Microwave-assisted green synthesis of bile acid derivatives and evaluation of glucocorticoid receptor binding†
Abstract
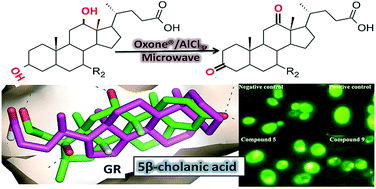
Herein, we present microwave-assisted AlCl3 catalyzed oxidation of bile acid hydroxyl groups in the presence of Oxone® in water media. Significant rate enhancements were observed for Wolff–Kishner reduction of synthesized bile acids oxo derivatives to the 5β-cholanic acid. Reaction of amidation of the simplest bile acid and aminolysis of the deoxycholic acid was accomplished in the absence of solvent and catalysts under sealed vessel microwave conditions. Because 5β-cholanic acid reportedly modulates glucocorticoid receptor signaling in cell models of Parkinson's disease, we tested the affinity of 5β-cholanic acid and deoxycholic acid derivatives for the glucocorticoid receptor in vitro using a yeast-based fluorescent screen. Treatment of GR-expressing yeast with prednisolone resulted in a dose-dependent increase in fluorescence; whereas 5β-cholanic acid binds to the glucocorticoid receptor with more moderate affinity. Similarly, molecular docking also suggests that 5β-cholanic acid can bind to the glucocorticoid receptor, with similar geometry to known GR ligands.


 Please wait while we load your content...
Please wait while we load your content...