Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organic double D–π–A sensitizers based on 2,2′-(2,2 diphenylethene-1,1-diyl)dithiophene: π-conjugation fragment effect on the photovoltaic properties†
Pengjuan
Zhou
a,
Bobing
Lin
a,
Ran
Chen
a,
Jianying
Liang
a,
Zhongwei
An
*ab,
Qiang
Weng
*a,
Xinbing
Chen
 a and
Pei
Chen
a
a and
Pei
Chen
a
aKey Laboratory of Applied Surface and Colloid Chemistry (MOE), Shaanxi Key Laboratory for Advanced Energy Devices, Shaanxi Engineering Lab for Advanced Energy Technology, School of Materials Science and Engineering, Shaanxi Normal University, Xi’an 710062, P. R. China. E-mail: gmecazw@163.com
bXi’an Modern Chemistry Research Institute, Xi’an, 710065, China
First published on 31st August 2021
Abstract
The development of new dye sensitizers to further reveal the influence of changes in structural components on the photovoltaic performance is of great significance to dye-sensitized solar cells (DSSCs). The propeller-shaped 2,2′-(2,2-diphenylethene-1,1-diyl)dithiophene (DPDT) unit was introduced to construct sensitizers for the first time. Three DPDT-bridged double D–π–A organic sensitizers (A6, A8, A9) were prepared by altering the π-conjugation fragment. The photophysical, electrochemical and photovoltaic properties of the sensitizers were systematically investigated to assess the role of the terminal fragment of dyes in DSSCs. The results show that, compared to the mono-anchoring congener AZ6, the di-anchoring sensitizer A6 displayed a comparable power conversion efficiency (PCE = 8.21%) and a higher short-circuit current density (JSC). Replacement of the terminal thiophene with a phenyl ring (A9) can effectively increase the photovoltage by 70 mV with an efficiency of 8.14%, which is 1.5 times higher than that of A8 (5.36%) with 2-cyanoacrylic acid at the meta-position of the phenyl ring. These results indicate that the terminal fragments of sensitizers have a significant effect on the photovoltaic performance.
Introduction
The field of dye-sensitized solar cells (DSSCs) has grown at an alarming speed and significant achievements have been made in metal complex-sensitized1,2 and pure organic dye-sensitized solar cells in the last three decades.3–5 As a crucial assembly unit, sensitizers fundamentally affect the performance of DSSCs and have grown rapidly in recent decades.6–8 Numerous ruthenium complex dyes and zinc porphyrin sensitizers with high efficiency such as CYC-B119 (11.5%), SM31510 (13.0%), XW5111 (11.1%), and so on have been reported. Among various dyes,12,13 metal-free photosensitizers have also aroused great research interest due to the flexible molecular design, high molar extinction coefficients, and cost-effectiveness. In addition to the donor–π–acceptor (D–π–A) featured sensitizers,14 new types of dyes have been developed to seek ideal sensitizers with the characteristics of being aggregation-resistant and having strong absorption. Recently, some studies have found that di-anchoring dyes15,16 have a stronger affinity to photoanodes, are more stable than the corresponding mono-anchored dyes, and can provide more electron injection pathways.However, compared with mono-anchoring dyes, di-anchoring dyes17–19 usually suffer a decreased open-circuit voltage (VOC) due to the change of the conduction band edge (ECB) of TiO2 and dark current. Therefore, more new fragment compositions were introduced into the di-anchoring dyes to overcome this shortcoming.20–22 Recently, the light-emitting molecule tetraarylethylene23 has attracted our interest, which can effectively promote the orderly aggregation of dyes without introducing aggregation-induced emission (AIE) characteristics. Since the Su group24 first introduced the tetraphenylethylene (TPE) core into X-shaped dyes, more dyes have been introduced into this fragment at different sites. For example, the Lin group25 synthesized a series of di-anchoring TPE-tethered YL dyes, providing a stronger ability to suppress charge recombination compared to the congener dyes. Recently, Zheng and co-workers found that different triarylethylene (TAE)26 units, when used as a π-spacer fragment of dyes, have an impact on photovoltaic properties. The DPTP unit has similar AIE properties, which may be more beneficial to the light absorption of dyes due to the electron-rich characteristics of thiophene. Therefore, it is still potentially valuable to introduce the DPTP unit into dye molecules.
