Open Access Article
Open Access ArticleProtonation- and electrostatic-interaction-based fluorescence probes for the selective detection of picric acid (2,4,6-trinitrophenol) – an explosive material
Sukhvinder
Dhiman
 ,
Nancy
Singla
,
Nancy
Singla
 ,
Manzoor
Ahmad
,
Manzoor
Ahmad
 ,
Prabhpreet
Singh
,
Prabhpreet
Singh
 and
Subodh
Kumar
and
Subodh
Kumar
 *
*
Department of Chemistry, Center of Advanced Studies, Guru Nanak Dev University, Amritsar – 143 005, India. E-mail: subodh_gndu@yahoo.co.in; subodh.chem@gndu.ac.in
First published on 19th August 2021
Abstract
Picric acid, due to its low pKa value, possesses distinct physicochemical features from all other nitroaromatic derivatives, enabling the design of fluorescent probes for its sensitive and selective detection. The electrostatic interactions between the positive charge on the fluorescent probe, which arises due to protonation or the presence of a quaternary nitrogen, and the picrate anion increase the proximity between the fluorescent probe and the analyte, thus increasing the efficiency of the electron or energy transfer processes. This provides a salient feature for the design of fluorescent probes for the selective detection of picric acid over other nitroaromatic compounds. Here, the literature on the use of fluorescent small organic molecules for the selective detection of picric acid based on electrostatic interactions between positively charged/protonated fluorophores and their ability to selectively detect picric acid has been discussed. The fluorescent probes have been classified into three broad categories – neutral fluorophores, which are selectively protonated by picric acid; positively charged probes, which exhibit electrostatic interactions with the picrate anion; and metal complexes with protonatable sites. This comprehensive review will prove a benchmark for the future design of fluorescent probes for picric acid.
1. Introduction
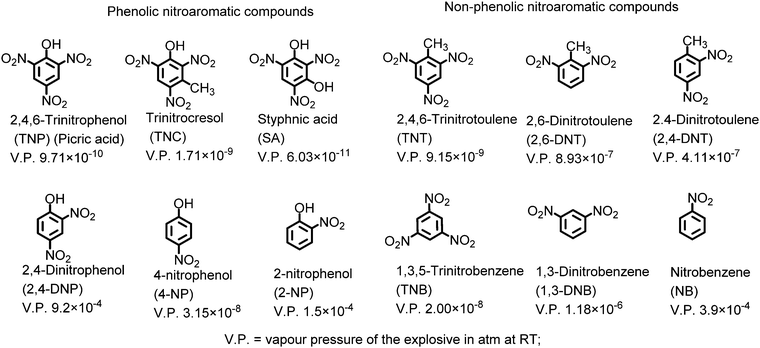
Anti-terrorism and security concerns at places of people movement and other locations have necessitated the development of methods for the trace detection of hazardous explosive materials. The nitroaromatic compounds (NACs) constitute a broad class of widely used explosive materials. In recent years, fluorescence-based detection of NACs has received remarkable attention due to its ease of execution, high sensitivity and the compatibility of technique with the solution, solid and vapor state. These nitroaromatic compounds can be classified into two major categories: (i) phenolic nitroaromatic compounds and (ii) non-phenolic nitroaromatic compounds (Chart 1). Structurally, these nitroaromatic compounds are electron-deficient π-systems, and in general, electron deficiency increases with the increase in the number of nitro groups on the benzene ring.The phenolic nitroaromatic compound 2,4,6-trinitrophenol (TNP), which is also known as picric acid (PA), is superior to even TNT in energy content and was widely used as an explosive material during the World Wars.1,2 PA finds applications in the manufacture of colored glass and the synthesis of dyes.3,4 It is also a key component in the rocket fuel, matchbox, electric battery and fireworks industries.5 Due to these industries, PA is released into the environment via anthropogenic activities, resulting in its presence in wastewater, aquatic systems6 and soil7 and as a contaminant in the air. Its high solubility in basic solutions and water (12.7 g l−1)8 and estimated soil absorption coefficient of 180 can lead to its easy transfer into the environment via groundwater, soil etc.9 PA causes skin sensitization, immunotoxicity and methemoglobinemia, headaches, liver damage, and anemia in humans and can also have a negative effect on the respiratory organs due to the effects of bio-amplification and bioaccumulation. It is a dermal sensitizer and strong eye irritant for animals such as rats.10,11 Additionally, PA can be metabolised to picramic acid, which has a higher mutagenic activity than PA itself.12 PA can also be degraded by the Rhodococcus sp. strain in PA-contaminated soil.13
In the literature, fluorescence-based molecular probes for the detection of NACs have been reviewed, but in not even a single case has the focus been on the selective recognition of picric acid.14,15
Conventional analytical methods, including chromatographic methods such as gas chromatography16 and liquid chromatography,17 mass spectrometry,18 electrochemical techniques19 and Raman spectroscopy20 have been applied for the recognition of picric acid, but have found find limited use due to their low sensitivity, interference from other compounds sample pre-treatment requirements, use of expensive and sophisticated equipment, lack of portability, and difficult on-site operation. Numerous fluorescence-based platforms involving small organic molecules, supramolecules,21 metal complexes,22 metal organic frameworks (MOFs),23 and quantum dots/nanoclusters,24,25 coordination polymers,26 among others, have been synthesized for the real-time detection of picric acid.
In order to improve the sensitivity of fluorescent sensors towards PA, the primary focus must be either increasing the quenching efficiency by increasing the binding affinity for picric acid, or increasing the energy/electron transfer rate between the fluorophore and PA through appropriate design. The ability of picric acid (pKa 0.42)27 to protonate basic sites such as amines or heterocyclic nitrogens present in the fluorophore can induce electrostatic interactions between the protonated fluorophore and the picrate anion. Further, due to its low pKa value, PA remains in dissociated form in water or buffer solutions and electrostatic interactions can form between positively charged fluorescent probes and the picrate anion. Such electrostatic interactions lead to proximity between the electron-rich fluorophore and the picrate anion and positively affect the electron/energy transfer process and sensitivity of the probe toward picric acid. The general mechanistic approach involved in the selective detection of picric acid by such fluorophores is presented in Scheme 1. Other nitrophenol derivatives have poor electron/energy transfer processes in comparison to picric acid, which is due to the difficulty in the protonation of the basic site(s) in the fluorophore in the case of 2,4-DNP (pKa 4.04) and 4-NP (pKa 7.07) and the absence of electrostatic interactions in the case of neutral NACs; thus, they may not interfere in the detection of picric acid.
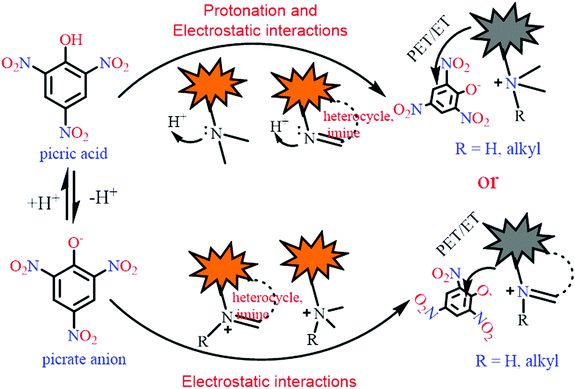 | ||
| Scheme 1 A schematic presentation of the mechanisms involved in the selective fluorescence quenching of different probes by picric acid. | ||
Generally, fluorophores without any protonation sites undergo fluorescence quenching with TNT and PA with equal ease28–30 and display strong TNT-interference in the detection of picric acid. Furthermore, there are number of references in which the quenching of a fluorophore by PA has been evaluated, but the effect of other NACs has not been studied.31,32 Such literature has been omitted in the present discussion.
The discussion of the fluorescent probes (listed in Table 1–4) for picric acid has been classified into following sections:
| S. no. | Probe | Solvent | LOD (μM) | K SV (M−1) | λ ex (nm) | λ em (nm) | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|
| Protonation of amine-based fluorescent probes for picric acid | ||||||||
| 1 | 1 | THF–H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.004 | 3.2 × 106 | 368 | 485 | Paper strips | 33 |
| 398 | 510 | |||||||
| 2 | 2 | THF–H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.009 | 2.0 × 106 | 370 | — | Paper strips | 33 |
| 3 | 3 | CH3OH | 4.89 | 7.203 × 104 | 340 | 385 | TLC plate | 34 |
H2O–CH3OH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 4 | 4 | CH3OH | 4.16 | 3.903 × 104 | 345 | 397 | TLC plate | 34 |
H2O–CH3OH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 5 | 5a | EtOH | 1 | 4.14 × 105 | 362 | 417 | — | 35 |
| 6 | 5b | EtOH | — | 3.34 × 104 | 362 | 417 | — | 35 |
| 7 | 5c | EtOH | — | 5.95 × 103 | 362 | 417 | — | 35 |
| 8 | 6 | THF | 0.3 | 4.51 × 105 | 410 | 520 | Paper strips | 36 |
| 9 | 7 | THF | 0.08 | 1.0 × 105 | 354 | 435 | Test strips | 37 |
THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 460 | TLC plate | |||||
THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
371 | 438 | ||||||
| 10 | 8 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (1 THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.04 | 1.11 × 105 | 306 | 393 | — | 38 |
| 11 | 9 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (7 THF (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.035 | 1.95 × 105 | 322 | 458 | Paper strips | 38 |
| 12 | 10a | CH3CN | — | 1.2 × 105 | 290 | 405 to 480 | — | 39,40 |
| 13 | 10b | CH3CN | 9.82 | 2.8 × 105 | 302 | 415 | — | 42 |
| 14 | 10c | CH3CN | 6.55 | 3.31 × 105 | 295 | 425 | — | 41 |
| 14 | 11 | H2O | 0.0042 | 4.82 × 105 | 460 | 550 | Paper strips | 43 |
| Real sample | ||||||||
| 15 | 12 | H2O | 0.0116 | 5.232 × 105 | 550 | 650 | Paper strips | 43 |
| Real sample | ||||||||
| 16 | 13 | THF | 16 | 0.4 × 104 | 398 | 512 | Paper strips | 44 |
THF–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
2 | 2.6 × 104 | 550 | |||||
| 17 | 14 | EtOH–H2O (5%) | 1.3 | — | 387 | 540 | — | 45 |
| 18 | 15 | THF | 5.7 | — | — | 454 | Paper strips | 46 |
| TLC plate | ||||||||
| 19 | 16 | THF | — | — | — | 454 | — | 46 |
| 20 | 17 | EtOH | 0.079 | 6.1 × 103 | 354 | 410 | — | 47 |
| 21 | 18 | THF–HEPES (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.0578 | 1.05 × 105 | 366 | 408 | Paper strips | 48 |
| Real sample | ||||||||
| 22 | 19 | THF | 26 | — | 384 to 400 | 467 to 576 | — | 49 |
THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
38 | |||||||
| 23 | 20 | THF | — | — | 390 | 471 | — | 49 |
THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
400 → 490 | 490 | ||||||
| 24 | 21 | THF | — | — | 392 | 490 → 540 | — | 49 |
THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
400 → 490 | |||||||
| 25 | 22 | THF | — | — | 402 | 490 → 540 | — | 49 |
THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
400 → 490 | |||||||
| 26 | 23 | CH3CN | 7.2. | 1.4 × 104 | 260 | 350 | — | 50 |
| 520 | ||||||||
| 27 | 24 | CH3CN–H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.44 | — | 365 | 540 | 51 | |
| 28 | 25 | EtOH | 0.0028 | 1.91 × 104 | 499 | 513 | — | 52 |
| 29 | 26 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.0032 | 4.37 × 104 | 367 | 496 to 454 | Real water sample | 53 |
| Paper strips | ||||||||
| 30 | 27 | Aqueous media | 0.0135 | — | 430 | 540 | Real sample | 54 |
| 31 | 28 | Aqueous media | 0.0135 | — | 430 | 535 | Real sample | 54 |
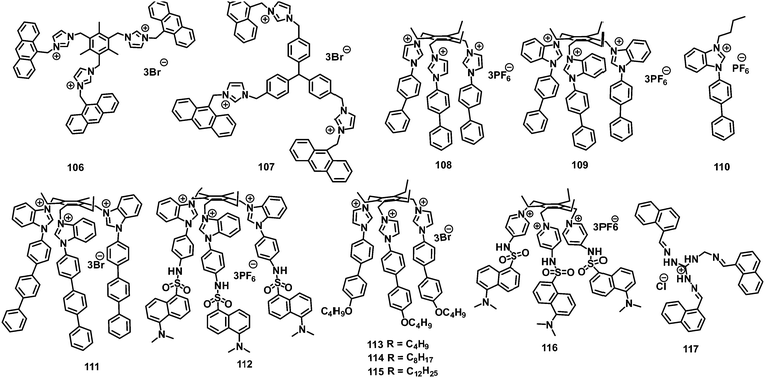
2. Neutral fluorescent probes for the detection of picric acid
2.1 Protonation of amine-based fluorescent probes for picric acid
2.2 Protonation of pyridine-based fluorescent probes for picric acid
2.3 Protonation of imidazole-based fluorescent probes for picric acid
2.4 Protonation of imine-based fluorescent probes for picric acid
2.5 Protonation of amide-based fluorescent probes for picric acid
3. Positively charged probes for the detection of picric acid
3.1 Monopodal cationic fluorescent probes for picric acid
3.2 Dipodal dicationic fluorescent probes for picric acid
4.3 Tripodal tricationic fluorescent probes for picric acid
4. Metal complexes for the detection of picric acid
44. Miscellaneous
2. Neutral fluorescent probes for the detection of picric acid
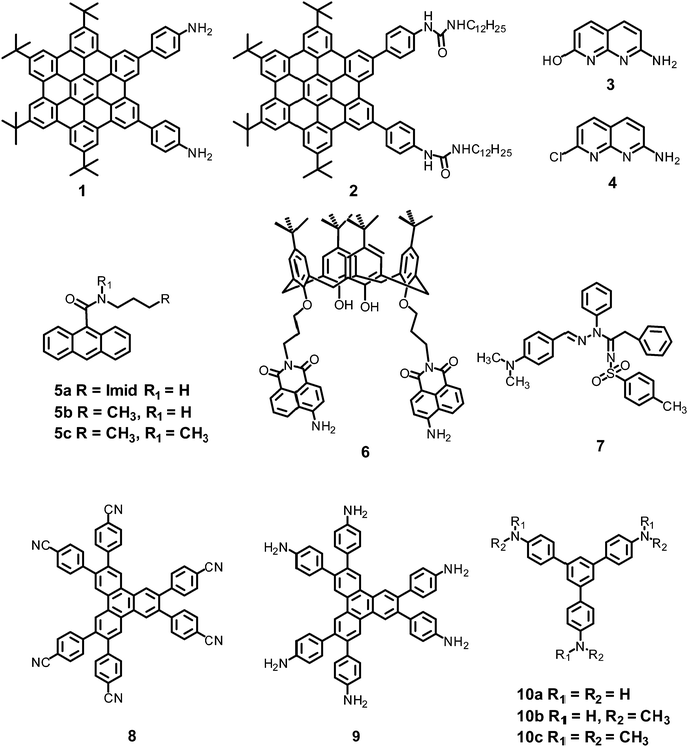
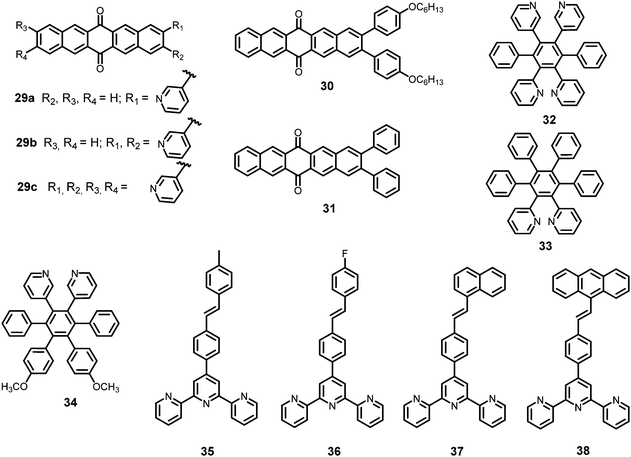
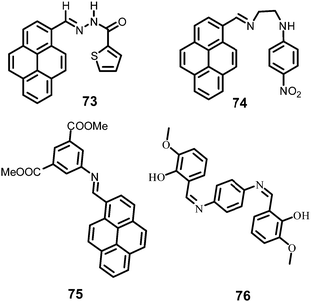
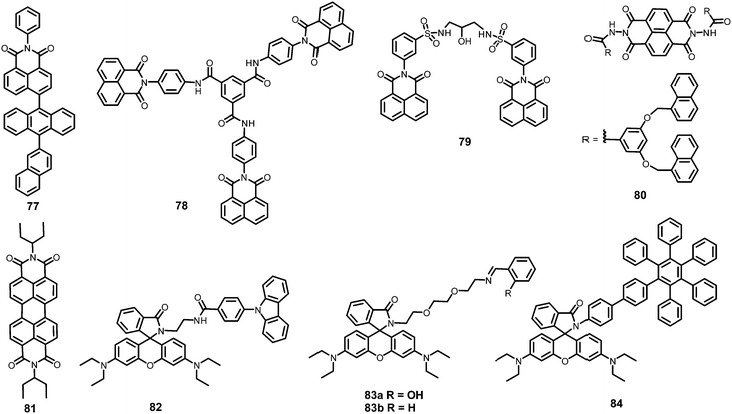
In this section, the fluorescent probes 1–2833–54 (Charts 2–4) undergo protonation by picric acid at the amine nitrogen to form quaternary anilinium picrate salts, and electron or energy transfer from the photo-excited probe to PA results in the formation of a non-emitting complex. However, the strong inhibition of PET in BODIPY-based probe 2552 and the aggregation of curcumin-based probes 27 and 2854 upon protonation with picric acid result in fluorescence enhancement. A comparison of the solvents, LODs, KSV values, emission maxima and applications of probes 1–28 has been presented in Table 1.2.1 Protonation of amine-based fluorescent probes for picric acid
Hexa-peri-hexabenzocoronene (HBC) probes 1 and 233 (Chart 2) formed spherical nano-aggregates with respective diameters of 200 and 150 nm and exhibited 67% and 85% enhancements in fluorescence intensity between 460–600 nm in water–THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) owing to the restriction in rotation. Further increasing the amount of water caused lowered emission intensity due to π–π interactions between the coronene cores. Probe 1 exhibited 98% fluorescence quenching in water–THF (4
6) owing to the restriction in rotation. Further increasing the amount of water caused lowered emission intensity due to π–π interactions between the coronene cores. Probe 1 exhibited 98% fluorescence quenching in water–THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) at pH 7.0, turning from a yellow fluorescent solution to a non-fluorescent one with 5 equivalents of PA. The formation of an electrostatic complex by proton transfer from PA to the basic amino group and electron transfer from photo-excited 1 to PA were responsible for the formation of the non-emitting complex. As the pH of the solution was lowered, the quenching efficiency of 1 by PA gradually decreased owing to the weaker interaction of PA with protonated NH2 and reduced electron transfer from the excited-state HBC core to PA. Probe 2 with no protonation site showed lower quenching efficiency by PA, and its ability to interact with PA remained unaffected by lowering of the pH of the solution. Probe 2 underwent fluorescence quenching by PA through an intermolecular charge transfer mechanism. Test paper strips coated with solutions of 1 and 2 detected PA by contact modes. A solution of 1 could detect PA vapor through fluorescence quenching.
