 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-flammable liquid electrolytes for safe batteries
Ritambhara
Gond†
,
Wessel
van Ekeren†
 ,
Ronnie
Mogensen
,
Andrew J.
Naylor
,
Ronnie
Mogensen
,
Andrew J.
Naylor
 * and
Reza
Younesi
* and
Reza
Younesi
 *
*
Department of Chemistry – Ångström Laboratory Uppsala University, Box 538, 751 21 Uppsala, Sweden. E-mail: reza.younesi@kemi.uu.se; andy.naylor@kemi.uu.se
First published on 16th September 2021
Abstract
With continual increments in energy density gradually boosting the performance of rechargeable alkali metal ion (e.g. Li+, Na+, K+) batteries, their safe operation is of growing importance and needs to be considered during their development. This is essential, given the high-profile incidents involving battery fires as portrayed by the media. Such hazardous events result from exothermic chemical reactions occurring between the flammable electrolyte and the electrode material under abusive operating conditions. Some classes of non-flammable organic liquid electrolytes have shown potential towards safer batteries with minimal detrimental effect on cycling and, in some cases, even enhanced performance. This article reviews the state-of-the-art in non-flammable liquid electrolytes for Li-, Na- and K-ion batteries. It provides the reader with an overview of carbonate, ether and phosphate-based organic electrolytes, co-solvated electrolytes and electrolytes with flame-retardant additives as well as highly concentrated and locally highly concentrated electrolytes, ionic liquids and inorganic electrolytes. Furthermore, the functionality and purpose of the components present in typical non-flammable mixtures are discussed. Moreover, many non-flammable liquid electrolytes are shown to offer improved cycling stability and rate capability compared to conventional flammable liquid electrolytes.
1. Introduction
Lithium-ion batteries (LIBs) have achieved widespread application in portable electronics and have demonstrated great potential in many other uses, particularly in the electric vehicle (EV) market.1,2 Sodium and potassium-ion batteries (SIBs, KIBs) are currently being explored, and are mostly based on analogous materials to LIBs.3,4 These new battery chemistries show great promise for cost-effective and more sustainable stationary energy storage. One major shortcoming of alkali metal-ion batteries is the flammability of currently used organic liquid electrolytes. These batteries are subject to catastrophic ‘thermal runaway’ events if they experience a deviation from their metastable state. Such an event could be caused, for example, by a short circuit, mechanical abuse, or overcharging, and lead to thermal ignition of the carbonate-based electrolyte.5,6 Also, layered oxides cathode materials, such as the state-of-the-art LiNi0.8Mn0.1Co0.1O2 (NMC811), can contribute significantly to the onset of thermal runaway. The oxygen and heat released due to phase transformations of the layered oxides can cause further reactions with the electrolyte and the anode, leading to tremendous heat generation and thereby increasing the risk of fire in the battery.7 Overall, battery safety is a complex issue, but one of the key factors on the road towards safe battery cells is to design non-flammable electrolytes. The main research focus for non-flammable electrolytes currently deals with how to enhance safety without compromising electrochemical performance.There are several promising strategies to develop non-flammable liquid electrolytes, such as incorporating non-flammable (co-)solvents or flame-retardant additives into the electrolyte, use of ionic liquids, or by opting for high salt to solvent ratios.8–10 Moreover, the introduction of gel/polymer electrolytes or inorganic ceramic/glass electrolytes reduces or even eliminates the flammable liquid component.11–14 However, fully solid-state cells suffer from some other issues, such as poor ionic conductivity and wettability, high costs and challenges with upscaling.15 Such topics are beyond the scope of this review and thus not included here. Herein, we summarize state-of-the-art developments on non-flammable organic liquid electrolytes.
Although international industry standards for testing flammability do exist (such as EN-ISO 2719:2016 and ISO 9038:2021), it should be emphasized that such widely-accepted experimental standards are rarely used to report on non-flammability in literature. The definitions and quantifications of flammability in terms of key metrics such as flashpoint and self-extinguishing time (SET) vary and make interpretation of non-flammability sometimes difficult.16 According to ISO 2719:2016, the flashpoint of a liquid can be determined by means of a Pensky-Martens Closed Cup Flash test. The flashpoint can be used as a rough guideline for non-flammability, but it does not give the full description. For example, from two liquids with similar flash points, one might continue burning after initial ignition, whilst the other does not. Therefore, an additional test is recommended to determine if a (flammable) liquid produces sufficient flammable vapor to continuously ignite even when the ignition source is removed. In the international standard ISO 9038:2021, a pass/fail methodology is described in which a 2.0 mL test sample is maintained at a specified test temperature (at temperatures up to 100 °C) and exposed to an ignition source for 15 s. The electrolyte is spontaneously combustible if it ignites without exposing the ignition source or sustains combustion if it remains burning for more than 15 s. Application of these industry standards, with minor modifications to fulfill lab conditions, could lead to more easily interpretable non-flammability results. A standardized way of addressing non-flammability in electrolytes would be highly desired, and is something for the research community of this field to consider. It is also worth mentioning that even electrolytes which are described as non-flammable in this review paper, might still catch fire under certain conditions (for example, if vapor pressure builds up in a sealed battery with limited available volume).
This review is an exhaustive account of studies within the relatively undeveloped, but increasingly important research field of non-flammable electrolytes, excluding solid or water-based electrolytes, which exhibit their own unique challenges. The studies included in this review are categorized based on the strategy used to obtain non-flammability of the electrolyte: (1) non-flammable solvent or co-solvent, (2) flame retardant additive, (3) highly concentrated electrolytes, (4) locally highly concentrated electrolytes, (5) ionic liquids, and (6) inorganic electrolytes. A summary of the non-flammable electrolytes discussed here is provided in Table 1.
| Approach | Electrolyte | Solv. ratio (vol) | Battery type | Cutoff volt. (V) | Dis. cap. (mA h g−1) | Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Non-flammable or flame-retardant solvents | 1.0 M LiPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FEMC + VC FEMC + VC |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2 wt% 1 + 2 wt% |
LiNi0.6Co0.2Mn0.2O2|graphite full cell | 2.5–4.5 | 186 | 0.1C | 25 |
| 0.95 M LiFSI/FEMC + TFEP | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Graphite|Li half-cell | 0.01–2.5 | 350 | 0.05C | 26 | |
| LiNi1/3Mn1/3Co1/3O2|Li half-cell | 3.0–4.3 | 150 | 0.1C | ||||
| LiNi0.5Mn1.5O4|Li half-cell | 3.5–4.9 | 120 | 0.1C | ||||
| 0.5 M NaBOB/TMP | 1 | Prussian white|hard carbon | 1.0–3.8 | 130 | 30 mA g−1 | 28 | |
| 1.0 M LiPF6/TMP + FEPE | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
LiNi0.5Mn1.5O4|Li4Ti5O12 full cell | 2.0–3.5 | 140.3 | 1C | 30 | |
| 1.5 M NaPF6/TMP + FEPE + FEC | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2 wt% 1 + 2 wt% |
NaNi1/3Fe1/3Mn1/3O2|Na half cell | 2.0–3.8 | 129.9 | 1C | 31 | |
| NaNi1/3Fe1/3Mn1/3O2|hard carbon full cell | 1.5–3.8 | 0.65 A h | 1C | ||||
| 3.0 M LiTFSI/TEP | 1 | LiNi0.8Co0.1Mn0.1O2|Li half cell | 2.8–4.3 | 150 | 0.2C | 32 | |
| 1.0 M LiTFSI/TEP + VC | 1 + 2 wt% | LiFePO4|Li half cell | 2.5–3.7 | 139.87 | 0.2C | ||
| 1.0 M LiTFSI/TEP + FEC | 1+ 2 wt% | Li|Li4Ti5O12 half cell | 1.0–2.5 | 163 | 0.2C | ||
| 2.0 M KFSI/TEP | 1 | PTCDA|K half cell | 1.5–3.5 | 175 | 0.2C | 23 | |
| K|graphite half cell | 0.01–2.0 | 275 | 0.2C | ||||
| PTCDA|potassiated graphite full cell | 0.8–2.8 | 127 | 0.2C | ||||
| 0.9 M NaFSI/TFP | 1 | Na|hard carbon half cell | 0–2 | 238 | 20 mA g−1 | 33 | |
| NaV2(PO4)3|Na half cell | 2.3–4 | 110.3 | 24 mA g−1 | ||||
| NaV2(PO4)3|hard carbon full cell | 2–3.4 | 221.5 | 20 mA g−1 | ||||
| 1.0 M NaBF4 in tetraglyme | 1 | M-Na2Fe2(CN)6.2H2O|graphite full cell | 2.0–3.7 | 68 W h kg−1 | 0.22C | 34 | |
| R-Na2Fe2(CN)6|graphite full cell | 2.0–3.7 | 79 W h kg−1 | 0.25C | ||||
| R-Na2Fe2(CN)6|Na2Ti3O7 → Na3-xTi3O7 | 2.0–3.7 | 88 W h kg−1 | 0.67C | ||||
| 0.8 M LiPF6/DMMP + FEC | 1 + 10 wt% | Li|SiO–C half cell | 0.01–1.5 | 1825 | 100 mA g−1 | 19 | |
| LiFePO4|Li half cell | 3.0–4.0 | 123 | 40 mA g−1 | ||||
| LiFePO4|SiO–C full cell | 2.0–3.48 | ∼800 | 100 mA g−1 | ||||
| 1.0 M LiClO4/DMMP + Cl-EC | 1 + 10 wt% | LiCoO2|graphite full cell | 2.8–4.3 | 450 | 0.2C | 57 | |
| Non-flammable co-solvents | 1.0 M LiPF6/PC + DFDEC | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Li1.13Mn0.463Ni0.203Co0.203O2|Li half-cell | 2.0–5.0 | 280 | 0.2C | 42 |
| 1.0 M LiPF6/PC + DFDEC + FEC | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 + 1 wt% 7 + 1 wt% |
Li1.13Mn0.463Ni0.203Co0.203O2|graphite full cell | 2.5–4.85 | 255 | 0.2C | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M LiPF6/FEC + FEMC + TTFE M LiPF6/FEC + FEMC + TTFE |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
LiNi0.8Mn0.1Co0.1O2|Li half cell | 2.7–4.4 | 200 | 0.5C | 41 | |
| LiCoPO4|Li half cell | 3.5–5.0 | 120 | 1C | ||||
| 1.0 M LiBETI/MFE + EMC | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
LiCoO2|graphite | 2.8–4.2 | 1400 mA h | 0.1C | 42 | |
| 0.8 M LiTFSI/G2E + MFE + FEC | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 + 5 wt% 4 + 5 wt% |
LiFePO4|graphite full cell | 2.5–4.2 | 129 | 0.2C | 43 | |
| 1.0 M LiPF6/FEC + DMC + EMC + HFPM | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
LiNi0.5Mn1.5O4|Li half cell | 3.0–5.0 | 128.9 | 40 mA g−1 | 44 | |
| Li|MCMB half cell | 0–2.0 | 352.6 | |||||
| LiNi0.5Mn1.5O4|MCMB graphite full cell | 3.5–4.9 | 1.176 A h | 0.5C | ||||
| Non-flammable additives (≤10%) | 1.0 M LiPF6/DMC + EMC/PFPN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 5 wt% 1 5 wt% |
LiCoO2|Li half cell | 3.0–4.3 | 150.7 | 0.1C | 46 |
| 1.0 M LiPF6/EC + DEC + DMC/PFN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 5 wt% 1 + 5 wt% |
LiNi0.5Mn1.5O4|graphite full cell | 3.5–4.9 | 114.2 | 1C | 49 | |
| 1.0 M NaPF6/EC + DEC/EFPN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 5 wt% 1 + 5 wt% |
Na0.44MnO2|Na half cell | 2.0–4.0 | 110 | 20 mA g−1 | 54 | |
| Na|AB half cell | 0.01–3.0 | 94 | |||||
| 1.0 M LiPF6 GBL/PFPN + LiODFB | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 + 2 wt% 4 + 2 wt% |
NMC532|graphite full cell | 2.5–4.3 | 139.4 | 1C | 48 | |
| 1.0 M LiPF6/EC + DMC + EEEP | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 + 5 wt% 7 + 5 wt% |
LiCoO2|Li half cell | 3.0–4.4 | 166.1 | 40 mA g−1 | 56 | |
| Highly concentrated electrolytes (>1 M) | 2.0 M LiPF6 PC + EC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
LiFePO4|graphite full cell | 2.2–4.1 | 360 | 0.05 | 62 |
| 2.3 M LiTFSI EC + DME | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NCM622|graphite full cell | 2.75–4.2 | 169.3 | 0.5 | 59 | |
| 3.3 M NaFSI/TMP | 1 | Na|hard carbon half cell | 0.01–2.5 | 250 | 1.0C | 63 | |
| 1 | Na3V2(PO4)3|hard carbon full cell | 1.8–3.5 | 250 | 1.0C | |||
| 5.3 M LiFSI/TMP | 1 | Li|graphite half cell | 0.01–2.5 | 372 | 1.0C | ||
| 1 | LiNi0.5Mn1.5O4|graphite full cell | 3.5–4.8 | 147 | 1.0C | |||
| Locally highly concentrated electrolytes | 1.0 M LiFSI/OFE + DME | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Li|S | 1.0–3.0 | 775 | 100 mA g−1 | 65 |
| 1.2 M LiFSI TEP/BFTE | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
LiNi0.6Mn0.2Co0.2O2|Li half cell | 2.8–4.4 | 190 | 1C | 66 | |
| Ionic liquids | NaCl-buffered AlCl3/EMImCl | 1 | Na|NVP | 2.7–3.7 | 92 | 25 mA g−1 | 75 |
| Na|NVPF | 2.5–4.25 | 115 | 50 mA g−1 | ||||
| Dicationic IL 1.0 M LiPF6/EC + DMC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NMC111|graphite full cell | 3.0–4.0 | 150 | 10 mA g−1 | 73 | |
| NaFSI/KFSI | 56 mol%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 mol% 44 mol% |
Na|NaCrO2 | 2.5–3.5 | 77.3 | 15 mA g−1 | 76 | |
| Inorganic liquid electrolytes | LiAlCl4·3SO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
LFP|Li half cells | 0–2 | 148 | 1C | 79 |
| LiAlCl4·3SO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Li|graphite half cell | 0.005–2.0 | 350.7 | 0.5C | 80 | |
| NaI·3.3NH3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 |
Na|Al/C | 0.05–1.0 | N/A | 0.01 A cm−2 | 81 | |
| NaBF4·2.5NH3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
Na|Al/C | |||||
| NaBF4·2.5NH3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
Na|stainless steel |
2. Non-flammability strategies
2.1. Non-flammable or flame-retardant solvents
An effective strategy to develop non-flammable electrolytes is to use flame retarding components in the electrolyte, either by completely substituting the flammable solvent or in the form of co-solvents. Fluorinated and/or phosphorous containing (co-) solvents can suppress the flammability of the flammable components of the electrolyte by means of radical quenching.17 Common carbonate-based solvents will produce hydrogen radicals upon heating, which will further react with oxygen to produce oxygen free radicals. This triggers the generation of more free radicals, eventually leading to a self-sustaining fire. An effective way of terminating this radical formation chain is by introducing hydrogen or oxygen radical scavengers. It is generally accepted that fluorinated or phosphorus-containing materials effectively act as radical scavengers when the electrolyte breaks down. The fluorine and phosphorus radicals, part of the electrolyte decomposition products, can react with hydrogen radicals and inhibit the radical linear chain reaction, which suppresses the combustion of the electrolyte solvent.18,19 It is important to note that flame retardants can still burn if their flame quenching properties are overloaded. Thus, in order to completely remove flammability from electrolytes, the solvents must be completely incombustible. The electrolytes which are discussed here do have flash points, and are therefore not guaranteed to work in all conditions. For instance, when the operating temperature reaches the flash point of the electrolyte, the material might still be prone to ignite.12 It is of utmost importance to carefully consider the battery chemistry and operating conditions which will influence the electrolyte performance.Phosphorous containing organic compounds are the most common class of flame retardants, well-known for a variety of different applications. They offer good thermal stability, low toxicity and low volatility.20 Several formulations of non-flammable electrolytes using alkyl phosphates, phosphazenes, and fluorinated phosphate-based organic solvents have been demonstrated to perform well not only in LIBs, but also in SIBs and KIBs.16,21–23
Trimethyl phosphate (TMP), triethyl phosphate (TEP) and tripropyl phosphate (TPrP) have received marked attention as flame-retardant electrolyte components. Xu et al. reported the flame-retarding properties of these alkyl phosphates when used in non-flammable electrolytes for LIBs.16 In this work Xu et al. described several important findings. Firstly, it was found that even though TEP and TMP reduced SET times even at low concentrations, amounts of 40 vol% were needed to approach non-flammability. Secondly, although the alkyl-phosphates had good oxidative stability they were not compatible with graphite and caused inferior cycling.
Zeng et al. first reported the use of TMP with 10 wt% of FEC in SIBs with a Sb-based anode and NaNi0.35Mn0.35Fe0.3O2 cathode.24 The use of FEC additive enabled the alkyl phosphate to provide stable stripping and plating of sodium and provided performance on-par with EC:DEC in terms of cyclability and ionic conductivity. The phosphate-based electrolytes seem to be promising in terms of non-flammability, but suffer from long-term stability issues in batteries with carbonaceous anodes, most likely due to unstable SEI formation.16
Chung et al. modified a 1.0 M LiPF6 and EC:EMC-based electrolyte system, by substituting a hydrogen atom with a fluorinated methyl group in the EMC molecule, resulting in 1.0 M LiPF6 in EC:FEMC.25 The electrolyte not only suppressed flammability, but was also shown to be highly effective in terms of improving the cycling performance of graphite//Li-Ni0.6Co0.2Mn0.2O2 cells. With addition of the commonly used vinylene carbonate (VC) additive, the full cell even outperformed conventional electrolytes with VC (186 mA h g−1, 11% increase) and remained non-flammable (Fig. 1). The underlying mechanisms and fundamental understanding of why this fluorinated 1.0 M LiPF6 electrolyte outperforms the conventional LiPF6 based electrolyte remains unanswered. The study of possible degradation mechanism and gas evolution in this system are interesting directions for further research to gain a better understanding of this promising performance in graphite//NMC622 full cells.
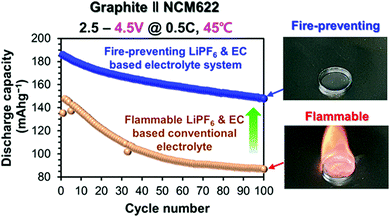 | ||
| Fig. 1 Discharge capacity vs. cycle number for conventional electrolyte and non-flammable electrolyte. Reproduced from Chung et al.,25 with permission from American Chemical Society. | ||
The aforementioned novel electrolyte uses the most common lithium salt, LiPF6. However, lithium bis(fluorosulfonyl)imide (LiFSI) salt has recently been used in a fluorinated cyclic phosphate-based solvent, 2-(2,2,2-trifluoroethoxy)-1,3,2-dioxaphospholane 2-oxide (TFEP) mixed with a low-viscosity carbonate-based solvent, FEMC.26 The results showed that 0.95 M LiFSI in TFEP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FEMC (1
FEMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) exhibits non-flammability during flame tests, whereas the electrolyte 0.98 M LiFSI in EMC immediately catches fire on ignition. Furthermore, a detailed ring-opening mechanism for TFEP was proposed, suggesting the formation of a cathode passivation layer that prevents transition metal dissolution from LiNi1/3Mn1/3Co1/3O2.26 The novel electrolyte exhibited excellent capacity retention and thermal stability in graphite, LiNi1/3Mn1/3Co1/3O2, and LiNi0.5Mn1.5O4 half-cells when compared to conventional electrolytes.26,27 The only apparent drawback in terms of performance of this formulation was the high viscosity (6.2 mPa s) and low ionic conductivity of (2.19 mS cm−1).
3) exhibits non-flammability during flame tests, whereas the electrolyte 0.98 M LiFSI in EMC immediately catches fire on ignition. Furthermore, a detailed ring-opening mechanism for TFEP was proposed, suggesting the formation of a cathode passivation layer that prevents transition metal dissolution from LiNi1/3Mn1/3Co1/3O2.26 The novel electrolyte exhibited excellent capacity retention and thermal stability in graphite, LiNi1/3Mn1/3Co1/3O2, and LiNi0.5Mn1.5O4 half-cells when compared to conventional electrolytes.26,27 The only apparent drawback in terms of performance of this formulation was the high viscosity (6.2 mPa s) and low ionic conductivity of (2.19 mS cm−1).
Recently, Mogensen et al. showed for the first time the solubility of sodium bis(oxalato)borate (NaBOB) salt in TMP.28 The electrolyte 0.5 M NaBOB in TMP was demonstrated to be non-flammable while providing reasonable ionic conductivity. This enabled relatively high coulombic efficiencies in full-cell SIBs with a hard carbon anode and Prussian white cathode (Fig. 2). However, due to the high viscosity of TMP the conventional electrolytes still perform better in terms of ionic conductivity.
 | ||
| Fig. 2 (a) Ionic conductivity in solutions containing NaBOB and NaPF6 dissolved in TMP. (b) Flame tests for 0.5 M NaBOB in TMP (left) electrolyte and PC solvent (right). Modified from Mogensen et al.,28 with permission from American Chemical Society. | ||
This motivated the research to enhance the ionic conductivity of NaBOB-TMP electrolytes. In a follow-up study it has been demonstrated that NaBOB-TMP electrolytes remain non-flammable by the addition of N-methyl-2-pyrrolidone (NMP) up to 60 vol%, whilst increasing the ionic conductivity from 4.5 to 7.4 mS cm−1.29 The NaBOB-TMP based electrolyte is promising in terms of compatibility in full cells, low costs and environmentally friendliness, but can even be further improved if long-term stability is obtained.
To achieve better separator wettability of TMP, which has high viscosity, Zheng et al. took the approach of co-solvating the non-flammable electrolyte with 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (FEPE) and tested it in LiNi0.5Mn1.5O4//Li4Ti5O12 full cells.30 Three different volume ratios of FEPE in TMP with 1.0 M LiPF6 were investigated. It was found that 20 wt% FEPE in TMP showed excellent rate performance with reduced polarization and improved oxidation stability, retaining the flame-retardant properties of the individual solvents. Although these results are promising it would be interesting for future research to understand the electrolyte compatibility with graphite (rather than with the high voltage anode LTO), i.e. stability at low potentials.
Yu et al. also showed the application of such a non-flammable electrolyte for SIBs, which was composed of 1.5 M NaPF6 in TMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FEPE (2
FEPE (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) along with 2 wt% of FEC.31 This electrolyte was tested in NFM//HC full cells and showed promising capacity and capacity retention (129.9 mA h g−1, 70.8% retention after 500 cycles). The addition of FEPE not only results in enhanced wettability, but also decreases the conductivity of the electrolyte due to solubility power of FEPE. The electrochemical performance and good separator wettability are noteworthy, but since this electrolyte consists of rather high salt concentration and low conductivity, further research should aim to enhance the conductivity.
1 v/v) along with 2 wt% of FEC.31 This electrolyte was tested in NFM//HC full cells and showed promising capacity and capacity retention (129.9 mA h g−1, 70.8% retention after 500 cycles). The addition of FEPE not only results in enhanced wettability, but also decreases the conductivity of the electrolyte due to solubility power of FEPE. The electrochemical performance and good separator wettability are noteworthy, but since this electrolyte consists of rather high salt concentration and low conductivity, further research should aim to enhance the conductivity.
Triethyl phosphate (TEP) is another non-flammable phosphate-based electrolyte which has been investigated with 2 wt% of VC or FEC additives.32 In another study, TEP was used as the main solvent in a non-flammable electrolyte for KIBs and compared to a conventional electrolyte 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC (vol% 1
DEC (vol% 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The investigated electrolyte, 2.0 M potassium bis(fluorosulfonyl)imide (KFSI) salt in TEP, showed superior electrochemical performance for various half- and full-cell KIBs when compared with conventional carbonate-based electrolyte (Fig. 3).23
1). The investigated electrolyte, 2.0 M potassium bis(fluorosulfonyl)imide (KFSI) salt in TEP, showed superior electrochemical performance for various half- and full-cell KIBs when compared with conventional carbonate-based electrolyte (Fig. 3).23
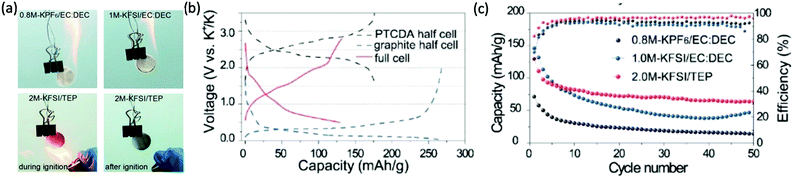 | ||
| Fig. 3 (a) Flame tests of glass fiber separators soaked with 0.8 M KPF6 in EC:DEC, 1 M KFSI in EC:DEC, and 2 M KFSI in TEP electrolytes. (b) Charge–discharge curves of a 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) half-cell, graphite half-cell, and PTCDA//graphite full-cell. (c) Cycling performances and coulombic efficiencies of 0.8 M KPF6 in EC:DEC, 1.0 M KFSI in EC:DEC, and 2 M KFSI in TEP in PTCDA//graphite full cells. Reproduced from Liu et al.,23 with permission from John Wiley and Sons. | ||
One of the issues with TEP-based electrolytes is the chemical stability against Na metal. However, the fluorinated version of TEP, tris(2,2,2-trifluoroethyl)phosphate (TFP), has been demonstrated to be non-reactive against Na metal.33 Also, ignition tests revealed excellent flame-retardant properties of TFP. However, to enhance its performance for high power applications, the conductivity should be significantly improved (0.43 mS cm−1).
Besides the phosphate-based electrolytes discussed above, an ether-based electrolyte of 1.0 M NaBF4 in tetraglyme has been demonstrated as non-flammable.34 This is quite surprising given that ethers are known to have low flash points. The thermal safety of 1.0 M NaBF4 in tetraglyme stems from the high flash point of the solvent tetraglyme (141 °C), which is significantly higher than the flashpoint of diglyme (57 °C).34 The glyme-based electrolyte was shown to be not only non-flammable, but also exhibited promising capacity retention in Na2Fe2(CN)6·2H2O//graphite full cells although the conductivity was very low (1.3 mS cm−1).34
Fluorinated and phosphate-based electrolytes are a promising route towards safer battery electrolytes, because of their non-flammable capabilities. However, sustainability issues (environmentally benignity) of fluorinated compounds and long-term stability of phosphate-based electrolytes are to be considered when these electrolytes are developed/investigated.
2.2. Non-flammable Co-solvents
Since the commercialization of the LiCoO2//C rocking-chair cell by Sony Corporation in 1991, non-aqueous electrolyte systems based on organic carbonate solvents and PF6− salt proved to be the most interesting when designing any rechargeable battery technology.35 This is evident in the development of electrolytes for SIBs, which builds on the more than 30 years’ experience in advancement of LIB electrolytes.36,37 Ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) are some of the most commonly-used organic solvents in LIB electrolytes, all of which display high flammability.38 Due to their inherent flammability, it is challenging to base a non-flammable electrolyte on existing carbonate solvents. However, some investigations demonstrate suppressed flammability through co-solvating flammable carbonate-based solvents with flame-retardant solvents. The co-solvents that are most widely applied nowadays consist of fluorinated compounds. The flame-retardant mechanism of these co-solvents can be mainly ascribed to the radical scavenging ability of fluorine.Pham et al. introduced a non-flammable carbonate-based organic liquid electrolyte comprising 1.0 M LiPF6 in various ratios of PC and di-(2,2,2 trifluoroethyl)carbonate (DFDEC).39 The authors demonstrated flammability tests where electrolytes containing more than 60% v/v DFDEC did not catch fire, confirming its flame-retardant properties. Furthermore, the non-flammable electrolyte with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v PC
7 v/v PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DFDEC and 1 wt% fluoroethylene carbonate (FEC) outperformed a conventional electrolyte in Li1.13Mn0.463Ni0.203Co0.203O2//graphite cells. A further study demonstrated the improved capacity retention of NMC811//Li cells through use of another non-flammable electrolyte comprising 1.0 M LiPF6 in PC
DFDEC and 1 wt% fluoroethylene carbonate (FEC) outperformed a conventional electrolyte in Li1.13Mn0.463Ni0.203Co0.203O2//graphite cells. A further study demonstrated the improved capacity retention of NMC811//Li cells through use of another non-flammable electrolyte comprising 1.0 M LiPF6 in PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methyl(2,2,2-trifluoroethyl)carbonate (FEMC)
methyl(2,2,2-trifluoroethyl)carbonate (FEMC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DFDEC in a 3
DFDEC in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 volume ratio.40Fig. 4a and b show flammability tests of a conventional flammable electrolyte and of this electrolyte, while Fig. 4c–e present their cycling performance. It should be noted that although the DFDEC certainly provides mitigation of flammability as a co-solvent, part of the effect stems from removing the highly volatile carbonate EMC and instead using PC with much lower flammability. Secondly, it is again worth mentioning that in the two examples ionic conductivity was lower than 3.4 mS cm−1 and although very good rate performance was shown the mass-loading of 3 mg cm−1 is far from practical.
5 volume ratio.40Fig. 4a and b show flammability tests of a conventional flammable electrolyte and of this electrolyte, while Fig. 4c–e present their cycling performance. It should be noted that although the DFDEC certainly provides mitigation of flammability as a co-solvent, part of the effect stems from removing the highly volatile carbonate EMC and instead using PC with much lower flammability. Secondly, it is again worth mentioning that in the two examples ionic conductivity was lower than 3.4 mS cm−1 and although very good rate performance was shown the mass-loading of 3 mg cm−1 is far from practical.
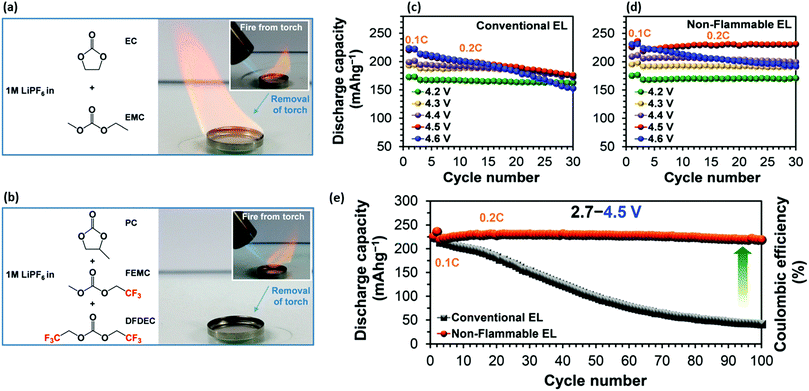 | ||
| Fig. 4 (a) Chemical structure of conventional electrolyte, 1.0 M LiPF6/EC:EC along with the photograph of flammability test during and after exposure to a torch. (b) Chemical structure of non-flammable electrolyte, 1.0 M LiPF6/PC:FEMC:DFDEC, along with the photograph of flammability test during and after exposure to a torch. (c) Discharge capacity at different charge cut-off voltages of Li//LiNi0.8Co0.1Mn0.1O2 half-cells with (c) conventional electrolyte and (d) non-flammable electrolyte. (e) Long-term cycling performance of the half-cells between 2.7 and 4.5 V. Reproduced from Pham et al.,40 with permission from Royal Society of Chemistry. | ||
Unlike the previous example where FEC was used as an additive, Fan et al. formulated an electrolyte for high voltage/high capacity by co-solvating FEC with FEMC and 1,1,2,2-tetrafluoroethyl-2′,2′,2′-trifluoroethyl ether (TTFE) solvents and LiPF6.41 The flammability of 1.0 M LiPF6 dissolved in FEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FEMC
FEMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TTFE (2
TTFE (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by weight) was compared with 1.0 M LiPF6 in EC
2 by weight) was compared with 1.0 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (2
DMC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 by weight) as well as in FEC
8 by weight) as well as in FEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (2
DMC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 by weight), where it was found that the carbonate–ether mixture displayed fire-retardant properties. The non-flammability was attributed to the fluorine substitution on the alkyl moiety, serving as an inhibitor of oxygen radical propagation.41 Furthermore, they reported excellent capacity retention and cycling stability even at high voltage (3.5 to 5.0 V) in LiCoPO4//Li half-cells. Although this electrolyte shows promising electrochemical results in Li half cells, it should be further investigated in full cells with graphite.
8 by weight), where it was found that the carbonate–ether mixture displayed fire-retardant properties. The non-flammability was attributed to the fluorine substitution on the alkyl moiety, serving as an inhibitor of oxygen radical propagation.41 Furthermore, they reported excellent capacity retention and cycling stability even at high voltage (3.5 to 5.0 V) in LiCoPO4//Li half-cells. Although this electrolyte shows promising electrochemical results in Li half cells, it should be further investigated in full cells with graphite.
While ethers are generally highly flammable solvents, fluorinated ethers can be non-flammable and are therefore explored as components in non-flammable electrolytes. Arai et al. explored a novel fluorinated ether, methyl/ethyl nonafluorobutyl ether (MFE/EFE), as co-solvent in a non-flammable electrolyte for LIBs. For demonstration, a LiCoO2//graphite 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cell using the 1.0 M lithium bispentafluoroethylsulfonyl imide (LiBETI) in MFE
650 cell using the 1.0 M lithium bispentafluoroethylsulfonyl imide (LiBETI) in MFE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC (80
EMC (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) electrolyte was fabricated.42 Nail penetration tests of the cells containing MFE or EFE-based electrolytes demonstrated their non-flammability. The fire retarding ability of the fluorinated compound is suggested to be contingent on the ratio of fluorine atoms to hydrogen atoms (F/H ratio) in the chemical structure. A stronger fire retarding ability of MFE compared to EFE was attributed to the higher F/H ratio. In electrochemical testing at a rate of 0.1C, the cycling was shown to be stable up to 30 cycles. It is promising to see that this electrolyte works in a full cell; however, long-term cycling stability is still lacking. Further research is required to enhance the long-term electrochemical performance.
20) electrolyte was fabricated.42 Nail penetration tests of the cells containing MFE or EFE-based electrolytes demonstrated their non-flammability. The fire retarding ability of the fluorinated compound is suggested to be contingent on the ratio of fluorine atoms to hydrogen atoms (F/H ratio) in the chemical structure. A stronger fire retarding ability of MFE compared to EFE was attributed to the higher F/H ratio. In electrochemical testing at a rate of 0.1C, the cycling was shown to be stable up to 30 cycles. It is promising to see that this electrolyte works in a full cell; however, long-term cycling stability is still lacking. Further research is required to enhance the long-term electrochemical performance.
Later, Fang et al. developed a non-flammable electrolyte containing the aforementioned MFE as a co-solvent.43 In their work LiFePO4//graphite full cells with 0.8 M LiTFSI in diethylene glycol diethyl ether (G2E), MFE and FEC (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 wt%) showed promising electrochemical performance close to cells with conventional electrolytes (1.0 M LiPF6–EC
5 wt%) showed promising electrochemical performance close to cells with conventional electrolytes (1.0 M LiPF6–EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC
DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC) at room temperature, but even at elevated and low temperatures. The capacity retention at −20 °C was about 46% after 200 cycles (62 mA h g−1). However, the conductivity of this electrolyte at room temperature is 3.8 mS cm−1, which is not within the range of commercial electrolytes and might not be sufficient for fast charging applications. In general, lithium salts show poor solubility in fluorinated ethers. However, by co-solvating with ethers and carbonates, one can increase the solubility.42
DEC) at room temperature, but even at elevated and low temperatures. The capacity retention at −20 °C was about 46% after 200 cycles (62 mA h g−1). However, the conductivity of this electrolyte at room temperature is 3.8 mS cm−1, which is not within the range of commercial electrolytes and might not be sufficient for fast charging applications. In general, lithium salts show poor solubility in fluorinated ethers. However, by co-solvating with ethers and carbonates, one can increase the solubility.42
An electrolyte consisting of 1.0 M LiPF6 in FEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC
DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC
EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,1,1,3,3,3-hexafluoroisopropyl methyl ether (HFPM) (2
1,1,1,3,3,3-hexafluoroisopropyl methyl ether (HFPM) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 vol%), was found to be non-flammable, exhibited remarkably high electrochemical stability (up to 5.5 V) and excellent electrochemical cycling in LiNi0.5Mn1.5O4//mesocarbon microbeads (MCMB) graphite full cells.44 Excellent electrochemical cycling stability was demonstrated also for LiNi0.5Mn1.5O4 half-cells against Li, when compared with conventional electrolytes (see Fig. 5).
4 vol%), was found to be non-flammable, exhibited remarkably high electrochemical stability (up to 5.5 V) and excellent electrochemical cycling in LiNi0.5Mn1.5O4//mesocarbon microbeads (MCMB) graphite full cells.44 Excellent electrochemical cycling stability was demonstrated also for LiNi0.5Mn1.5O4 half-cells against Li, when compared with conventional electrolytes (see Fig. 5).
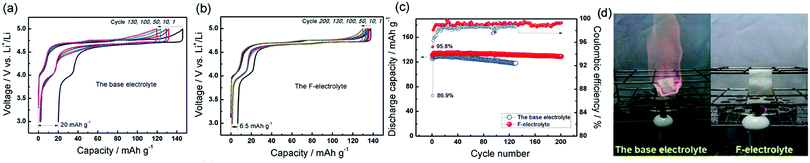 | ||
Fig. 5 Comparison of the conventional base electrolyte (1.0 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC) (3 DMC) (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, by volume) and fluorinated electrolyte ‘F-electrolyte’ (1.0 M LiPF6 in FEC 7, by volume) and fluorinated electrolyte ‘F-electrolyte’ (1.0 M LiPF6 in FEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HFPM), (2 HFPM), (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, by volume). (a) Li//LiNi0.5Mn1.5O4 half-cell galvanostatic charge–discharge curves in the base electrolyte at 40 mA g−1 rate. (b) Galvanostatic charge–discharge curves of a Li//LiNi0.5Mn1.5O4 half-cell with F-electrolyte cycled at 40 mA g−1 rate. (c) Discharge capacity and coulombic efficiency versus cycle number for a Li//LiNi0.5Mn1.5O4 half-cell cycled between 3.0 and 5.0 V in the base as well as F-electrolyte. (d) Ignition test. Modified from Xia et al.,44 with permission from John Wiley and Sons. 4, by volume). (a) Li//LiNi0.5Mn1.5O4 half-cell galvanostatic charge–discharge curves in the base electrolyte at 40 mA g−1 rate. (b) Galvanostatic charge–discharge curves of a Li//LiNi0.5Mn1.5O4 half-cell with F-electrolyte cycled at 40 mA g−1 rate. (c) Discharge capacity and coulombic efficiency versus cycle number for a Li//LiNi0.5Mn1.5O4 half-cell cycled between 3.0 and 5.0 V in the base as well as F-electrolyte. (d) Ignition test. Modified from Xia et al.,44 with permission from John Wiley and Sons. | ||
Although the fluorine containing additives show promising electrochemical and safety performance, they are currently associated negatively with environment, toxicity and high costs. This highly motivates researchers to find fluorine-free alternatives as non-flammable co-solvents.
2.3. Non-flammable additives (fire retardant or completely non-flammable) (≤10%)
Flame retardants as electrolyte additives have shown to be effective in making electrolytes non-flammable. Flame retardants provide improved thermal stability due to their higher flash point and enhanced non-flammability due to radical quenching. In Fig. 6 an overview is shown of the additives discussed here. The concentration of flame retardants is often required to be as high as 20% to obtain non-flammability, and therefore, this leads to an increase in the electrolyte cost and often inferior electrochemical performance. Therefore, research interest has grown to develop electrolytes with non-flammable additives in low concentrations which positively affect the non-flammability characteristics of the cell, whilst maintaining or improving cell performance. Since there is currently a lack of clear distinction between an additive and a co-solvent, here a threshold value of 10% is used, following the suggestion by Kang Xu.45 | ||
| Fig. 6 An overview of the chemical structures of the non-flammable additives discussed in this review. PFPN is also often referred to as PFN or EFPN. | ||
Recently, the additive ethoxy(pentafluoro)cyclotriphosphazene (PFPN) has gained research interest as flame-retardant. The addition of 5 wt% ethoxy(pentafluoro)cyclotriphosphazene (PFPN) can suppress flammability of 1.0 M LiPF6 in EC:DMC and showed both outstanding cycling stability as well as capacity retention in LiCoO2//Li half cells.46 During the first charge/discharge cycles the electrolyte is reduced and forms a passivation layer on the negative electrode, the solid electrolyte interphase (SEI). Ideally, this SEI is ionically conducting, electronically insulating and inhibits further decomposition of the electrolyte. In the study by Feng Wu et al. it has been argued that nitrogen and fluorine elements in PFPN can synergistically suppress the flammability and positively affect the composition and morphology of the SEI (i.e. form a more stable and dense inorganic passivation film).47 The same flame-retardant additive was investigated in a recent work of Gu et al. in an organic gamma butyrolactone (GBL) based electrolyte.48 The salt LiODFB was added as an additive to enhance electrode interfacial properties and promote cycle performance. The obtained electrolyte was a 1.0 M LiPF6 GBL/PFPN (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) + 2 wt% LiODFB showed to be non-flammable, have good conductivity (9.95 mS cm−1) and remarkable prolonged cycle performance in graphite//NMC532 full cells (85.4% capacity retention after 500 cycles). This study shows the compatibility with graphite anodes and the potential of synergistically adding additives to an organic based electrolyte.
4) + 2 wt% LiODFB showed to be non-flammable, have good conductivity (9.95 mS cm−1) and remarkable prolonged cycle performance in graphite//NMC532 full cells (85.4% capacity retention after 500 cycles). This study shows the compatibility with graphite anodes and the potential of synergistically adding additives to an organic based electrolyte.
This novel electrolyte additive (also known as PFN, and EFPN) was proven to exhibit excellent flame retardancy and even improved electrochemical performance at high voltages.49 According to the authors, the excellent flame retardancy is explained by a combination of the radical quenching of phosphorus and the lower saturated vapor pressure, which effectively inhibits the evaporation of solvent in the electrolyte, thus preventing the combustion risk in a flammable solvent. In our opinion the lowering of vapour pressure using only 5 vol% PFN is less impactful than the phosphorous–halogen synergy that amplifies the radical quenching of the additive.50 Furthermore, the nitrogen can form a protective char layer by the production of N2 and NH3 during the combustion process, which inhibits the oxygen supply.51 This synergistic effect was also observed in an extensive study of Dagger et al. in which five flame retardants were investigated in the standard 1 M LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%) electrolyte (TFP, TTFPi, TFMP, PFPN and FPPN).52 Among these five flame retardants it was shown that the fluorinated cyclophosphazenes (PFPN and FPPN) outperform the other additives (phosphates, phosphites and phosphonates) both in terms of electrolyte safety and electrochemical performance.53 Although the fluorinated cyclophosphazenes are most expensive, they are promising for future investigations. Future work on this electrolyte may include the application in larger cells and compatibility with other electrode configurations.
1 wt%) electrolyte (TFP, TTFPi, TFMP, PFPN and FPPN).52 Among these five flame retardants it was shown that the fluorinated cyclophosphazenes (PFPN and FPPN) outperform the other additives (phosphates, phosphites and phosphonates) both in terms of electrolyte safety and electrochemical performance.53 Although the fluorinated cyclophosphazenes are most expensive, they are promising for future investigations. Future work on this electrolyte may include the application in larger cells and compatibility with other electrode configurations.
An addition of 5 wt% PFN resulted in better cycling performance, rate capability, shortened Li-ion diffusion paths, decreased interfacial resistance and suppressed dissolution and corrosion in LiNi0.5Mn1.5O4//graphite full cells. The additive PFPN is also shown to be stable against sodium metal and improved the cyclability of both acetylene black anode and Na0.44MnO2 cathode.54 The reason behind improved electrochemical performance may be ascribed to the fluorine rich structure, which result in a stable inorganic SEI layer. The phosphate and phosphazene compounds make the electrolyte non-flammable due to the H radical capture mechanism.
A similar flame-retardant additive (phenoxy)pentafluorocyclotriphosphazene (FPPN) was analyzed by Dagger et al. in standard 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%) and used in MCMB graphite//NMC111 full cells.55 The additive was shown to be electrochemically stable against graphite and significantly enhanced the safety of the electrolyte. As was pointed out by the authors future work should include the influence of the additive during abuse tests (thermal, mechanical and electrical).
1 wt%) and used in MCMB graphite//NMC111 full cells.55 The additive was shown to be electrochemically stable against graphite and significantly enhanced the safety of the electrolyte. As was pointed out by the authors future work should include the influence of the additive during abuse tests (thermal, mechanical and electrical).
Zhou et al. showed that 5 wt% polybis-(ethoxyethoxyethoxy)phosphazene (EEEP) in 1.0 M LiPF6 not only significantly reduced the flammability (due to synergistic flame-retardant effect of P and N elements), but also improved cycling performance of LiCoO2 cathode when charged up to 4.4 V.56 To further investigate the practical application of this electrolyte, it would be interesting to analyse this electrolyte in full cell chemistries.
Another non-flammable additive is the relatively new fire-retardant dimethyl phosphate (DMMP).19 It has been used as an electrolyte additive along with the salt LiPF6 since 2007 and appears promising in terms of non-flammability characteristics. The additive has recently been studied in the baseline electrolyte 1.0 M LiPF6 in EC:EMC.57 The electrochemical performance was analyzed in LiCoO2//surface modified graphite (SMG) full cells and the electrolyte with 10 wt% DMMP did not adversely affect the capacity. However, it should be noted that the addition of DMMP in the electrolyte diminishes the compatibility with untreated graphite, because it could lead to graphite exfoliation.
A very promising additive combination, in terms of non-flammability characteristics, in a 1.0 M LiPF6 electrolyte with a double safety protection mechanism was studied by Jiang et al.58 The additive DMAC was added (5 vol%) to act as a Lewis base and thus reduce the attack ability of the Lewis acid (decomposition products of LiPF6 reacting with the electrolyte) and PFMP was added (10 vol%) to establish self-cooling. The safety mechanism of this electrolyte is encouraging and effective even in full cell systems, but the electrochemical performance should be improved since only 85% of the capacity of the full cell with the conventional electrolyte could be achieved.
Non-flammable electrolytes obtained by adding flame-retardants seems extremely promising in terms of facile design, low costs, and good electrochemical performance. Of course, the non-flammability characteristic of a single additive might be limited compared to co-solvation, simply because of the lower concentration of flame-retardant components. However, the balanced trade-off between electrochemical performance, enhanced safety and low costs make this a promising approach towards the next non-flammable electrolyte.
2.4. Highly concentrated electrolytes (>1 M)
High concentrations of salts in liquid battery electrolytes can provide non-flammability. This type of electrolyte, known as highly concentrated electrolytes (HCE), have recently attracted attention amongst researchers.59,60 Due to a high salt concentration, most of the solvent molecules form solvation pairs with the cation in the HCE system, which decreases the number of free solvent molecules and leads to a unique solvation structure. The solvation structure is predominantly composed of contact ion pairs and aggregates, which causes the interface reactions between solvent and electrodes to be significantly suppressed and hence reduce the flammability of the battery. The reduction in flammability is primarily based on two factors. Firstly, the volatility and vapour pressure of the solvent is significantly reduced if salt concentration reaches the levels typically used in HCE systems. Secondly, a significant amount of the electrolyte (up to circa 60 wt%) is actually non-combustible salt and thus the energy produced per mL of burning electrolyte is reduced.61 Additional benefits of highly concentrated electrolytes are the inorganic anion-derived SEI and reduced degradation of the Al current collector. Although highly concentrated electrolytes can reduce flammability and sometimes even enhance electrochemical performance, they also come with some drawbacks. The high viscosity, poor wettability and high costs will still impede their implementation in commercial LIBs or SIBs.The fire-retardant capability of such highly concentrated electrolytes was demonstrated by increasing the LiPF6 salt concentration up to 2.5 M in a PC-based electrolyte (i.e. EC/PC). The highly concentrated electrolyte was shown to have a significantly longer ignition time and shorter SET time (26.8 s and 22.2 s respectively). The electrolytes with 2 M LiPF6 did not only show suppressed flammability, but also showed superior cycling performance compared to the “standard” electrolyte of 1.0 M LiPF6 in EC/DEC in both Li//graphite half-cells and LiFePO4//graphite full-cells.62 This was ascribed to enhanced shuttling of abundant Li+ between cathode and anode.
A high concentration of 2.3 mol kg−1 LiTFSI salt in EC:DME was investigated as a non-flammable electrolyte based by Liang et al.59 The electrolyte showed excellent thermal stability and non-flammability characteristics. With Raman spectroscopy it was demonstrated that upon increase of salt concentration, the solvation number for EC and DME decreased and increased, respectively. The authors suggest that Li+ bonds with fewer EC but more DME molecules in a concentrated electrolyte, leading to improved thermal stability and non-flammability. Besides its excellent thermal stability, this electrolyte possesses electrochemical performance comparable to conventional carbonate-based electrolytes.
As earlier discussed, phosphate-based electrolytes tend to form unstable SEIs, and therefore have rather limited long-term cycling stability. However, based on the flame retarding phosphate TMP, Wang et al. reported that increasing the salt concentration preserved its flame-retarding properties and resulted in excellent cycling stability of the carbonaceous anode (Fig. 7).63 By testing different salts (sodium bis(fluorosulfonyl)imide, NaFSI, and lithium bis(fluorosulfonyl)imide, LiFSI) in varying concentrations, the group concluded that 3.3 M NaFSI in TMP and 5.3 M LiFSI in TMP offer the best performance for hard carbon and graphite, respectively. These electrolyte formulations are not only non-flammable, but also deliver superior electrochemical performance when compared with conventional dilute electrolytes, although the ionic conductivity is rather low due to high viscosity.63 NaFSI and LiFSI salts are known to have weak cation–anion interaction which offers high ion transport even in high concentrations. Remarkably, it was shown that the concentrated electrolyte formulation does not have any flashpoint, whereas many previously reported non-flammable blends of TMP still showed low flash points, responsible for the flammability of the electrolyte.6 This behaviour was explained by the contribution of dominant Na+-TMP solvation, with a low concentration of free solvent molecules.6 The concentrated electrolyte not only suppressed flammability, it also allowed charge–discharge cycling of hard carbon or graphite anodes comparable or superior to conventional flammable carbonate electrolytes. They also showed, by applying density functional theory molecular dynamics simulations that most of the TMP molecules are coordinated with Na+ and 80% of the FSI− anions are in an aggregate state. The researchers claim that this leads to a non-flammable electrolyte, because no free solvent is present. The charge–discharge test also shows an improved initial coulombic efficiency of 75%.
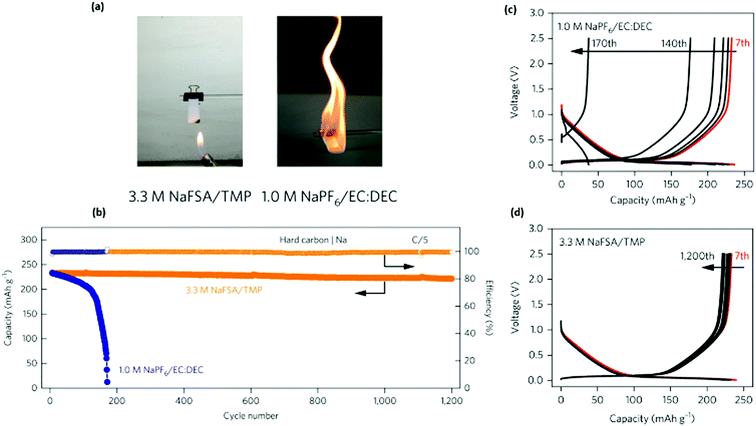 | ||
Fig. 7 (a) Flame tests of 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M NaFSI in TMP and conventional 1.0 M NaFSI in TMP and conventional 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M NaPF6 in EC M NaPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC (1 DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by vol) electrolytes. (b) Cycling performance and coulombic efficiency of the HC electrode in a half-cell using concentrated 3.3 1 by vol) electrolytes. (b) Cycling performance and coulombic efficiency of the HC electrode in a half-cell using concentrated 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M NaFSI in TMP electrolyte (orange) and conventional 1.0 M NaFSI in TMP electrolyte (orange) and conventional 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M NaPF6 in EC M NaPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC (1 DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by vol) electrolyte (blue). Charge–discharge curves (C/5 rate), for the half-cell using 1.0 1 by vol) electrolyte (blue). Charge–discharge curves (C/5 rate), for the half-cell using 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M NaPF6 in EC M NaPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC (1 DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by vol) (c) and 3.3 1 by vol) (c) and 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M NaFSI in TMP (d). Reproduced from Wang et al.,63 with permission from Nature. M NaFSI in TMP (d). Reproduced from Wang et al.,63 with permission from Nature. | ||
Researchers often prove that electrolytes are non-flammable by showing their low ignition ability, low SET times or thermogravimetric analysis. But, safety verification tests in practical batteries are often lacking. As earlier mentioned in the introduction, it is not solely the electrolyte that causes the battery to catch fire, but the reaction at the interface of the anode or cathode material is also of major importance. In a recent study by Hou et al.64 it was shown that graphite//NMC (both 811 and 532) full cells with the non-flammable highly concentrated electrolytes LiFSI in DMC and LiFSI in TMP still catch fire. It is demonstrated that the heat generated up to 250 °C is dominated by the reaction between the HCE and the anode. After the onset of thermal runaway, the highly concentrated electrolyte is combustible and the battery remains burning. This study emphasizes that the onset and propagation of thermal runaway is not determined by the flammability properties of the electrolyte alone. In this case the practical safety characteristics were demonstrated for concentrated electrolytes, but the interactions between charged electrodes and non-flammable electrolyte should always be considered when the battery safety is assessed.
2.5. Locally highly concentrated electrolytes
To overcome above mentioned challenges regarding HCEs, locally highly concentrated electrolytes (LHCE) have gained research attention. To preserve the structure of the solvated ion pairs obtained in the HCE system, it is key to add an inert diluent to the electrolyte. This means that, in contrast to previously mentioned co-solvents, it is imperative that the diluent does not itself coordinate or dissolve the salt. The coordination structure of the LHCE is similar to that of the HCE, and therefore remains non-flammable if an inert non-flammable diluent is added.A new kind of LHCE was investigated in Li–S batteries, where they applied an inert diluent with low donor ability (to reduce the shuttle effect), low permittivity, low viscosity and high wettability.65 The DME based HCE was diluted with 1H,1H,5H-octafluoropentyl-1,1,2,2-tetrafluoroethyl ether (OFE) which has the additional characteristic of non-flammability due to its high degree of fluorination. The Li–S cells containing 1.0 M LiFSI/OFE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME (OFE
DME (OFE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME 95
DME 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 vol. ratio) electrolyte showed excellent cycle performance with a capacity retention of 775 mA h g−1 at 100 mA g−1 current after 150 cycles.
5 vol. ratio) electrolyte showed excellent cycle performance with a capacity retention of 775 mA h g−1 at 100 mA g−1 current after 150 cycles.
In a study by Chen et al.66 a non-flammable localized high-concentration electrolyte containing 1.2 M LiFSI in TEP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTFE (1
BTFE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by mol) was investigated, based on their earlier studies of the flammable electrolytes NaFSI and LiFSI in DME
3 by mol) was investigated, based on their earlier studies of the flammable electrolytes NaFSI and LiFSI in DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTFE.67,68 A highly concentrated electrolyte with 3.2 M LiFSI was diluted with the electrochemically stable (but flammable) BTFE. Although their novel electrolyte formulation was diluted with the flammable BTFE, it still resisted burning (no ignition during flammability tests). Raman spectroscopy showed that BFTE had minimal effect on the solvation structure, and thus Li(TEP)1.33-FSI solvates were preserved accounting for stabilization of the solvent molecules. Compared to conventional and HCE phosphate-based non-flammable electrolytes the LHCEs seem to be promising in terms of safety, electrochemical stability and reduced costs. However, the latter only holds true if the inert diluent is significantly cheaper than the salt added. Also, adding an inert diluent which in itself is flammable, might not be the most ideal solution to obtain a non-flammable electrolyte. Although BTFE shows promising results as an inert diluent in LHCE electrolytes, it is expensive, has a high fluorination degree and is flammable, which establishes the need to investigate alternative and more environmentally friendly inert diluents.
BTFE.67,68 A highly concentrated electrolyte with 3.2 M LiFSI was diluted with the electrochemically stable (but flammable) BTFE. Although their novel electrolyte formulation was diluted with the flammable BTFE, it still resisted burning (no ignition during flammability tests). Raman spectroscopy showed that BFTE had minimal effect on the solvation structure, and thus Li(TEP)1.33-FSI solvates were preserved accounting for stabilization of the solvent molecules. Compared to conventional and HCE phosphate-based non-flammable electrolytes the LHCEs seem to be promising in terms of safety, electrochemical stability and reduced costs. However, the latter only holds true if the inert diluent is significantly cheaper than the salt added. Also, adding an inert diluent which in itself is flammable, might not be the most ideal solution to obtain a non-flammable electrolyte. Although BTFE shows promising results as an inert diluent in LHCE electrolytes, it is expensive, has a high fluorination degree and is flammable, which establishes the need to investigate alternative and more environmentally friendly inert diluents.
2.6. Ionic liquids
Generally, ionic liquids (ILs) are defined as salts (mostly organic) consisting entirely of ions below the arbitrary temperature of 100 °C. Their most promising properties are high electrochemical and thermal stability, ultralow volatility and intrinsically high ionic conductivity. Although ILs are generally accepted to be safe in terms of flammability (negligible vapor pressures), they should not be considered intrinsically non-flammable, especially when applied in conditions where heat or ignition sources are present.69 The decomposition products (ignitable gasses) formed during the thermal decomposition of some ILs are sensitive to combustion and therefore not all ILs are applicable as a non-flammable battery electrolyte. In a study performed by Arbizzani et al. it was shown that organic carbonate-based electrolytes with high IL contents are more difficult to ignite, but do burn longer (up to twice the SET time of the carbonate-based electrolyte).70 However, if the selection and design of ionic liquids is carefully made, they appear to be very promising as non-flammable battery electrolytes. One could distinguish two main classes of non-flammable ionic liquids; pristine ionic liquids and hybrid ionic liquids (ionic liquids mixed with organic liquids). The characteristic properties of ionic liquids can be finely tuned to a targeted application, for instance by varying the combination of the cations with the anions. Some challenges when employing ionic liquids are low ionic conductivity, high cost, and their rare tendency to form SEI layers. The most effective strategy to overcome the high costs is to explore and develop the usage of less expensive ions.Most of the recent IL electrolytes used in batteries are comprised of the cations ammonium, imidazolium (CnMIm), piperidinium (CnMPip), pyrrolidinium (CnMPyr), sulfonium and the anions tetrafluoroborate (BF4−), FSI−, TFSI− or triflate (CF3SO3−).71
In a characterization study by Wilken et al.72 two types of hybrid ionic liquid electrolytes, 1-ethyl-3-methylimidazolium (EMIm)FSI and EMImPF6 added in additive concentrations (≤10%) up to co-solvent concentrations, were studied in LiPF6/EC:DEC electrolytes. The composition of 2 M LiPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC
DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL (1
IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 wt%) was found to be non-flammable for both ionic liquids. This fundamental research highlights the importance of trade-off between properties, such as safety and viscosity and ionic conductivity. As follow-up to this research, it is important to investigate these electrolytes in terms of electrochemical performance.
3 wt%) was found to be non-flammable for both ionic liquids. This fundamental research highlights the importance of trade-off between properties, such as safety and viscosity and ionic conductivity. As follow-up to this research, it is important to investigate these electrolytes in terms of electrochemical performance.
Recently a novel room temperature ionic liquid with 1,1′-(5,14-dioxo-4,6,13,15-tetraazaoctadecane-1,18-diyl) bis(3-(sec-butyl)-1H-imidazol-3-ium) as cation and TFSI− as anion was synthesized and demonstrated to have suppressed flammability in a LiPF6/EC:DMC based organic liquid electrolyte.73 The electrochemical properties were tested with and without IL in NMC//graphite full cells. The electrolyte with IL additive outperformed the cell without IL in terms of (suppressed) flammability, capacity retention and coulombic efficiency. Although this electrolyte is not completely non-flammable, it has promising balanced properties in terms of safety and electrochemical performance.
A non-flammable ionic liquid based on NaCl-buffered AlCl3/EMImCl has been applied in Na metal batteries.74 To achieve a stable SEI, EtAlCl2 and (EMImFSI) were added as additives. By means of X-ray photoelectron spectroscopy (XPS) and cryo-Transmission Electron Microscopy (cryo-TEM) analysis it was shown that the SEI consisted mainly of inorganic components, such as NaCl, Al2O3, and NaF. The spacing of lattice fringes obtained from high-resolution cryo-TEM images confirmed the composition of Al2O3, which was in line with their observations from X-ray diffraction measurements. Employing sodium vanadium phosphate (NVP) and sodium vanadium phosphate fluoride (NVPF) cathodes in half-cells with Na metal counter electrode and the non-flammable IL electrolyte showed high coulombic efficiency, excellent cycling stability, and good rate performance from 50 to 500 mA g−1. A very similar KCl-buffered AlCl3/1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) electrolyte with EtAlCl2 and KFSI as additives, was also demonstrated for a potassium-based system.75 Both of these chloroaluminate IL electrolytes share some attractive properties that are unusual for IL electrolytes such as high ionic-conductivity (9.2–13.1 mS cm−1) and low cost due to low concentrations of expensive components. One major detrimental aspect of using chloroaluminate is the corrosion issues that are common for this class of electrolytes. For both examples shown above the current collectors consisted of carbon and used nickel tabs.
A binary eutectic IL electrolyte consisting of 56 mol% NaFSI and 44 mol% KFSI with a melting point of 60 °C has shown promising results at elevated temperatures.76 The binary eutectic electrolyte was shown to be non-flammable and exhibited good electrochemical performances at 80 °C in Na//NaCrO2 half-cells (89% of initial discharge capacity 77.3 mA h g−1 after 100 cycles). This study demonstrates that the concept of ionic liquids as non-flammable electrolytes is not limited to room temperature applications, but can be extended to applications at elevated temperatures. The low conductivity of 3.3 mS cm−1 achieved at 90 °C, very expensive composition, and high melting point means that the electrolyte if quite far from the perfect. However, this study is still rather interesting due to the completely carbon free and inorganic nature of the electrolyte.
Ionic liquids show great potential in terms of their electrochemical performance, acceptable ionic conductivity and intrinsic non-flammability, but costs and viscosity need to be reduced. The use of ILs as an additive or co-solvent in conventional organic electrolytes appears promising. However, future work should elucidate on possible ignitable gasses that might be formed during combustion of ILs, especially in operating batteries.
2.7. Inorganic liquid electrolytes (IEs)
Inorganic liquid electrolytes (IEs) are usually intrinsically non-flammable i.e. non-combustible, so they can provide an alternative strategy to prevent the combustion of the electrolyte without compromising the electrochemical performance. To the best of our knowledge these types of electrolytes, typically based on either liquid/gaseous SO2 or NH3, were initially reported by Badoz-Lambling et al. in 1987 and Foster et al. in 1988.77,78 Nowadays, this type of electrolyte has gained research attention again for the purpose of non-flammability and high ionic conductivity. The mechanism of non-flammability is attributed to the non-combustible nature of SO2 and NH3. Sulphur dioxide reacts extremely slow with oxygen and ammonia does not react exothermically.The inorganic liquid electrolyte LiAlCl4 with SO2 was recently studied in LIBs.79 This IE displayed an exceptionally high Li+ ion conductivity of 121 mS cm−1 at 22 °C, remarkable longevity in LFP//graphite prismatic cells (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles reaching 20% of residual capacity) and outstanding discharge and rate capability in LFP//Li half cells up to 8C with a capacity of 74 mA h g−1.
000 cycles reaching 20% of residual capacity) and outstanding discharge and rate capability in LFP//Li half cells up to 8C with a capacity of 74 mA h g−1.
Similar behaviour was shown by Kim et al. for LiAlCl4·3SO2. The intrinsically non-flammable IE was demonstrated with high ionic conductivity of about 80 mS cm−1 and promising cycling stability (Fig. 8).80 By means of XPS it was determined that the SEI was mainly composed of the inorganic reduction products of the SO2-based inorganic electrolyte such as lithium chloride, lithium sulfide, lithium oxide, and lithium sulfur-oxy compounds. The remarkable electrochemical performance was attributed to the high conductivity and formation of a highly efficient SEI layer.80
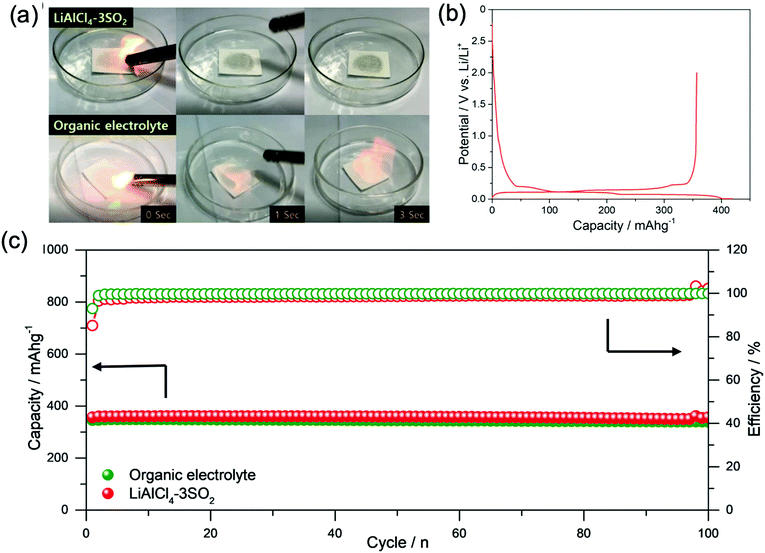 | ||
Fig. 8 (a) Flammability test of the LiAlCl4·3SO2 inorganic electrolyte and 1.0 M LiPF6 dissolved in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC (1 EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with 2 wt% FEC. (b) First cycle voltage profiles of graphite half-cell cycled in LiAlCl4·3SO2 electrolyte, and (c) galvanostatic cycling performance of graphite half-cell in LiAlCl4·3SO2 electrolyte and organic electrolyte. Modified from Kim et al.,80 with permission from American Chemical Society. 2) with 2 wt% FEC. (b) First cycle voltage profiles of graphite half-cell cycled in LiAlCl4·3SO2 electrolyte, and (c) galvanostatic cycling performance of graphite half-cell in LiAlCl4·3SO2 electrolyte and organic electrolyte. Modified from Kim et al.,80 with permission from American Chemical Society. | ||
An ammonia-based (NaY·xNH3) electrolyte is a promising alternative route towards safe, cheap, fast-charging and high-power SIBs.81 This type of electrolyte has the distinctive feature of being non-flammable (although having high volatility), high Na+ concentration (7 M), and high ionic conductivity (65–105 mS cm−1). Cyclic voltammetry experiments indicated promising electrochemical performances of the electrolytes in two-electrode split cells using sodium metal and Cu foil. To the best of our knowledge there are currently no reports on how inorganic non-flammable electrolytes perform in full cells, which opens pathways for further research.
Although the inorganic liquid electrolytes appear to be promising in terms of electrochemical properties and high ionic conductivity there are challenges when it comes to its stability against aluminium current collectors, the electrochemical oxidation of AlCl4− above 4.0 V (vs. Li/Li+) leading to Cl2 gas evolution and preparation of the electrolyte (SO2 is a toxic gas and should be handled with care).82 Also, the inorganic electrolyte might be intrinsically non-flammable, but the potential formation of toxic gasses and the pressure evolution needs to be further studied to understand and fully mitigate other safety issues (such as potential explosion hazards).
3. Conclusion and outlook
Overall, the design of non-flammable electrolytes is achievable by employing different strategies. Regardless of the strategy to develop non-flammable electrolytes, it is suggested to determine the flammability properties (such as flash point and combustibility) by means of standard tests, such as EN-ISO 2719:2016 and ISO 9038:2021. Furthermore, when designing non-flammable electrolytes, the safety tests should not be limited to the determination of electrolyte flammability, but preferably also include the interaction (compatibility) of electrolytes with (charged) anodes, cathodes and separators. So, when interpreting non-flammability properties, the reader should be aware of the limitations of the performed and reported tests.Non-flammable electrolytes can be developed by using suitable non-flammable or flame-retardant solvents. These solvents can be co-solvated with the conventional carbonate-based solvents, thereby providing overall high conductivity, better wettability and improved electrochemical performance. Within this direction there has been growing interest in fluorinated solvents as flame-retardants containing carbonate/ether/phosphate functional group. However, fluorine-substituted compounds offer advantages when used as components in electrolytes by preventing severe structural degradation at high voltage. This is motivating research towards development of further F-containing solvents to meet compatibility and cost. Also, non-flammable additives are a cost-effective option through their addition to electrolytes in low concentrations without decreasing the electrochemical performance.
The fundamental concepts of HCE and LHCE open up new avenues for further development of highly stable and safe electrolytes for high-energy rechargeable batteries. However, high concentrated electrolyte salt may act as strong oxidation agent, thus thermal stability charged cathodes in contact with such electrolytes should be carefully studied. The costs of HCE needs to be reduced, promoting the research towards developing cheap, inert and environmentally benign diluents in LHCEs. Current inert diluents mainly consist of expensive and highly fluorinated components, which are not fulfilling industry requirements.
Further alternative routes could also be considered, such as intrinsically non-flammable ionic liquids or inorganic liquid electrolytes, which could potentially open new doors towards the next non-flammable electrolyte. Both fundamental and practical studies of inorganic electrolytes should be performed, to deepen the understanding of potential toxic gas evolution and explosion hazards. Within the development of non-flammable ionic liquids attention should be paid to reducing viscosity and costs.
Among the various strategies discussed in this review, the fluorine-free phosphate-based flame-retarding solvents appear very promising. They showed encouraging electrochemical results with carbonaceous electrode compounds and are also often found to be environmentally benign. Despite this being a young field of research, this already provides justification to move away from F-containing non-flammable solvents for potentially safer electrolytes.
Abbreviations
| BTFE | Bis(2,2,2-trifluoroethyl)ether |
| Cl-EC | Chloro-ethylene carbonate |
| DEC | Diethyl carbonate |
| DFDEC | Di-(2,2,2-trifluoroethyl)carbonate |
| DMAC | N,N-Dimethylacetamide |
| DMC | Dimethyl carbonate |
| DMMP | Dimethyl methyl phosphate |
| EC | Ethyl carbonate |
| EEEP | Polybis-(ethoxyethoxyethoxy)phosphazene |
| EFE | Ethyl nonafluorobutyl ether |
| EFPN | Ethoxy(pentafluoro)cyclotriphosphazene |
| EMC | Ethyl methyl carbonate |
| FEC | Fluoroethylene carbonate |
| FEMC | FEMC |
| FEPE | 1,1,2,2-Tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether |
| G2E | Diethylene glycol diethyl ether |
| TFTFE | 1,1,2,2-Tetrafluoroethyl-2′,2′,2′-trifluoroethyl ether |
| HFPM | 1,1,1,3,3,3-Hexafluoroisopropyl methyl ether |
| KFSI | Potassium bis(fluorosulfonyl)imide |
| KPF6 | Potassium hexafluorophosphate |
| LiClO4 | Lithium perchlorate |
| LiBETI | Lithium bis(pentafluoroethylsulfonyl)imide |
| LiFSI | Lithium bis(fluorosulfonyl)imide |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| LiPF6 | Lithium hexafluorophosphate |
| LiAlCl4·3SO2 | Lithium tetrachloroaluminate·sulfur dioxide |
| MCMB | Mesocarbon microbeads |
| MFE | Methyl nonafluorobutyl ether |
| NaBOB | Sodium bis(oxalato)borate |
| NaFSI | Sodium bis(fluorosulfonyl)imide |
| NaTMSI | Sodium bis(trimethylsulfonyl)imide |
| NaPF6 | Sodium hexafluorophosphate |
| NVP | Sodium vanadium phosphate |
| NVPF | Sodium vanadium phosphate fluoride |
| TEP | Triethyl phosphate |
| TFEP | 2-(2,2,2-Trifluoroethoxy)-1,3,2-dioxaphospholane 2-oxide |
| TFP | Tris(2,2,2-trifluoroethyl)phosphate |
| TFPi | Tris(2,2,2-trifluoroethyl)phosphite |
| TFMP | Bis(2,2,2-trifluoroethyl)methylphosphonate |
| TMP | Trimethyl phosphate |
| TmdSx | (1,3-Bis(cyanopropyl)tetramethyl disiloxane) |
| TPrP | Tripropyl phosphate |
| OFE | 1H,1H,5H-Octafluoropentyl-1,1,2,2-tetrafluoroethyl ether |
| PTCDA | 3,4,9,10-Perylenetetracarboxylic dianhydride |
| PFMP | Perfluoro-2-methyl-3-pentanone |
| PFN | Ethoxy(pentafluoro)cyclotriphosphazene |
| PFPN | Ethoxy(pentafluoro)cyclotriphosphazene |
| PC | Propylene carbonate |
| VC | Vinylene carbonate |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support by the ÅForsk Foundation via the grant no. 20-675, by the Swedish Energy Agency via projects 48198-1, 51989-1, and 50177-1, by the Batteries in Sweden (BASE) supported by VINNOVA, and by STandUP for Energy.References
- Y. Liang, C.-Z. Zhao, H. Yuan, Y. Chen, W. Zhang, J.-Q. Huang, D. Yu, Y. Liu, M.-M. Titirici, Y.-L. Chueh, H. Yu and Q. Zhang, InfoMat, 2019, 1, 6–32 CrossRef CAS.
- M. Armand, P. Axmann, D. Bresser, M. Copley, K. Edström, C. Ekberg, D. Guyomard, B. Lestriez, P. Novák, M. Petranikova, W. Porcher, S. Trabesinger, M. Wohlfahrt-Mehrens and H. Zhang, J. Power Sources, 2020, 479, 228708 CrossRef CAS.
- N. Tapia-Ruiz, A. R. Armstrong, H. Alptekin, M. A. Amores, H. Au, J. Barker, R. Boston, W. R. Brant, J. M. Brittain, Y. Chen, M. Chhowalla, Y.-S. Choi, S. I. R. Costa, M. Crespo Ribadeneyra, S. A. Cussen, E. J. Cussen, W. I. F. David, A. V. Desai, S. A. M. Dickson, E. I. Eweka, J. D. Forero-Saboya, C. P. Grey, J. M. Griffin, P. Gross, X. Hua, J. T. S. Irvine, P. Johansson, M. O. Jones, M. Karlsmo, E. Kendrick, E. Kim, O. V. Kolosov, Z. Li, S. F. L. Mertens, R. Mogensen, L. Monconduit, R. E. Morris, A. J. Naylor, S. Nikman, C. A. O’Keefe, D. M. C. Ould, R. G. Palgrave, P. Poizot, A. Ponrouch, S. Renault, E. M. Reynolds, A. Rudola, R. Sayers, D. O. Scanlon, S. Sen, V. R. Seymour, B. Silván, M. T. Sougrati, L. Stievano, G. S. Stone, C. I. Thomas, M.-M. Titirici, J. Tong, T. J. Wood, D. S. Wright and R. Younesi, J. Phys. Energy, 2021, 3, 031503 CrossRef.
- T. Hosaka, K. Kubota, A. S. Hameed and S. Komaba, Chem. Rev., 2020, 120, 6358–6466 CrossRef CAS PubMed.
- K. Liu, Y. Liu, D. Lin, A. Pei and Y. Cui, Sci. Adv., 2018, 4, eaas9820 CrossRef PubMed.
- S. Hess, M. Wohlfahrt-Mehrens and M. Wachtler, J. Electrochem. Soc., 2015, 162, A3084–A3097 CrossRef CAS.
- X. Liu, D. Ren, H. Hsu, X. Feng, G.-L. Xu, M. Zhuang, H. Gao, L. Lu, X. Han, Z. Chu, J. Li, X. He, K. Amine and M. Ouyang, Joule, 2018, 2, 2047–2064 CrossRef CAS.
- N. Chawla, N. Bharti and S. Singh, Batteries, 2019, 5, 19 CrossRef CAS.
- Q. Wang, L. Jiang, Y. Yu and J. Sun, Nano Energy, 2019, 55, 93–114 CrossRef CAS.
- Y. E. Hyung, D. R. Vissers and K. Amine, J. Power Sources, 2003, 119–121, 383–387 CrossRef CAS.
- Y. Cui, J. Wan, Y. Ye, K. Liu, L.-Y. Chou and Y. Cui, Nano Lett., 2020, 20, 1686–1692 CrossRef CAS PubMed.
- P. Jaumaux, J. Wu, D. Shanmukaraj, Y. Wang, D. Zhou, B. Sun, F. Kang, B. Li, M. Armand and G. Wang, Adv. Funct. Mater., 2021, 31, 2008644 CrossRef CAS.
- A. Agrawal, S. Choudhury and L. A. Archer, RSC Adv., 2015, 5, 20800–20809 RSC.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2, 16103 CrossRef CAS.
- J. Janek and W. G. Zeier, Nat. Energy, 2016, 1, 1–4 CrossRef.
- K. Xu, M. S. Ding, S. Zhang, J. L. Allen and T. R. Jow, J. Electrochem. Soc., 2002, 149, A622 CrossRef CAS.
- J. W. Hastie, J. Res. Natl. Bur. Stand., Sect. A, 1973, 77A, 733 CrossRef CAS PubMed.
- G. Nagasubramanian and K. Fenton, Electrochim. Acta, 2013, 101, 3–10 CrossRef CAS.
- H. F. Xiang, H. Y. Xu, Z. Z. Wang and C. H. Chen, J. Power Sources, 2007, 173, 562–564 CrossRef CAS.
- S. V. Levchik and E. D. Weil, J. Fire Sci., 2006, 24, 345–364 CrossRef CAS.
- K. Xu, M. S. Ding, S. Zhang, J. L. Allen and T. R. Jow, J. Electrochem. Soc., 2003, 150, A161 CrossRef CAS.
- L. O. S. Colbin, R. Mogensen, A. Buckel, Y.-L. Wang, A. J. Naylor, J. Kullgren and R. Younesi, Adv. Mater. Interfaces DOI:10.1002/admi.202101135.
- S. Liu, J. Mao, Q. Zhang, Z. Wang, W. K. Pang, L. Zhang, A. Du, V. Sencadas, W. Zhang and Z. Guo, Angew. Chem., Int. Ed., 2020, 59, 3638–3644 CrossRef CAS PubMed.
- Z. Zeng, X. Jiang, R. Li, D. Yuan, X. Ai, H. Yang and Y. Cao, Adv. Sci., 2016, 3, 1600066 CrossRef PubMed.
- G. J. Chung, J. Han and S.-W. Song, ACS Appl. Mater. Interfaces, 2020, 12, 42868–42879 CrossRef CAS PubMed.
- Q. Zheng, Y. Yamada, R. Shang, S. Ko, Y.-Y. Lee, K. Kim, E. Nakamura and A. Yamada, Nat. Energy, 2020, 5, 291–298 CrossRef CAS.
- C.-C. Su, M. He, C. Peebles, L. Zeng, A. Tornheim, C. Liao, L. Zhang, J. Wang, Y. Wang and Z. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 30686–30695 CrossRef CAS PubMed.
- R. Mogensen, S. Colbin, A. S. Menon, E. Björklund and R. Younesi, ACS Appl. Energy Mater., 2020, 3, 4974–4982 CrossRef CAS.
- R. Mogensen, A. Buckel, S. Colbin and R. Younesi, Chem. Mater., 2021, 33, 1130–1139 CrossRef CAS.
- H. Zheng, W. Fang, Y. Sun, X. Liang, H. Xiang, L. Jiang and Q. Wang, Fire Technol., 2020, 56, 2349–2364 CrossRef.
- Y. Yu, H. Che, X. Yang, Y. Deng, L. Li and Z.-F. Ma, Electrochem. Commun., 2020, 110, 106635 CrossRef CAS.
- L. Jiang, C. Liang, H. Li, Q. Wang and J. Sun, ACS Appl. Energy Mater., 2020, 3, 1719–1729 CrossRef CAS.
- X. Jiang, X. Liu, Z. Zeng, L. Xiao, X. Ai, H. Yang and Y. Cao, iScience, 2018, 10, 114–122 CrossRef CAS PubMed.
- A. Rudola, K. Du and P. Balaya, J. Electrochem. Soc., 2017, 164, A1098–A1109 CrossRef CAS.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- A. Ponrouch, D. Monti, A. Boschin, B. Steen, P. Johansson and M. R. Palacín, J. Mater. Chem. A, 2015, 3, 22–42 RSC.
- R. Mogensen, S. Colbin and R. Younesi, Batteries Supercaps, 2021, 4, 791–814 CrossRef CAS.
- Q. Wang, P. Ping, X. Zhao, G. Chu, J. Sun and C. Chen, J. Power Sources, 2012, 208, 210–224 CrossRef CAS.
- H. Q. Pham, H.-Y. Lee, E.-H. Hwang, Y.-G. Kwon and S.-W. Song, J. Power Sources, 2018, 404, 13–19 CrossRef CAS.
- H. Q. Pham, E.-H. Hwang, Y.-G. Kwon and S.-W. Song, Chem. Commun., 2019, 55, 1256–1258 RSC.
- X. Fan, L. Chen, O. Borodin, X. Ji, J. Chen, S. Hou, T. Deng, J. Zheng, C. Yang, S.-C. Liou, K. Amine, K. Xu and C. Wang, Nat. Nanotechnol., 2018, 13, 715–722 CrossRef CAS PubMed.
- J. Arai, J. Appl. Electrochem., 2002, 32, 1071–1079 CrossRef CAS.
- S. Fang, G. Wang, L. Qu, D. Luo, L. Yang and S. Hirano, J. Mater. Chem. A, 2015, 3, 21159–21166 RSC.
- L. Xia, Y. Xia, C. Wang, H. Hu, S. Lee, Q. Yu, H. Chen and Z. Liu, ChemElectroChem, 2015, 2, 1707–1712 CrossRef CAS.
- K. Xu, Chem. Rev., 2014, 114, 11503–11618 CrossRef CAS PubMed.
- X. Li, W. Li, L. Chen, Y. Lu, Y. Su, L. Bao, J. Wang, R. Chen, S. Chen and F. Wu, J. Power Sources, 2018, 378, 707–716 CrossRef CAS.
- L. Xia, Y. Xia and Z. Liu, J. Power Sources, 2015, 278, 190–196 CrossRef CAS.
- Y. Gu, S. Fang, L. Yang and S. Hirano, J. Mater. Chem. A, 2021, 9, 15363–15372 RSC.
- J. Liu, X. Song, L. Zhou, S. Wang, W. Song, W. Liu, H. Long, L. Zhou, H. Wu, C. Feng and Z. Guo, Nano Energy, 2018, 46, 404–414 CrossRef CAS.
- J. Green, J. Fire Sci., 1996, 14, 426–442 CrossRef CAS.
- R. Liu and X. Wang, Polym. Degrad. Stab., 2009, 94, 617–624 CrossRef CAS.
- T. Dagger, B. R. Rad, F. M. Schappacher and M. Winter, Energy Technol., 2018, 6, 2011–2022 CrossRef CAS.
- T. Dagger, P. Niehoff, C. Lürenbaum, F. M. Schappacher and M. Winter, Energy Technol., 2018, 6, 2023–2035 CrossRef CAS.
- J. Feng, Y. An, L. Ci and S. Xiong, J. Mater. Chem. A, 2015, 3, 14539–14544 RSC.
- T. Dagger, C. Lürenbaum, F. M. Schappacher and M. Winter, J. Power Sources, 2017, 342, 266–272 CrossRef CAS.
- M. Zhou, C. Qin, Z. Liu, L. Feng, X. Su, Y. Chen, L. Xia, Y. Xia and Z. Liu, Appl. Surf. Sci., 2017, 403, 260–266 CrossRef CAS.
- J. K. Feng, X. J. Sun, X. P. Ai, Y. L. Cao and H. X. Yang, J. Power Sources, 2008, 184, 570–573 CrossRef CAS.
- L. Jiang, Q. Wang, K. Li, P. Ping, L. Jiang and J. Sun, Sustainable Energy Fuels, 2018, 2, 1323–1331 RSC.
- H. Liang, X. Zuo, L. Zhang, W. Huang, Q. Chen, T. Zhu, J. Liu and J. Nan, J. Electrochem. Soc., 2020, 167, 90520 CrossRef CAS.
- Z. Zeng, V. Murugesan, K. S. Han, X. Jiang, Y. Cao, L. Xiao, X. Ai, H. Yang, J.-G. Zhang, M. L. Sushko and J. Liu, Nat. Energy, 2018, 3, 674–681 CrossRef CAS.
- D. W. McOwen, D. M. Seo, O. Borodin, J. Vatamanu, P. D. Boyle and W. A. Henderson, Energy Environ. Sci., 2014, 7, 416–426 RSC.
- Y. Ding, J. Yun, H. Liu, Z. Wan, M. Shen, L. Zhang, Q. Qu and H. Zheng, Pure Appl. Chem., 2014, 86, 585–591 CAS.
- J. Wang, Y. Yamada, K. Sodeyama, E. Watanabe, K. Takada, Y. Tateyama and A. Yamada, Nat. Energy, 2018, 3, 22–29 CrossRef CAS.
- J. Hou, L. Lu, L. Wang, A. Ohma, D. Ren, X. Feng, Y. Li, Y. Li, I. Ootani, X. Han, W. Ren, X. He, Y. Nitta and M. Ouyang, Nat. Commun., 2020, 11, 5100 CrossRef CAS PubMed.
- J. Zheng, G. Ji, X. Fan, J. Chen, Q. Li, H. Wang, Y. Yang, K. C. DeMella, S. R. Raghavan and C. Wang, Adv. Energy Mater., 2019, 9, 1803774 CrossRef.
- S. Chen, J. Zheng, D. Mei, K. S. Han, M. H. Engelhard, W. Zhao, W. Xu, J. Liu and J.-G. Zhang, Adv. Mater., 2018, 30, 1706102 CrossRef PubMed.
- J. Zheng, S. Chen, W. Zhao, J. Song, M. H. Engelhard and J.-G. Zhang, ACS Energy Lett., 2018, 3, 315–321 CrossRef CAS.
- S. Chen, J. Zheng, L. Yu, X. Ren, M. H. Engelhard, C. Niu, H. Lee, W. Xu, J. Xiao, J. Liu and J.-G. Zhang, Joule, 2018, 2, 1548–1558 CrossRef CAS.
- M. Smiglak, W. M. Reichert, J. D. Holbrey, J. S. Wilkes, L. Sun, J. S. Thrasher, K. Kirichenko, S. Singh, A. R. Katritzky and R. D. Rogers, Chem. Commun., 2006, 2554–2556 RSC.
- C. Arbizzani, G. Gabrielli and M. Mastragostino, J. Power Sources, 2011, 196, 4801–4805 CrossRef CAS.
- M. Watanabe, M. L. Thomas, S. Zhang, K. Ueno, T. Yasuda and K. Dokko, Chem. Rev., 2017, 117, 7190–7239 CrossRef CAS PubMed.
- S. Wilken, S. Xiong, J. Scheers, P. Jacobsson and P. Johansson, J. Power Sources, 2015, 275, 935–942 CrossRef CAS.
- K. Chatterjee, A. D. Pathak, A. Lakma, C. S. Sharma, K. K. Sahu and A. K. Singh, Sci. Rep., 2020, 10, 9606 CrossRef PubMed.
- H. Sun, G. Zhu, X. Xu, M. Liao, Y.-Y. Li, M. Angell, M. Gu, Y. Zhu, W. H. Hung, J. Li, Y. Kuang, Y. Meng, M.-C. Lin, H. Peng and H. Dai, Nat. Commun., 2019, 10, 3302 CrossRef PubMed.
- H. Sun, P. Liang, G. Zhu, W. H. Hung, Y.-Y. Li, H.-C. Tai, C.-L. Huang, J. Li, Y. Meng, M. Angell, C.-A. Wang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 27847–27853 CrossRef CAS PubMed.
- A. Fukunaga, T. Nohira, Y. Kozawa, R. Hagiwara, S. Sakai, K. Nitta and S. Inazawa, J. Power Sources, 2012, 209, 52–56 CrossRef CAS.
- J. Badoz-Lambling, M. Bardin, C. Bernard, B. Fahys, M. Herlem, A. Thiebault and G. Robert, J. Electrochem. Soc., 1988, 135, 587–591 CrossRef CAS.
- D. L. Foster, H. C. Kuo, C. R. Schlaikjer and A. N. Dey, J. Electrochem. Soc., 1988, 135, 2682–2686 CrossRef CAS.
- V. Ramar, C. Pszolla, M. Rapp, M. Borck and L. Zinck, J. Electrochem. Soc., 2020, 167, 70521 CrossRef CAS.
- A. Kim, H. Jung, J. Song, H. J. Kim, G. Jeong and H. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 9054–9061 CrossRef CAS PubMed.
- D. Ruiz-Martínez, A. Kovacs and R. Gómez, Energy Environ. Sci., 2017, 10, 1936–1941 RSC.
- C. Wan Park and S. M. Oh, J. Power Sources, 1997, 68, 338–343 CrossRef.
Footnote |
| † Equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2021 |