Up to now, di-anchoring dyes27–30 have not been extensively studied due to the difficulties in the design and synthesis of di-anchoring dyes. It is easy to find that little attention was paid to the modulation of the π-spacer fragment, which dramatically affected the optical properties and the interfacial charge recombination. Thiophene and phenyl rings are the most commonly used π-spacer components. Dyes containing thiophene as a π-spacer component always display a higher JSC value but a lower open-circuit voltage,31,32 while dyes with phenyl as a π-spacer component usually exhibit opposite results.33–35 By inserting an additional terminal phenyl ring, the reported dyes GY5036 and 237 can greatly suppress the charge recombination and significantly improve the VOC and JSC. Simultaneously, the position of the acceptor in sensitizers has an influence on the binding mode and photovoltaic properties.38 For instance, Galoppini et al.39 reported that the anchor group of sensitizers in the meta position (m-ZnTCPP) favored a planar binding mode to the metal oxide surfaces. D’Souza and Gao40,41 revealed that dyes with a para- or meta-position acceptor showed better DSSC performance than dyes with an ortho-position acceptor. However, related investigations are still scant, especially in metal-free di-anchoring dyes.
Based on the above background, we introduced the propeller-shaped DPDT unit into double D–π–A sensitizers (A6, A8, A9) for the first time and connected it with different terminal fragments of dyes (Fig. 1). The cyclic thiourea functionalized triphenylamine was selected as the donor unit due to its excellent optical characteristics and the ability to inhibit charge recombination.42 Bithiophene or 2-phenylthiophene was selected as the π-spacer with cyanoacrylic acid as the acceptor at the meta- or para-position of the phenyl ring. The dyes are applied in DSSCs to understand which is the dominant factor affecting the VOC of double D–π–A sensitizers: the conduction band edge (ECB) of TiO2 or the ability to suppress the dark current? In addition, it is important to clarify that the different geometric configurations caused by the positions of the para- or meta-anchoring group of benzene may affect the loading mode of dyes on TiO2 film. The reported D–π–A sensitizer AZ6 was synthesized as a reference. The photophysical, electrochemical and photovoltaic properties of the dyes were studied systematically. In particular, the synthetic method provides more space and feasibility for further modulation of the tetraarylethylene fragment.
Results and discussion
Material synthesis and characterization
The synthetic routes of A6, A8, and A9 are shown in Scheme 1. The raw material C6S2TPAB(OH)2 was synthesized according to our previously reported method.42 Intermediates 3a–3c and 4a–4c were synthesized via Suzuki coupling. The target dye molecules A6, A8, and A9 were obtained from 4a–4cvia the Knoevenagel condensation reaction. All of the new compounds were characterized by 1H NMR, 13C NMR, IR spectroscopy and HRMS or MALDI-TOF-MS (see the ESI†).Photophysical properties
The UV-vis absorption spectra of the dyes measured in dichloromethane and on TiO2 films are shown in Fig. 2. The detailed parameters are summarized in Table 1. All the dyes showed two major absorption bands. The absorption band at 300–400 nm is attributed to the π–π* transition of the conjugated system and the absorption band at 400–670 nm is ascribed to the intramolecular charge transfer (ICT) from the functionalized triphenylamine donor to the cyanoacetic acid acceptor. Compared with the single D–π–A dye AZ6, the double D–π–A dye A6 exhibited broader absorption characteristics with a higher molar extinction coefficient (ε) in the whole absorption region due to conjugation extension. It is worth noting that the π–π* electron transition band of the three D–π–A dyes showed a higher ε than their ICT bands, which indicated that a stronger π–π* interaction occurred in the dyes due to the bulky donor configuration. This phenomenon can also be found in other reported dyes.34,43,44 Dye A8 showed a narrower and less intense ICT band than A9, which was caused by the poor coplanarity of the π-spacer and the meta-position acceptor molecular configuration may weaken the charge transfer. When the phenyl ring was replaced with a thiophene group, the absorption properties of sensitizer A6 were further enhanced. The ε values at λmax of the absorption spectra for A6, A8, and A9 were 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 M−1 cm−1, 34
210 M−1 cm−1, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 M−1 cm−1, and 41
556 M−1 cm−1, and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 648 M−1 cm−1, respectively, suggesting that the absorption characteristics can be effectively fine-tuned by adjustment of the π-spacer segments and the acceptor position.
648 M−1 cm−1, respectively, suggesting that the absorption characteristics can be effectively fine-tuned by adjustment of the π-spacer segments and the acceptor position.
| Dye | λ max (nm) | ε (M−1 cm−1) | E ox (eV) | E red (eV) | E HOMO (eV) | E LUMO (eV) | E g (eV) |
|---|---|---|---|---|---|---|---|
| a Absorption spectra of sensitizers in CH2Cl2 (10−5 M). b E HOMO = −e (Eox + 4.71) (eV) and ELUMO = −e (Ered + 4.71) (eV); Eg = e(Eox − Ered) (eV). | |||||||
| A6 | 432 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
0.21 | −0.99 | −4.92 | −3.72 | 1.20 |
| 484 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
||||||
| A8 | 413 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 556 |
0.25 | −1.18 | −4.96 | −3.53 | 1.43 |
| A9 | 447 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 648 648 |
0.27 | −1.14 | −4.98 | −3.57 | 1.41 |
| AZ6 | 501 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 939 |
0.31 | −1.06 | −5.02 | −3.65 | 1.37 |
In the case of the dyes absorbed on the TiO2 surface, the absorption bands were broader and red-shifted (ca. 50 nm) than those in solution, which is favorable to improving the light-harvesting ability. In order to further verify the binding mode of the sensitizers on the TiO2 film, the FT-IR analysis of the dyes anchored on the TiO2 film was carried out.45 As shown in Fig. S2 (ESI†), asymmetric stretching (υas, around 1598 cm−1) and symmetric stretching (υs, around 1408 cm−1) bands appeared, whereas the –COOH peak (around 1724 cm−1) of the pure dyes disappeared. This result indicates that all three double D–π–A dyes adsorbed on the TiO2 film in the bidentate adsorption mode, which may improve the affinity of the dye to the TiO2 film. The desorption experiment25 of the dyes in an alkaline solution (Fig. S3, ESI†) showed that the di-anchoring dyes were more difficult to extract than the mono-anchoring dyes. The result is consistent with its binding mode.
Electrochemical properties
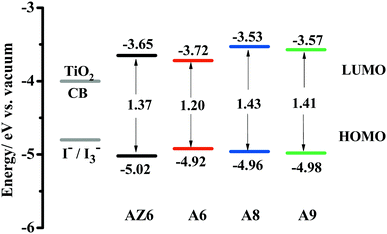
Cyclic voltammetry (CV) was performed to evaluate the feasibility of the electron injection and dye regeneration processes.46 As shown in Fig. 3, all dyes had sufficiently high LUMO levels, which indicates that electrons can be effectively injected from the excited dyes into the conduction band of TiO2 (−4.0 eV vs. vacuum). Meanwhile, the HOMO levels of the dyes were more negative than the redox potential of the iodide/triiodide electrolyte (−4.80 eV vs. vacuum), ensuring that these oxidized dyes could be easily regenerated. For A8 and A9, similar energy levels demonstrate that the acceptor position has a slight influence on the energy levels. Replacing the phenyl with a thiophene ring, the LUMO level of dye A6 significantly shifted down, thus leading to a narrow energy gap, which may be favorable for generating a higher photocurrent. The HOMO–LUMO energy gap values decreased in the order: A8 (1.43 eV) > A9 (1.41 eV) > AZ6 (1.37 eV) > A6 (1.20 eV), which is consistent with the absorption characteristics.DFT calculations
The molecular geometry conformations and electronic properties of dyes A6–A9 were simulated by density functional theory (DFT) and time-dependent density functional theory (TD-DFT). The frontier molecular orbitals of the dyes are shown in Fig. 4 and the corresponding energy levels are listed in Table S2 (ESI†). Compared with A9, A8 presents a more twisted molecular configuration and a shorter distance between the two acceptors (13.9 Å for A8 and 20.0 Å for A9), which has an advantage in inhibiting charge recombination. In contrast, A6 shows better coplanarity from the bithiophene to the acceptor, which is favorable for its effective intramolecular charge transfer process. The HOMO levels of the three dyes are mainly concentrated on the donor moieties. For di-anchoring dyes, the charges are mainly located on both sides of their two branches, implying double electron injection channels. According to TD-DFT theoretical calculations, the ICT in A8 and A9 is largely from the HOMO to LUMO+2 (Table S3, ESI†), while for A6 is largely from the HOMO to the LUMO.Photovoltaic performance of DSSCs
The DSSC performances of the dyes were evaluated under simulated AM 1.5G solar light (100 mW cm−2) and the relevant parameters are tabulated in Table 2. The photocurrent density–voltage (J–V) curves and incident photon to current conversion efficiency (IPCE) spectra are plotted in Fig. 5. The JSC values increased in the sequence: A8 (10.26 mA cm−2) < A9 (16.60 mA cm−2) < AZ6 (16.84 mA cm−2) < A6 (18.63 mA cm−2). Obviously, the A8-based DSSC displayed an inferior photocurrent, although A8 has a higher loading amount (0.74 × 10−7 mol cm−2) than A6. This result indicates that the loading amount has a small influence on its photocurrent as observed in other reported dyes.47,48 The narrow UV-vis absorption band of A8 in solution and on the TiO2 film is consistent with the integration and intensity of the IPCE spectrum. All the factors contribute to a low JSC value of A8. It is worth noting that the VOC values showed an opposite tendency: A8 (731.8 mV) > A9 (717 mV) > AZ6 (683.7 mV) > A6 (647.0 mV). Also compared with the mono-anchoring dye AZ6, the A6-based DSSC showed a lower VOC, which may be caused by the following two aspects. Firstly, compared with the mono-anchoring dye AZ6 (0.84 × 10−7 mol cm−2), the di-anchoring dye A6 released more protons (2 × 0.51 × 10−7 mol cm−2) to the TiO2 surface and thus decreased the ECB of the TiO2. Furthermore, the dye A6 with more active sulfur sites is easier to form dye–iodine complexes,49,50 causing serious charge recombination. However, replacing the thiophene with a phenyl ring in the π-spacer, higher VOC values were observed in both A8- and A9-based DSSCs. Compared with dyes AZ6 and A6, dyes A8 and A9 have a better loading capacity, indicating that more protons were released and then the VOC was decreased. The higher VOC values indicate that the benzene ring in the π-spacer effectively inhibits charge recombination and then reduces dark current. The DSSC based on A8 showed a slightly higher VOC value than that based on A9, which may be due to the larger steric hindrance. This reasonable speculation is also supported by the dark current test results (Fig. 5). Overall, the DSSC based on A6 obtained a power conversion efficiency (PCE = 8.21%) with the highest photocurrent. When the acceptor position was altered from the meta- to the para-position, the efficiency increased by 52% from A8 (PCE = 5.36%) to A9 (PCE = 8.14%), which confirmed that the suitable acceptor position is crucial for the device performance. The A6-based DSSC presented a broad spectral response and extended the spectral region to 750 nm. The integration area of the IPCE spectra of the three dyes is in good accordance with the light-harvesting characteristics and JSC values.| Dye | J SC (mA cm−2) | V OC (mV) | FF | PCE (%) | Dye loading (10−7 mol cm−2) |
|---|---|---|---|---|---|
| a Performances of DSSCs were measured with a 0.25 cm2 working area under AM 1.5G solar light irradiation (100 mW cm−2), the TiO2 layer thickness is 13 μm and the photovoltaic data are the averaged values of six parallel cells. | |||||
| A6 | 18.63 (18.11 ± 0.96) | 647.0 (648.2 ± 7.5) | 0.68 (0.69 ± 0.01) | 8.21 (8.08 ± 0.32) | 0.51 |
| A8 | 10.26 (10.01 ± 0.26) | 731.8 (733.0 ± 6.7) | 0.71 (0.71 ± 0.01) | 5.36 (5.23 ± 0.15) | 0.74 |
| A9 | 16.60 (16.36 ± 0.47) | 717.0 (712.0 ± 9.4) | 0.68 (0.69 ± 0.01) | 8.14 (8.05 ± 0.11) | 0.91 |
| AZ6 | 16.84 (16.84 ± 0.50) | 683.7 (689.0 ± 20.2) | 0.72 (0.71 ± 0.02) | 8.31 (8.24 ± 0.27) | 0.84 |
In addition to the PCE of the DSSCs, the stability of the dye-based DSSCs was also evaluated to further reveal the differences between mono-anchoring dyes and di-anchoring dyes. The cells were stored at room temperature and approximately 25% relative humidity. Fig. S6 (ESI†) shows the variation of the photovoltaic parameters of DSSCs recorded under continuous light irradiation (AM 1.5G, 100 mW cm−2) over a period of 3000 h. The A6-based solar cell showed the best long-term stability, which can still retain 98% of the initial value after 3000 h. After 3000 hours of aging, the A8-based solar cell and A9-based solar cell retained 90% and 82% of the initial efficiencies, respectively. As a comparison, the AZ6-based solar cell could retain 78% of its initial value after 3000 h of one sun soaking. This result confirms that the bidentate binding mode of di-anchoring dyes is beneficial for enhancing the stability of DSSCs.
Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) analysis was performed to investigate the photovoltaic properties and charge transfer processes at the electrode/electrolyte interface of DSSCs under dark conditions. As shown in Fig. 5, the charge-transfer resistance at the dye-adsorbed TiO2/electrolyte interface was assessed through the diameter of the semicircle. The diameter of the semicircle increased in the order: A6 < AZ6 < A9 < A8. The electron recombination lifetime (τe) was obtained from the Bode phase plot (Fig. 5d) using the equation τe = 1/2πfmax, which increased in the order: A6 (6.5 ms) < AZ6 (8.0 ms) < A9 (21.3 ms) < A8 (29.9 ms). The larger semicircle and longer lifetime imply less interfacial charge recombination. The results coincided well with the VOC values and dark current test results, which further confirmed that the phenyl ring effectively retarded the charge recombination process.Conclusions
We synthesized three DPDT-based double D–π–A organic sensitizers by altering the terminal fragment. The di-anchoring sensitizers showed robust affinity to the TiO2 surface and better long-term stability than the corresponding mono-anchoring dye AZ6. Owing to the extension of conjugation, the A6-based DSSC achieved a high photocurrent. By altering the terminal fragment of the dyes, the photocurrent and voltage can be improved. As a result, the A6-based DSSC achieved an efficiency of 8.21% with a superior JSC of 18.63 mA cm−2. When replacing the terminal thiophene with a phenyl ring, the A9-based DSSC yielded a comparable efficiency of 8.14% with a higher VOC value of 717 mV. Further study on new double D–π–A sensitizers through a molecular engineering strategy is in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Science Foundation Committee of China (21673134 and 21543012), Program for Science & Technology Innovation Team of Shaanxi Province (2018TD-030), the International Science and Technology Cooperation Project of Shaanxi Province (2021KW-20) and the Fundamental Research Funds for the Central Universities (GK202101005).References
- S. Zhang, X. Yang, Y. Numata and L. Han, Energy Environ. Sci., 2013, 6, 1443–1464 RSC.
- K. Zeng, Z. Tong, L. Ma, W.-H. Zhu, W. Wu and Y. Xie, Energy Environ. Sci., 2020, 13, 1617–1657 RSC.
- N. Zhou, K. Prabakaran, B. Lee, S. H. Chang, B. Harutyunyan, P. Guo, M. R. Butler, A. Timalsina, M. J. Bedzyk, M. A. Ratner, S. Vegiraju, S. Yau, C.-G. Wu, R. P. H. Chang, A. Facchetti, M.-C. Chen and T. J. Marks, J. Am. Chem. Soc., 2015, 137, 4414–4423 CrossRef CAS PubMed.
- L. Zhang, X. Yang, W. Wang, G. G. Gurzadyan, J. Li, X. Li, J. An, Z. Yu, H. Wang, B. Cai, A. Hagfeldt and L. Sun, ACS Energy Lett., 2019, 4, 943–951 CrossRef CAS.
- J. An, X. Yang, B. Cai, L. Zhang, K. Yang, Z. Yu, X. Wang, A. Hagfeldt and L. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 46397–46405 CrossRef CAS PubMed.
- H. Song, Q. Liu and Y. Xie, Chem. Commun., 2018, 54, 1811–1824 RSC.
- Y. Kurumisawa, T. Higashino, S. Nimura, Y. Tsuji, H. Iiyama and H. Imahori, J. Am. Chem. Soc., 2019, 141, 9910–9919 CrossRef CAS PubMed.
- J. Zou, Q. Yan, C. Li, Y. Lu, Z. Tong and Y. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 57017–57024 CrossRef CAS PubMed.
- C.-Y. Chen, M. Wang, J.-Y. Li, N. Pootrakulchote, L. Alibabaei, C.-H. Ngoc-le, J.-D. Decoppet, J.-H. Tsai, C. Grätzel, C.-G. Wu, S. M. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103–3109 CrossRef CAS PubMed.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- K. Zeng, W. Tang, C. Li, Y. Chen, S. Zhao, Q. Liu and Y. Xie, J. Mater. Chem. A, 2019, 7, 20854–20860 RSC.