6) at pH 7.0, turning from a yellow fluorescent solution to a non-fluorescent one with 5 equivalents of PA. The formation of an electrostatic complex by proton transfer from PA to the basic amino group and electron transfer from photo-excited 1 to PA were responsible for the formation of the non-emitting complex. As the pH of the solution was lowered, the quenching efficiency of 1 by PA gradually decreased owing to the weaker interaction of PA with protonated NH2 and reduced electron transfer from the excited-state HBC core to PA. Probe 2 with no protonation site showed lower quenching efficiency by PA, and its ability to interact with PA remained unaffected by lowering of the pH of the solution. Probe 2 underwent fluorescence quenching by PA through an intermolecular charge transfer mechanism. Test paper strips coated with solutions of 1 and 2 detected PA by contact modes. A solution of 1 could detect PA vapor through fluorescence quenching.
Naphthyridine-based fluorescent probes 3 and 434 (Chart 2) were used for the detection of PA in both pure methanol and aqueous methanol solutions. Probes 3 and 4 showed complete fluorescence quenching with 4.5 and 4.0 equivalents of PA, respectively, due to the protonation of the amine and H-bonding between the nitrogen atom of the naphthyridine ring and PA. Similar fluorescence quenching of 3 and 4 was observed for 22 equivalents of 4-NP and significantly higher amounts of other NACs. Probes 3 and 4 formed ground-state complexes with PA and followed static quenching processes. TLC plates coated with probes 3 and 4 with PA exhibited a change in fluorescence colour from blue to non-fluorescent (Fig. 1).
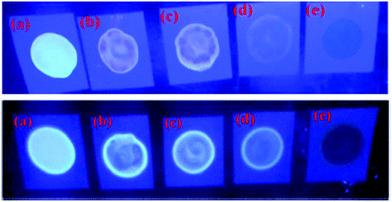 | ||
| Fig. 1 Fluorescence images of TLC plates coated with probe 3 (upper) and probe 4 (lower) with various amounts of PA: (a) 0 M; (b) 10−5 M; (c) 10−4 M; (d) 10−3 M; and (e) 10−2 M. Reprinted from ref. 34, Copyright 2015 Royal Society of Chemistry. | ||
Anthracenecarboxamide-based probe 5a35 (Chart 2) exhibited fluorescence quenching with 5 equivalents of nitrophenols; the fluorescence quenching followed the order PA (>96%) > 2,4-DNP (35%) > 3,4-DNP (17%) > 4-DNP (10%) in ethanol. A Job's plot analysis showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between probe 5a and PA with an association constant of 4.14 × 105 M−1. Probes 5b and 5c also showed formation of a 1
1 complexation between probe 5a and PA with an association constant of 4.14 × 105 M−1. Probes 5b and 5c also showed formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with lower binding constant values of 3.34 × 104 M−1 and 5.95 × 103 M−1, respectively. The 1H NMR downfield shift of the amide proton from δ 8.91 to 9.55 ppm and of the imidazole C2–H from δ 7.68 to 9.05 ppm for probe 5a upon the addition of PA revealed its multiple hydrogen bonding with PA. The fluorescence quenching of probes 5a–5c by PA could be assigned to multiple factors, including FRET, H-bonding and π–π interactions.
1 complex with lower binding constant values of 3.34 × 104 M−1 and 5.95 × 103 M−1, respectively. The 1H NMR downfield shift of the amide proton from δ 8.91 to 9.55 ppm and of the imidazole C2–H from δ 7.68 to 9.05 ppm for probe 5a upon the addition of PA revealed its multiple hydrogen bonding with PA. The fluorescence quenching of probes 5a–5c by PA could be assigned to multiple factors, including FRET, H-bonding and π–π interactions.
Calix[4]arene-based probe 636 (Chart 2) with aminonaphthalimide moieties conjugated at the 1 and 3 aryl rings displayed 97% fluorescence quenching at 520 nm (λex = 410 nm) by PA and resulted in a fluorescence colour change from green to non-florescent and a decrease in the quantum yield from 0.33 to 0.02. 4-Nitrophenol and neutral NACs did not cause any considerable fluorescence quenching of 6. The protonation of the amine by PA inhibited ICT and resulted in non-fluorescent complex formation. AFM studies revealed that the donut-like structures of 6 were transformed into extended ribbon-like structures (Fig. 2A) with a length of several microns, a width of 100–160 nm, and a height of 35–45 nm upon treatment with PA. A solution of 6 and cellulose filter paper strips coated with probe 6 (Fig. 2B) could detect 0.3 μM and 0.1 μM PA, respectively, as the lowest limits.
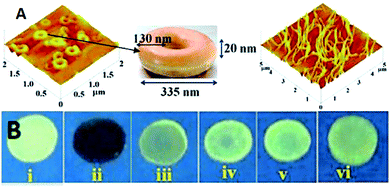 | ||
| Fig. 2 (A) The donut-like structure of 6 transformed into ribbon-like fibrils upon treatment with PA. (B) Fluorescence quenching of 6 at different concentrations of PA: (i) only 6; (ii–vi) {6 + PA}: (ii) 100 mm, (iii) 10 mm, (iv) 1 mm, (v) 100 nm, and (vi) 10 nm. Reprinted from ref. 36, Copyright 2015 Wiley Online. | ||
Hydrazono-sulfonamide adduct 737 (Chart 2), which is weakly emissive in THF, became strongly emissive with a maximum between 434–460 nm upon the addition of 50–100% water, owing to the inhibition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. Solutions of 7 in THF, THF–water (1
N isomerization. Solutions of 7 in THF, THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and THF–water (1
1) and THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) exhibited complete fluorescence quenching by 50, 30 and 30 equivalents of PA, respectively. However, the Stern–Volmer constant (1.0 × 105 M−1) was highest in the THF–water (1
9) exhibited complete fluorescence quenching by 50, 30 and 30 equivalents of PA, respectively. However, the Stern–Volmer constant (1.0 × 105 M−1) was highest in the THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. In the THF–water (1
1) mixture. In the THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture, the aggregates of 7 formed a greater number of voids to interact with PA, and this loose packing was responsible for the higher sensing performance compared to that observed in other solvents. The quenching efficiency of nitrophenols followed the order PA > 4-NP > 2,4-DNP. The protonation of the dimethylarylamine nitrogen associated with electron transfer from the excited state of fluorophore 7 to PA was responsible for the fluorescence quenching.
1) mixture, the aggregates of 7 formed a greater number of voids to interact with PA, and this loose packing was responsible for the higher sensing performance compared to that observed in other solvents. The quenching efficiency of nitrophenols followed the order PA > 4-NP > 2,4-DNP. The protonation of the dimethylarylamine nitrogen associated with electron transfer from the excited state of fluorophore 7 to PA was responsible for the fluorescence quenching.
Triphenylene derivatives 8 and 938 revealed a 1.5- to 1.6-fold fluorescence enhancement in an H2O–THF (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixture owing to the formation of aggregates (Fig. 3A and B) and the restriction of TICT. The fluorescent aggregates of 838 (Chart 2) with 20 equivalents PA in an H2O–THF (1
3) mixture owing to the formation of aggregates (Fig. 3A and B) and the restriction of TICT. The fluorescent aggregates of 838 (Chart 2) with 20 equivalents PA in an H2O–THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture displayed 98% fluorescence quenching following the energy transfer process at 393 nm, as observed from the overlap of the fluorescence spectrum of 8 with the absorbance spectrum of PA. The aggregates of 9 with 26 equivalents of PA in an H2O–THF (7
1) mixture displayed 98% fluorescence quenching following the energy transfer process at 393 nm, as observed from the overlap of the fluorescence spectrum of 8 with the absorbance spectrum of PA. The aggregates of 9 with 26 equivalents of PA in an H2O–THF (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixture exhibited fluorescence quenching at 458 nm due to the protonation of the basic NH2 groups and electrostatic interactions between the protonated aggregates of 9 and the picrate anion. Probe 9 displayed higher selectivity towards PA than derivative 8. Paper strips coated with 9 (Fig. 3C) could detect 10−11 M PA. Other NACs such as TNT, 2,4-DNT, 1,4-DNB, NB, 4-NT, BQ, NM and DMNB caused fluorescence quenching, but at significantly higher concentrations.
3) mixture exhibited fluorescence quenching at 458 nm due to the protonation of the basic NH2 groups and electrostatic interactions between the protonated aggregates of 9 and the picrate anion. Probe 9 displayed higher selectivity towards PA than derivative 8. Paper strips coated with 9 (Fig. 3C) could detect 10−11 M PA. Other NACs such as TNT, 2,4-DNT, 1,4-DNB, NB, 4-NT, BQ, NM and DMNB caused fluorescence quenching, but at significantly higher concentrations.
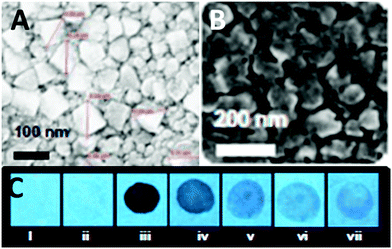 | ||
Fig. 3 Scanning electron microscopy (SEM) images of (A) 9 in a H2O–THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and (B) 9 in a H2O 1) mixture and (B) 9 in a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (7 THF (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixture. (C) Photographs of paper strips coated with 9 showing fluorescence quenching (under 365 nm light): (i) blank paper strip and (ii) paper strip with a drop of water; paper strips upon the addition of 10 μl of solutions of PA with different concentrations: (iii) 10−3 M, (iv) 10−5 M, (v) 10−7 M, (vi) 10−9 M, and (vii) 10−11 M. Reprinted from ref. 38, Copyright 2015 Royal Society of Chemistry. 3) mixture. (C) Photographs of paper strips coated with 9 showing fluorescence quenching (under 365 nm light): (i) blank paper strip and (ii) paper strip with a drop of water; paper strips upon the addition of 10 μl of solutions of PA with different concentrations: (iii) 10−3 M, (iv) 10−5 M, (v) 10−7 M, (vi) 10−9 M, and (vii) 10−11 M. Reprinted from ref. 38, Copyright 2015 Royal Society of Chemistry. | ||
The C3-symmetric 1,3,5-tris(4′-aminophenyl)benzene 10a39,40 (Chart 2) exhibited a red shift in its emission maximum from 405 nm to 480 nm due to PA. A solution of 10a with 32 equivalents of PA and 450–640 equivalents of TNT, DNT and 1,4-DNB exhibited >80% quenching of fluorescence intensity, pointing to the selective fluorescence quenching by PA (Fig. 4A). The X-ray crystal structures of 10a with PA (Fig. 4B) and TNT revealed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexes through strong intermolecular π–π interactions between 10a and the NACs. The layers of the probe could intercalate three molecules of 1,3-DNB. The crystal lattices are further stabilized through inter-stack hydrogen bonds (N–H⋯N and N–H⋯O) between the amino groups of the probe and nitro groups of the NACs (Fig. 4B). Thin films prepared from 10a could detect TNT and DNT vapor, but not PA vapor.
1 stoichiometric complexes through strong intermolecular π–π interactions between 10a and the NACs. The layers of the probe could intercalate three molecules of 1,3-DNB. The crystal lattices are further stabilized through inter-stack hydrogen bonds (N–H⋯N and N–H⋯O) between the amino groups of the probe and nitro groups of the NACs (Fig. 4B). Thin films prepared from 10a could detect TNT and DNT vapor, but not PA vapor.
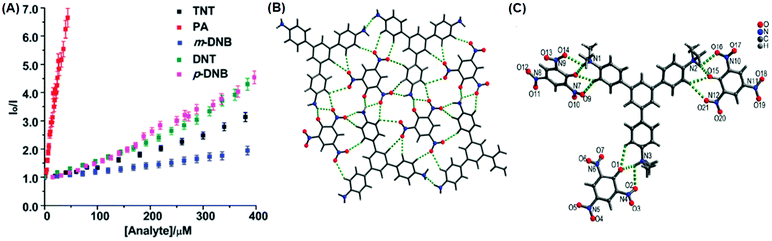 | ||
| Fig. 4 (A) Stern–Volmer plots of the fluorescence quenching of 10a (1.0 mM in acetonitrile) with TNT, PA, m-DNB, DNT, and p-DNB. (B) The crystal structure of the 10a(PA) complex showing hydrogen bonds. (C) The crystal structure of the 10c(PA)3 complex showing hydrogen bonds (H-bonds are shown as dashed bonds). Reprinted from ref. 39, Copyright 2014 Royal Society of Chemistry. Reprinted from ref. 40, Copyright 2014 American Chemical Society. Reprinted from ref. 41, Copyright 2015 Royal Society of Chemistry. | ||
Probe 10c,41 which exhibited reduced H-bond donor characteristics in comparison to probe 10a, but increased basicity of the amine nitrogen, revealed 90% fluorescence quenching with only 10 equivalents of PA in comparison to the 32 equivalents of PA required by probe 10a. Furthermore, 10c formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (10c ∩ (PA)3) stoichiometric complex through proton transfer from three molecules of PA to the three amine nitrogens of 10c, as was clearly evident from the X-ray crystal structure (Fig. 4C). However, 10a formed only a 1
3 (10c ∩ (PA)3) stoichiometric complex through proton transfer from three molecules of PA to the three amine nitrogens of 10c, as was clearly evident from the X-ray crystal structure (Fig. 4C). However, 10a formed only a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex through H-bonding (Fig. 4B). The association constants of 10c with NACs followed the order PA ≫ 1,4-DNB > TNT ≈ 2,4-DNT ≈ 1,3-DNB, with PA being almost 25 times more effective as a quencher in comparison to the other NACs. Additionally, instead of the PET mechanism operative in the case of probe 10a, here, proton transfer is the actual quenching mechanism. The quenching efficiency of 10b42 was intermediate between those of probes 10a and 10c, and their quenching efficiency followed the same order as the basicity of the amine nitrogens. The emission spectrum of 10b showed 85% quenching of the emission maximum with 20 equivalents of PA with a detection limit of 9.82 μM.
1 stoichiometric complex through H-bonding (Fig. 4B). The association constants of 10c with NACs followed the order PA ≫ 1,4-DNB > TNT ≈ 2,4-DNT ≈ 1,3-DNB, with PA being almost 25 times more effective as a quencher in comparison to the other NACs. Additionally, instead of the PET mechanism operative in the case of probe 10a, here, proton transfer is the actual quenching mechanism. The quenching efficiency of 10b42 was intermediate between those of probes 10a and 10c, and their quenching efficiency followed the same order as the basicity of the amine nitrogens. The emission spectrum of 10b showed 85% quenching of the emission maximum with 20 equivalents of PA with a detection limit of 9.82 μM.
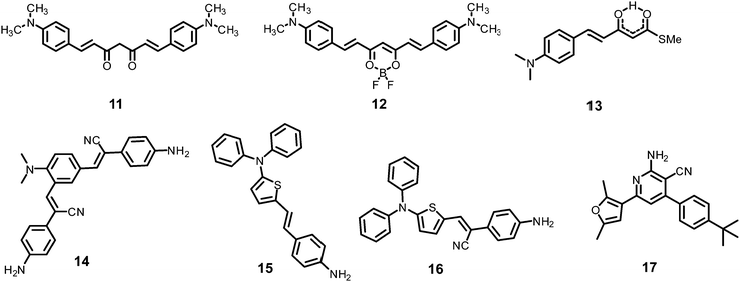
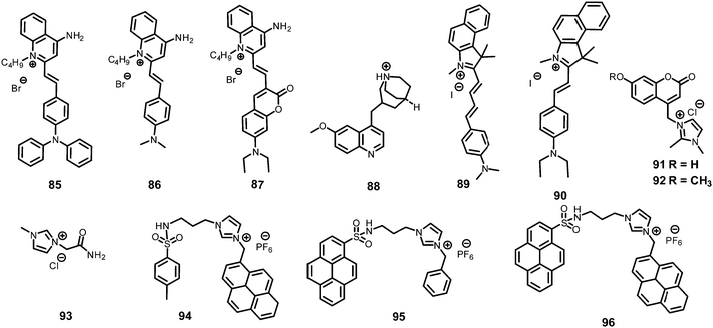
The curcumin-based fluorophores 11 and 1243 (Chart 3) with PA exhibited 92 and 93% quenching of the intense emission at 550 nm for 11 and 650 nm for 12, respectively, with a visible colour change from yellow to red for 11 and pink to bluish green for 12 in the aqueous solution. No change in the FI was observed with the addition of other NACs. The protonation of 11/12 by PA and electron transfer from the fluorophore to PA are responsible for fluorescence quenching via the static process. The probes were also used to detect PA in water samples.
Probe 1344 showed a red shift in its emission maximum from 485 nm to 543 nm when moving from the non-polar solvent toluene to the highly polar solvent methanol, indicating that due to increased ICT, the molecular geometry in the first excited state is more polarized than that in the ground state. A THF–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) solution of 13 displayed the highest emission intensity among the binary solutions owing to the formation of aggregates with restriction in the π–π stacking interactions and intramolecular rotations. The high emission of 13 in THF–H2O (4
6) solution of 13 displayed the highest emission intensity among the binary solutions owing to the formation of aggregates with restriction in the π–π stacking interactions and intramolecular rotations. The high emission of 13 in THF–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) decreased by 97.32% upon the addition of 30 equivalents of PA, whereas in pure THF, 95.43% fluorescence quenching was observed with 40 equivalents of PA. The emission quenching mechanism is explained by the electron transfer and/or energy transfer processes. Among PA, 2,4-DNP, 4-NP, TNT, 1,4-DNB and NB, PA had the greatest quenching efficiency (Fig. 5). Paper strips coated with 13 showed a dark spot for 10−3 M PA.
6) decreased by 97.32% upon the addition of 30 equivalents of PA, whereas in pure THF, 95.43% fluorescence quenching was observed with 40 equivalents of PA. The emission quenching mechanism is explained by the electron transfer and/or energy transfer processes. Among PA, 2,4-DNP, 4-NP, TNT, 1,4-DNB and NB, PA had the greatest quenching efficiency (Fig. 5). Paper strips coated with 13 showed a dark spot for 10−3 M PA.
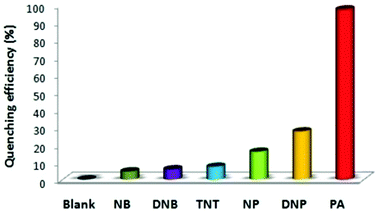 | ||
Fig. 5 Relative fluorescence quenching of 13 (10−5 M) in THF–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) upon the addition of various NACs (30 equivalents). Reprinted from ref. 44, Copyright 2018 Wiley Online. 6) upon the addition of various NACs (30 equivalents). Reprinted from ref. 44, Copyright 2018 Wiley Online. | ||
α-Cyanostilbene derivative 1445 (Chart 3) with 4 equivalents of PA in an ethanol–water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) mixture revealed 60% fluorescence quenching (at 540 nm), but the quenching did not exceed 20% with other NACs. Remarkable acidochromic behaviour was observed with TFA, hydrochloric acid, TEA and/or their vapors due to the basicity of the nitrogen atom. Using mechanical grinding, solvent writing and vapor erasing, a reversible fluorescence switching cycle was observed.
5) mixture revealed 60% fluorescence quenching (at 540 nm), but the quenching did not exceed 20% with other NACs. Remarkable acidochromic behaviour was observed with TFA, hydrochloric acid, TEA and/or their vapors due to the basicity of the nitrogen atom. Using mechanical grinding, solvent writing and vapor erasing, a reversible fluorescence switching cycle was observed.
Thiophene-based probes 15 and 1646 (Chart 3) were used for the detection of PA and intracellular pH. 15 was more selective for PA as compared to 16. Probe 15 showed a 30% reduction in emission intensity in THF with 0.1 equivalents of PA and could detect concentrations as low as 5.7 μM PA. Paper strips and TLC plates coated with probe 15 solution detected PA in visible light (colourless to yellow) and under 365 nm (blue fluorescent to non-fluorescent). The compound was also used for the fluorescence-based determination of pH in live cells.
Fluorophore 1747 (Chart 3) displayed quenching of its fluorescence intensity with no shift in the emission maximum with PA in ethanol. The quenching was due to the H-bonding interactions of the NH2 group of 17 with the phenolic –OH group of PA, as the singlet at δ 5.33 ppm for the free NH2 group shifted downfield to δ 6.48 ppm after interaction with PA in the 1H NMR experiment. Probe 17 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with PA with a Stern–Volmer constant of 6.1 × 103 M−1.
1 stoichiometric complex with PA with a Stern–Volmer constant of 6.1 × 103 M−1.
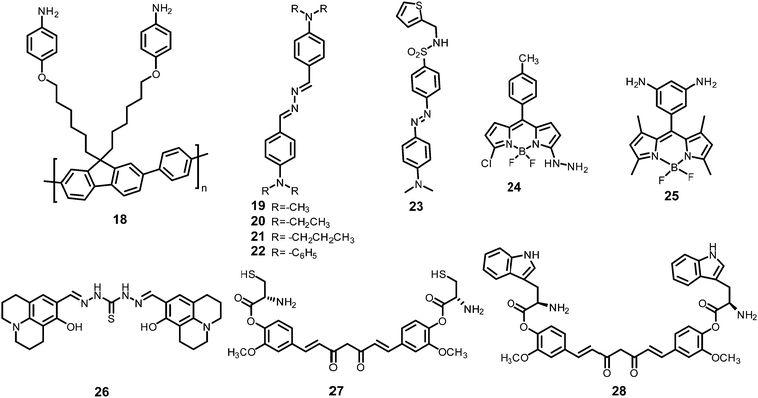
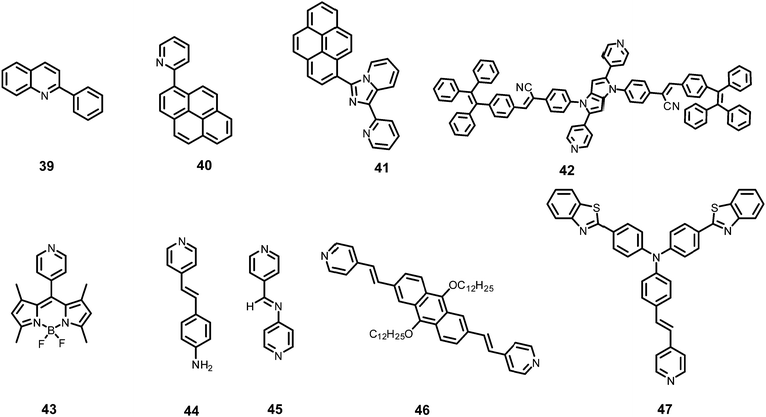
Inner filter effect and protonation assisted PET-based detection of PA using conjugated polymer 18 (Chart 4) was developed by Iyer et al.48 Upon excitation of a THF solution of 18 at 366 nm, emission was observed at 408 nm. The fluorescence of 18 in THF–HEPES (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) buffer solution was quenched by 25% with only 3.3 equivalents of PA, and was decreased by 95% with 50 equivalents of PA. The LOD was found to be 0.0578 μM. Metal ions, anions, and other nitroaromatics did not produce the same quenching phenomena as PA. The significant spectral overlap and insignificant lifetime change revealed the quenching mechanism to be the inner filter effect (IFE). Along with the IFE, the PET from polymer 18 to PA induced by acid–base interaction was also responsible for the quenching. The probe was able to detect PA in natural water samples, as well as on probe-18-coated test strips.
1) buffer solution was quenched by 25% with only 3.3 equivalents of PA, and was decreased by 95% with 50 equivalents of PA. The LOD was found to be 0.0578 μM. Metal ions, anions, and other nitroaromatics did not produce the same quenching phenomena as PA. The significant spectral overlap and insignificant lifetime change revealed the quenching mechanism to be the inner filter effect (IFE). Along with the IFE, the PET from polymer 18 to PA induced by acid–base interaction was also responsible for the quenching. The probe was able to detect PA in natural water samples, as well as on probe-18-coated test strips.
AIE-based azine derivatives 19–2249 (Chart 4) exhibited the appearance of a new absorption band with PA in THF. In the presence of PA, probe 19 displayed decreased absorption at 380 nm and the appearance of a new maximum at 480 nm with an isosbestic point at 415 nm and a colour change from colourless to yellow. Upon excitation at 415 nm, 19 displayed a gradual increase in fluorescence intensity at its emission maximum (576 nm) with increasing concentration of PA. Other NACs, such as 2-NP, 3-NP, 4-NP, 2,4-dinitrobenzoic acid and NB, did not induce any such change in the absorption and emission of the azine derivative in THF. This highly selective increase in fluorescence intensity by PA could be assigned to the formation of H-bonding between PA and the nitrogen of the N,N-dimethylamino moiety, resulting in increased internal charge transfer and the stabilization of the more-polarised excited state. In THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), probe 19 exhibited a similar red-shift in the absorption band to 490 nm with PA due to ground-state complex formation, but displayed fluorescence quenching at 567 nm in fluorescence studies.
7), probe 19 exhibited a similar red-shift in the absorption band to 490 nm with PA due to ground-state complex formation, but displayed fluorescence quenching at 567 nm in fluorescence studies.
The addition of picric acid to probe 2350 (Chart 4) caused a shift in the absorption maximum from 440 nm to 500 nm and fluorescence quenching at 350 nm and 520 nm. The 1H NMR spectrum of probe 23 with picric acid revealed the downfield shift of the –N(CH3)2 protons from δ 3.13 ppm to 3.25 ppm, as well as a shift in the aromatic protons of picric acid from δ 9.00 ppm to δ 9.10 ppm due to electrostatic and hydrogen-bond interactions.
Hydrazine-substituted BODIPY probe 2451 (Chart 4) displayed emission with a maximum at 540 nm in CH3CN–water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and distinctly differentiated RDX and PA from each other and from other explosives through different absorption and fluorescence signals. 40 μM of 2,4-DNT, PA, TNT and RDX were tested, and only RDX caused the fluorescence enhancement of probe 24, together with an obvious color change to bright yellow, while PA prominently decreased the fluorescence intensity (Fig. 6a). The fluorescence intensity of probe 24 declined linearly as the concentration of PA was increased from 0 to 40 μM, and a quenching efficiency of 65.14% was obtained. In the case of RDX, the HCHO released from RDX formed a Schiff base with the hydrazine derivative to inhibit the PET process and resulted in a fluorescence enhancement (Fig. 6b). In case of PA, the inner-filter effect caused fluorescence quenching of fluorophore 24.
2) and distinctly differentiated RDX and PA from each other and from other explosives through different absorption and fluorescence signals. 40 μM of 2,4-DNT, PA, TNT and RDX were tested, and only RDX caused the fluorescence enhancement of probe 24, together with an obvious color change to bright yellow, while PA prominently decreased the fluorescence intensity (Fig. 6a). The fluorescence intensity of probe 24 declined linearly as the concentration of PA was increased from 0 to 40 μM, and a quenching efficiency of 65.14% was obtained. In the case of RDX, the HCHO released from RDX formed a Schiff base with the hydrazine derivative to inhibit the PET process and resulted in a fluorescence enhancement (Fig. 6b). In case of PA, the inner-filter effect caused fluorescence quenching of fluorophore 24.
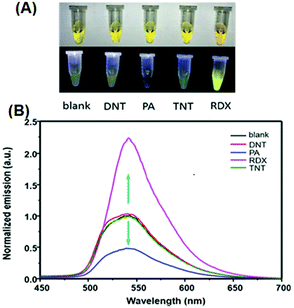 | ||
| Fig. 6 (A) Images of the products after the reaction of different explosives with probe 24 in daylight (top) and under a 365 nm UV lamp (bottom). (B) Normalized emission spectra in the presence of different explosives. The concentration of the explosives is 40 μM, λex = 365 nm, and λem = 540 nm. Reprinted from ref. 51, Copyright 2019 American Chemical Society. | ||
The “turn-on” fluorescent BODIPY probe 2552 (Chart 4) revealed a 300-fold enhancement in emission intensity at 513 nm with 10 equivalents of PA in ethanol upon excitation at 499 nm. The Job's plot showed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex between probe 25 and PA. Therefore, only one of the two amino groups could be protonated by PA. The Benesi–Hildebrand plot gave a binding constant of 1.91 × 104 M−1. Probe 25 could detect PA concentrations as low as 0.65 ppb.
1 stoichiometric complex between probe 25 and PA. Therefore, only one of the two amino groups could be protonated by PA. The Benesi–Hildebrand plot gave a binding constant of 1.91 × 104 M−1. Probe 25 could detect PA concentrations as low as 0.65 ppb.
Hydrazone-based probe 2653 (Chart 4) in DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) exhibited intense green fluorescence centred at 496 nm due to an ESIPT process. With 2 equivalents of PA, the intensity of the probe at the 496 nm emission maximum decreased with the appearance of a new maximum at 454 nm (Fig. 7). This quenching behaviour was due to the hydrogen bonding interactions between PA and the basic amine NH of probe 26, which resulted in inhibition of the ESIPT. The model compound O-methylated derivative of 26 was non-fluorescent and showed no interaction with PA. This proved the importance of the phenolic –OH group and the ESIPT mode for quenching. The quenching effect was found to be rapid (response time <1 min), selective, and pH independent. Furthermore, probe 26 was used to detect PA in the solid and vapor state, and in real water samples.
4) exhibited intense green fluorescence centred at 496 nm due to an ESIPT process. With 2 equivalents of PA, the intensity of the probe at the 496 nm emission maximum decreased with the appearance of a new maximum at 454 nm (Fig. 7). This quenching behaviour was due to the hydrogen bonding interactions between PA and the basic amine NH of probe 26, which resulted in inhibition of the ESIPT. The model compound O-methylated derivative of 26 was non-fluorescent and showed no interaction with PA. This proved the importance of the phenolic –OH group and the ESIPT mode for quenching. The quenching effect was found to be rapid (response time <1 min), selective, and pH independent. Furthermore, probe 26 was used to detect PA in the solid and vapor state, and in real water samples.
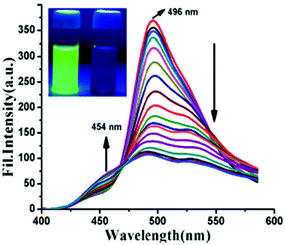 | ||
| Fig. 7 Emission spectra of probe 26 (λex = 367 nm) with PA (0–2 equiv.). The inset shows the fluorescence color change before (intense green) and after (low-intensity blue) the addition of PA to 26. Reprinted from ref. 53, Copyright 2017 American Chemical Society. | ||
The two curcumin-based probes 27 and 2854 (Chart 4) were synthesized by linking curcumin with L-cysteine in probe 27 and L-tryptophan in probe 28. These probes bind with PA due to the presence of basic amine groups. The weak emission bands at 540 nm and 535 nm for 27 and 28, respectively, were enhanced 26.5-fold after the addition of 70 nM PA. The electrostatic interactions of the protonated fluorophore with the picrate anion caused strong association and aggregation, which were responsible for the fluorescence enhancement. The probes had high selectivity, low interference, and a quick response. Practical applications for sensing PA in the solid state and in real water samples were also studied.
2.2 Protonation of pyridine-based fluorescent probes for picric acid
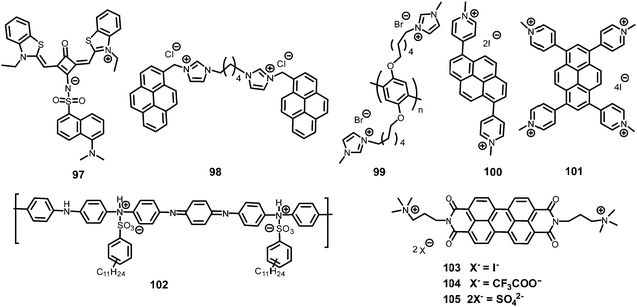
In general, picric acid can form a hydrogen bond with a pyridine nitrogen to stabilize the hydrogen-bonded complex or can completely transfer the proton to the pyridine nitrogen to form an ionic complex. These situations differ only in the extent of internal charge transfer. The fluorescent probes 29–60 (Charts 5–7) protonate the pyridine nitrogen, and a comparison of the solvents, LODs, KSV values, emission maxima and applications of probes 29–60 has been presented in Table 2.| Protonation of pyridine-based fluorescent probes for picric acid | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. no. | Probe | Solvent | LOD (μM) | K SV (M−1) | λ ex (nm) | λ em (nm) | Applications | Ref. |
| 1 | 29a | H2O–DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.720 | 3.40 × 103 | 310 | 481 | Paper strips | 55 |
| 2 | 29b | H2O–DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.650 | 4.36 × 103 | 310 | 481 | Paper strips | 55 |
| 3 | 29c | H2O–DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.600 | 6.52 × 103 | 310 | 492 | Paper strips | 55 |
| 4 | 30 | H2O–THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.250 | 8.44 × 103 | 300 | 475, 555 | Paper strips | 55 |
| 5 | 31 | H2O–THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.290 | 3.61 × 103 | 300 | 475, 555 | Paper strips | 55 |
| 6 | 32 | H2O–EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.026 | — | 273 | 339 to 460 | Paper strips | 56 |
| 7 | 33 | H2O–EtOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
— | — | 273 | 339 to 460 | — | 56 |
| 8 | 34 | H2O–EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
— | — | 290 | 345 | — | 56 |
| 9 | 35 | CHCl3 | 0.131–0.294 | 2.54 × 104 | 334 | 417 | Paper strips | 57 |
| 10 | 36 | CHCl3 | 0.131–0.294 | 2.63 × 104 | 339 | 435 | Paper strips | 57 |
| 11 | 37 | CHCl3 | 0.131–0.294 | 2.69 × 104 | 341 | 437 | Paper strips | 57 |
| 12 | 38 | CHCl3 | 0.131–0.294 | 8.08 × 104 | 390 | 498 | Paper strips | 57 |
| 13 | 39 | Hydrosol | 0.43 | 7.8 × 104 | 315 | 359 | — | 58 |
| 14 | 40 | MeOH | 0.056 | — | — | 405 to 522 | — | 59 |
| 15 | 41 | H2O–DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.063 | — | — | 530 | Smartphone device | 60 |
| 16 | 42 | DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
0.0315 | 2.3 × 106 | 380 | 527 | Water sample, test strips | 61 |
| 17 | 43 | DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.013 | 7.69 × 104 | 375 | 522 | Real sample | 62 |
| 18 | 44 | THF | 1.747 | 4.106 × 105 | 350 to 424 | 440 to 550 | — | 63 |
| 19 | 45 | CH3CN–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.5 | 4.4 × 105 | 330 | 410 | Water sample | 64 |
| 20 | 46 | THF | 2.184 | 4.3 × 104 | 360 to 500 | 500 | Paper strips | 65 |
| TLC plates | ||||||||
| 21 | 47 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8 H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
100.4 | — | 400 | 500 | — | 66 |
| 22 | 48 | DMF | 0.108 | 3.86 × 105 | 419 | 483 | — | 67 |
| 23 | 49 | DMF | 0.138 | 3.21 × 105 | 369 | 420 | — | 68 |
| 24 | 50 | DMF | 0.102 | 4.65 × 105 | 353 | 448 | — | 68 |
| 25 | 51 | DMF | 0.196 | 1.57 × 105 | 335 | 416, 559 | — | 68 |
| 26 | 52 | EtOH | 0.075 | 4.15 × 105 | 500 | 545 | Paper strips | 69 |
| H2O | 0.570 | 3.94 × 104 | 400 | 542 | ||||
| 27 | 53 | EtOH | 0.110 | 2.5 × 105 | 517 | 558 | Paper strips | 69 |
| H2O | 0.93 | 2.1 × 104 | 400 | 555 | ||||
| 28 | 54 | H2O | 2.62 | 5.78 × 104 | — | 420 | Paper strips | 70 |
| 29 | 55 | H2O | 6.98 | 3.26 × 104 | 450 | Paper strips | 70 | |
| 30 | 56 | MeOH | 0.0056 | 6.87 × 104 | 490 | 537, 576, 627 | — | 71 |
| 31 | 57 | CH3CN | 0.644 | 3.45 × 105 | 365 | 493 | Test strips | 72 |
| H2O | 0.586 | 2.18 × 105 | 365 | 460 | ||||
| 32 | 58 | CH3CN | — | — | 365 | 438 | — | 72 |
| 33 | 59a | EtOH | — | 67.6 × 103 | 370 | 474 | — | 73 |
| 34 | 59b | EtOH | — | 60.3× 103 | 370 | 474 | — | 73 |
| 35 | 59c | EtOH | — | 74.2 × 103 | 370 | 474 | — | 73 |
| 36 | 59d | EtOH | — | 62.2 × 103 | 370 | 474 | — | 73 |
| 37 | 60 | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
0.0373 | 6.4 × 107 | 540 | 581 | Paper strips, water samples | 74 |
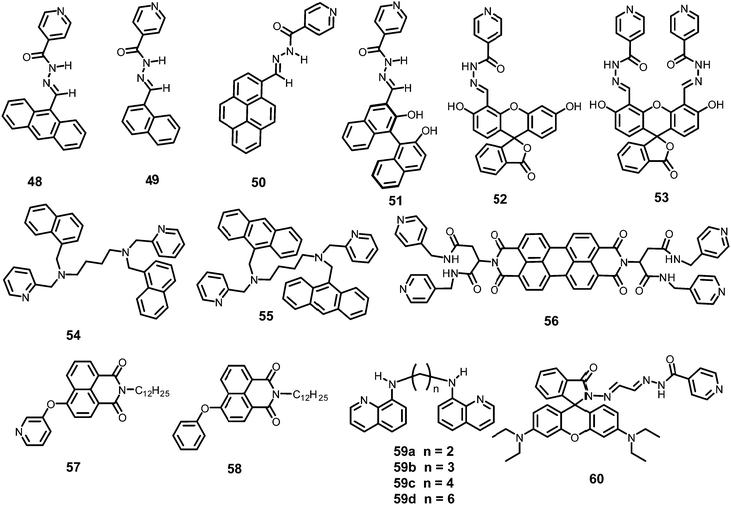
The AIEE-active mono-, di- and tetra-pyridine-substituted pentacenequinone-based probes 29a–29c55 (Chart 5) exhibited increased fluorescence intensity upon increasing the water fraction or viscosity and decreasing the temperature due to restriction in rotation (RIR). In DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures, probes 29a, 29b and 29c showed >95% fluorescence quenching with 62, 60 and 58 equivalents of PA, respectively. The fluorescence quenching by PA was attributed to electron and/or energy transfer mechanisms.
1) mixtures, probes 29a, 29b and 29c showed >95% fluorescence quenching with 62, 60 and 58 equivalents of PA, respectively. The fluorescence quenching by PA was attributed to electron and/or energy transfer mechanisms.
The minimum detection limit for PA was 600 nM with probe 29c. Probes 29a–29c were non-selective towards PA, and other NACs such as TNT, 2,4-DNT, 1,3-DNB, and DNBA also caused competitive fluorescence quenching in these probes. Probes 30–31 showed similar results with PA and other NACs. TLC strips coated with 29c could detect 10−8 M PA via fluorescence quenching.
