Phys., 2020, 241, 122371 CrossRef.
- B. Ouadil, O. Cherkaoui, M. Safi and M. Zahouily, Appl. Surf. Sci., 2017, 414, 292 CrossRef CAS.
- K. B. Yazhini and H. G. Prabu, RSC Adv., 2015, 5, 49062 RSC.
- K. Nasouri, A. M. Shoushtari, J. Mirzaei and A. A. Merati, Diamond Relat. Mater., 2020, 107, 107896 CrossRef CAS.
- J. Zhang, W. Lei, J. Schutz, D. Liu, B. Tang, C. H. Wang and X. Wang, J. Polym. Sci., Part B: Polym. Phys., 2019, 57, 406 CrossRef CAS.
- M. Lian, J. Fan, Z. Shi, H. Li and J. Yin, Polymers, 2014, 55, 2578 CrossRef CAS.
- Y. Hao, M. Tian, H. Zhao, L. Qu, S. Zhu, X. Zhang, S. Chen, K. Wang and J. Ran, Eng. Chem. Res., 2018, 57, 13437 CrossRef CAS.
- H. Kim, S. Lee and H. Kim, Sci. Rep., 2019, 9, 1511 CrossRef PubMed.
- Y. Zhang, T.-T. Li, B.-C. Shiu, F. Sun, H.-T. Ren, X.-F. Zhang, C.-W. Loue and J.-H. Lina, Polym. Int., 2021, 70, 308 CrossRef CAS.
- Y. Zheng, Y. Li, Y. Zhou, K. Dai, G. Zheng, B. Zhang, C. Liu and C. Shen, ACS Appl. Mater. Interfaces, 2020, 12, 1474 CrossRef CAS PubMed.
- H. J. Salavagione, P. S. Shuttleworth, J. P. Fernández-Blázquez, G. J. Ellis and M. A. Gómez-Fatou, Carbon, 2020, 167, 495 CrossRef CAS.
- S. Bhattacharjee, C. R. Macintyre, X. Wen, P. Bahl, U. Kumar, A. A. Chughtai and R. Joshi, Carbon, 2020, 166, 148 CrossRef CAS.
- X. Zhang, X. Pei and Q. Wang, Polym. Adv. Technol., 2011, 22, 2157 CrossRef CAS.
- A. B. Sengul and E. Asmatulu, Environ. Chem. Lett., 2020, 18, 1659 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |