Discovery of sustainable drugs for Alzheimer's disease: cardanol-derived cholinesterase inhibitors with antioxidant and anti-amyloid properties†
Abstract
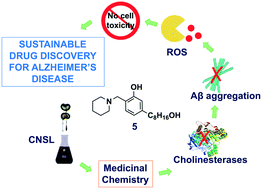
As part of our efforts to develop sustainable drugs for Alzheimer's disease (AD), we have been focusing on the inexpensive and largely available cashew nut shell liquid (CNSL) as a starting material for the identification of new acetylcholinesterase (AChE) inhibitors. Herein, we decided to investigate whether cardanol, a phenolic CNSL component, could serve as a scaffold for improved compounds with concomitant anti-amyloid and antioxidant activities. Ten new derivatives, carrying the intact phenolic function and an aminomethyl functionality, were synthesized and first tested for their inhibitory potencies towards AChE and butyrylcholinesterase (BChE). 5 and 11 were found to inhibit human BChE at a single-digit micromolar concentration. Transmission electron microscopy revealed the potential of five derivatives to modulate Aβ aggregation, including 5 and 11. In HORAC assays, 5 and 11 performed similarly to standard antioxidant ferulic acid as hydroxyl scavenging agents. Furthermore, in in vitro studies in neuronal cell cultures, 5 and 11 were found to effectively inhibit reactive oxygen species production at a 10 μM concentration. They also showed a favorable initial ADME/Tox profile. Overall, these results suggest that CNSL is a promising raw material for the development of potential disease-modifying treatments for AD.
- This article is part of the themed collection: NeuroMedChem


 Please wait while we load your content...
Please wait while we load your content...