- K. Zeng, Y. Chen, W.-H. Zhu, H. Tian and Y. Xie, J. Am. Chem. Soc., 2020, 142, 5154–5161 CrossRef CAS PubMed.
- H. Meier, Z.-S. Huang and D. Cao, J. Mater. Chem. C, 2017, 5, 9828–9837 RSC.
- Z. Wang, L. Miu, H. Yao, M. Liang, S. Yan, J. Wang, T. Guo, L. Zhang, J. Chen and S. Xue, Dyes Pigm., 2019, 162, 126–135 CrossRef CAS.
- Y.-F. Chen, J.-M. Liu, J.-F. Huang, L.-L. Tan, Y. Shen, L.-M. Xiao, D.-B. Kuang and C.-Y. Su, J. Mater. Chem. A, 2015, 3, 8083–8090 RSC.
- A. Abbotto, N. Manfredi, C. Marinzi, F. De Angelis, E. Mosconi, J.-H. Yum, Z. Xianxi, M. K. Nazeeruddin and M. Grätzel, Energy Environ. Sci., 2009, 2, 1094–1101 RSC.
- Z.-S. Huang, C. Cai, X.-F. Zang, Z. Iqbal, H. Zeng, D.-B. Kuang, L. Wang, H. Meier and D. Cao, J. Mater. Chem. A, 2015, 3, 1333–1344 RSC.
- X. Jiang, K. M. Karlsson, E. Gabrielsson, E. M. J. Johansson, M. Quintana, M. Karlsson, L. Sun, G. Boschloo and A. Hagfeldt, Adv. Funct. Mater., 2011, 21, 2944–2952 CrossRef CAS.
- W. Lee, S. B. Yuk, J. Choi, H. J. Kim, H. W. Kim, S. H. Kim, B. Kim, M. J. Ko and J. P. Kim, Dyes Pigm., 2014, 102, 13–21 CrossRef CAS.
- C.-Y. Lo, D. Kumar, S.-H. Chou, C.-H. Chen, C.-H. Tsai, S.-H. Liu, P.-T. Chou and K.-T. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 27832–27842 CrossRef CAS PubMed.
- X. Ren, S. Jiang, M. Cha, G. Zhou and Z.-S. Wang, Chem. Mater., 2012, 24, 3493–3499 CrossRef CAS.
- Y. Hong, J.-Y. Liao, D. Cao, X. Zang, D.-B. Kuang, L. Wang, H. Meier and C.-Y. Su, J. Org. Chem., 2011, 76, 8015–8021 CrossRef CAS PubMed.
- H.-T. Feng, Y.-X. Yuan, J.-B. Xiong, Y.-S. Zheng and B. Z. Tang, Chem. Soc. Rev., 2018, 47, 7452–7476 RSC.
- F. Zhang, J. Fan, H. Yu, Z. Ke, C. Nie, D. Kuang, G. Shao and C. Su, J. Org. Chem., 2015, 80, 9034–9040 CrossRef CAS PubMed.
- C.-T. Li, Y.-L. Kuo, C. H. P. Kumar, P.-T. Huang and J. T. Lin, J. Mater. Chem. A, 2019, 7, 23225–23233 RSC.
- Y.-Q. Yan, Y.-Z. Zhu, J. Han, P.-P. Dai, M. Yan and J.-Y. Zheng, Dyes Pigm., 2020, 183, 108630 CrossRef CAS.
- C.-T. Li, F.-L. Wu, C.-J. Liang, K.-C. Ho and J. T. Lin, J. Mater. Chem. A, 2017, 5, 7586–7594 RSC.
- D. Heredia, J. Natera, M. Gervaldo, L. Otero, F. Fungo, C.-Y. Lin and K.-T. Wong, Org. Lett., 2010, 12, 12–15 CrossRef CAS PubMed.
- G. Pozzi, S. Orlandi, M. Cavazzini, D. Minudri, L. Macor, L. Otero and F. Fungo, Org. Lett., 2013, 15, 4642–4645 CrossRef CAS PubMed.
- X. Qian, H.-H. Gao, Y.-Z. Zhu, L. Lu and J.-Y. Zheng, RSC Adv., 2015, 5, 4368–4375 RSC.
- G. Tian, S. Cai, X. Li, H. Ågren, Q. Wang, J. Huang and J. Su, J. Mater. Chem. A, 2015, 3, 3777–3784 RSC.
- J. He, F. Guo, X. Li, W. Wu, J. Yang and J. Hua, Chem. – Eur. J., 2012, 18, 7903–7915 CrossRef CAS PubMed.
- W. Ying, J. Yang, M. Wielopolski, T. Moehl, J.-E. Moser, P. Comte, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, Chem. Sci., 2014, 5, 206–214 RSC.
- R. Yeh-Yung Lin, F.-L. Wu, C.-H. Chang, H.-H. Chou, T.-M. Chuang, T.-C. Chu, C.-Y. Hsu, P.-W. Chen, K.-C. Ho, Y.-H. Lo and J. T. Lin, J. Mater. Chem. A, 2014, 2, 3092–3101 RSC.
- S. Qu, W. Wu, J. Hua, C. Kong, Y. Long and H. Tian, J. Phys. Chem. C, 2010, 114, 1343–1349 CrossRef CAS.
- A. Yella, C.-L. Mai, S. M. Zakeeruddin, S.-N. Chang, C.-H. Hsieh, C.-Y. Yeh and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 2973–2977 CrossRef CAS PubMed.
- S. Haid, M. Marszalek, A. Mishra, M. Wielopolski, J. Teuscher, J.-E. Moser, R. Humphry-Baker, S. M. Zakeeruddin, M. Grätzel and P. Bäuerle, Adv. Funct. Mater., 2012, 22, 1291–1302 CrossRef CAS.
- R. B. Ambre, S. B. Mane, G.-F. Chang and C.-H. Hung, ACS Appl. Mater. Interfaces, 2015, 7, 1879–1891 CrossRef CAS PubMed.
- J. Rochford, D. Chu, A. Hagfeldt and E. Galoppini, J. Am. Chem. Soc., 2007, 129, 4655–4665 CrossRef CAS PubMed.
- A. S. Hart, C. B. Kc, H. B. Gobeze, L. R. Sequeira and F. D’Souza, ACS Appl. Mater. Interfaces, 2013, 5, 5314–5323 CrossRef CAS PubMed.
- S. Liu, Y. Jiao, Y. Ding, X. Fan, J. Song, B. Mi and Z. Gao, Dyes Pigm., 2020, 180, 108470 CrossRef CAS.
- Z. Wu, Z. An, X. Chen and P. Chen, Org. Lett., 2013, 15, 1456–1459 CrossRef CAS PubMed.
- X. Lu, X. Jia, Z.-S. Wang and G. Zhou, J. Mater. Chem. A, 2013, 1, 9697–9706 RSC.
- X.-X. Dai, H.-L. Feng, Z.-S. Huang, M.-J. Wang, L. Wang, D.-B. Kuang, H. Meier and D. Cao, Dyes Pigm., 2015, 114, 47–54 CrossRef CAS.
- M. C. Sil, V. Sudhakar, M. F. Mele Kavungathodi, V. Punitharasu and J. Nithyanandhan, ACS Appl. Mater. Interfaces, 2017, 9, 34875–34890 CrossRef CAS PubMed.
- J. Hou, Z. a. Tan, Y. Yan, Y. He, C. Yang and Y. Li, J. Am. Chem. Soc., 2006, 128, 4911–4916 CrossRef CAS PubMed.
- H. Li, Y. Hou, Y. Yang, R. Tang, J. Chen, H. Wang, H. Han, T. Peng, Q. Li and Z. Li, ACS Appl. Mater. Interfaces, 2013, 5, 12469–12477 CrossRef CAS PubMed.
- X.-F. Zang, T.-L. Zhang, Z.-S. Huang, Z. Iqbal, D.-B. Kuang, L. Wang, H. Meier and D. Cao, Dyes Pigm., 2014, 104, 89–96 CrossRef CAS.
- Y. Cui, Y. Wu, X. Lu, X. Zhang, G. Zhou, F. B. Miapeh, W. Zhu and Z.-S. Wang, Chem. Mater., 2011, 23, 4394–4401 CrossRef CAS.
- M. Zhang, J. Liu, Y. Wang, D. Zhou and P. Wang, Chem. Sci., 2011, 2, 1401–1406 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00728a |
| This journal is © The Royal Society of Chemistry 2021 |