Among the hetero-oligophenylene derivatives 32–34,56 (Chart 5), derivative 32 bearing four 3-pyridyl moieties exhibited quenching of its fluorescence intensity at an emission maximum of 339 nm with 2.5 equivalents of PA, but with >2.5 equivalents of PA, a new emission band appeared at 446 nm. Its intensity increased gradually with increasing amounts of PA and was finally red-shifted to 460 nm (Fig. 8A and B). The π–π interactions of derivative 32 with PA probably caused the fluorescence quenching at 339 nm, and at higher amounts of PA, the excited-state intermolecular proton transfer from PA to the pyridyl nitrogen resulted in the new emission band at 460 nm. Derivative 33 with 2-pyridyl groups exhibited similar results with PA, but derivative 34 exhibited only fluorescence quenching. Paper strips (Fig. 8C) coated with 32 could detect 10−12 M PA.
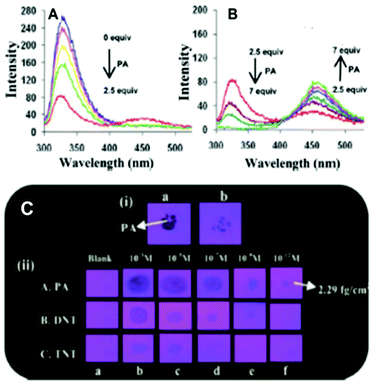 | ||
Fig. 8 (A) Fluorescence spectra of 32 (50 μM) with picric acid (125 μM) in H2O–EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in HEPES, pH = 7.0; λex = 273 nm. (B) Fluorescence spectra of 32 (50 μM) with 350 μM of PA in H2O–EtOH (6 4) in HEPES, pH = 7.0; λex = 273 nm. (B) Fluorescence spectra of 32 (50 μM) with 350 μM of PA in H2O–EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); λex = 273 nm. (C) Photographs of 32-coated TLC strips under different experimental conditions: (i) (a) PA crystals on top of TLC strips coated with derivative 32 and (b) the removal of the PA crystal after 5 s; (ii) photographs of the fluorescence quenching of 32-coated TLC strips by PA, DNT, and TNT in contact mode (10 μL of analyte with a contact area of 0.05 cm2). Reprinted from ref. 56, Copyright 2014 Royal Society of Chemistry. 4); λex = 273 nm. (C) Photographs of 32-coated TLC strips under different experimental conditions: (i) (a) PA crystals on top of TLC strips coated with derivative 32 and (b) the removal of the PA crystal after 5 s; (ii) photographs of the fluorescence quenching of 32-coated TLC strips by PA, DNT, and TNT in contact mode (10 μL of analyte with a contact area of 0.05 cm2). Reprinted from ref. 56, Copyright 2014 Royal Society of Chemistry. | ||
The extended π-conjugated arylene–vinylene probes 35–3857 (Chart 5) displayed a gradual red-shift of their emission maximum from 417 (in the case of 35) to 498 nm (in the case of 38). The maximum quantum yield and red-shift of the emission for probe 38 were found to be due to anthracene having the maximum π-conjugation and minimum HOMO–LUMO energy gap. The intensity of the emission for all four probes in chloroform solution decreased after the addition of PA, but there was no interference from any other nitroaromatics, such as NB, NT, 4-NP, 4-NB acid, or 2,4-DNT. With PA, the colour of the probe 38 solution turned an intense yellow owing to the formation of a charge-transfer complex with PA. Moreover, the spectral overlap of the absorption with the emission bands of the probes permitted energy transfer from the excited state of the arylene–vinylene unit of probes 35–38 to the ground state of PA and increased the fluorescence quenching efficiency. Paper strips (Fig. 9) coated with probe 38 could detect 10−9 M PA from 5 μl of solution.
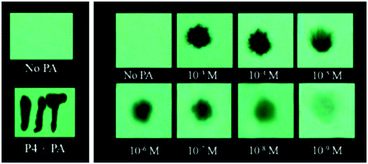 | ||
| Fig. 9 The visual detection of different concentrations of PA using paper test strips coated with probe 38, demonstrating its remarkable sensitivity towards trace PA down to the nanomolar level. Reprinted from ref. 57, Copyright 2017 Royal Society of Chemistry. | ||
The small molecule phenyl quinoline (39)58 (Chart 6) emits weakly at 359 nm in THF, but the addition of 90% water (SDS – 4 mM) resulted in an increase in the fluorescence intensity and quantum yield due to the formation of rod-like microstructures (Fig. 10). The addition of PA resulted in 80% quenching of the fluorescence intensity via a static quenching mechanism with no change in the emission maximum, whereas the other NACs exhibited lower fluorescence quenching, viz. 4-NP (34%), 2,4-DNP (15%), NA (12%), DNT (5%) and DNB (3%). Probe 39 was also used as an acid–base sensor due to its ratiometric fluorescence emission in presence of acids and bases.
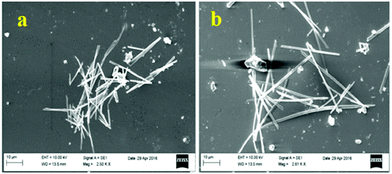 | ||
| Fig. 10 SEM images of probe 39 with 4 mM SDS. Reprinted from ref. 58, Copyright 2018 Royal Society of Chemistry. | ||
Probe 4059 (Chart 6) in methanol with PA displayed a linear relationship between the ratio of the emission intensities (I522/I405) and the concentration of PA in the range of 5–100 μM. In the 1H NMR spectrum of the probe–PA in CDCl3, the up-field shift of the OH signal of picric acid from δ 9.23 ppm to 8.56 ppm and the down-field shift of the pyridine protons supported the formation of a ground state charge transfer complex, and the overlap of the emission of probe with the absorption spectrum of PA supported a FRET process.
Pyrene-tethered probe 4160 (Chart 6) exhibited an emission maximum at 495 nm in THF, which gradually shifted to 515 nm with increasing amounts of DMF in THF–DMF binary mixtures and demonstrated the occurrence of an ICT transition in probe 41. A solution of probe 41 in DMSO displayed efficient fluorescence quenching at an emission maximum of 530 nm by PA. Among other nitroaromatics, only 2,4-DNP showed some interference. Theoretical studies clearly indicated that the pyridine nitrogen preferably formed a hydrogen bond with the OH group of PA and was primarily responsible for the fluorescence quenching. Probe 41 could also detect PA in a 50% DMSO–water medium. A portable fluorimeter module interfaced wirelessly with a smartphone could detect 99 nM PA.
TPE- and pyridine-unit-based probe 4261 (Chart 6) revealed strong AIE behaviour with a strong green emission maximum at 527 nm in a DMF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) mixture. These aggregates of probe 4261 with PA exhibited fluorescence quenching at 527 nm with a color change from yellow to dark with an LOD of 31.5 nM. 1H NMR and DFT studies clearly indicated that the protonation of the pyridine nitrogen by the OH group of PA followed by PET from the fluorophore to PA was primarily responsible for the fluorescence quenching. Paper strips coated with probe 42 detected 10−5 M of PA (Fig. 11).
99) mixture. These aggregates of probe 4261 with PA exhibited fluorescence quenching at 527 nm with a color change from yellow to dark with an LOD of 31.5 nM. 1H NMR and DFT studies clearly indicated that the protonation of the pyridine nitrogen by the OH group of PA followed by PET from the fluorophore to PA was primarily responsible for the fluorescence quenching. Paper strips coated with probe 42 detected 10−5 M of PA (Fig. 11).
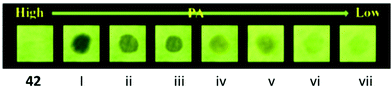 | ||
| Fig. 11 Photographs of the fluorescence quenching of probe 42 for detecting different concentrations of PA. From left to right: blank, (i) 5.0 × 10−3 M, (ii) 1.0 × 10−3 M, (iii) 5.0 × 10−4 M, (iv) 1.0 × 10−4 M, (v) 5.0 × 10−5 M, (vi) 1.0 × 10−5 M. Reprinted from ref. 61, Copyright 2019 Elsevier. | ||
Upon excitation at 375 nm, p-pyridine BODIPY probe 4362 (Chart 6) showed a 95.8% decrease in the emission intensity at 522 nm with 2 equivalents of picric acid in DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The bright green fluorescence color of probe 43 changed to dark in 30 s due to the protonation of the pyridine nitrogen followed by PET from the excited-state probe to the ground-state PA. The Stern–Volmer constant (KSV) of the probe was calculated to be 7.69 × 104 mol L−1, and it could detect PA concentrations as low as 0.013 μM.
1). The bright green fluorescence color of probe 43 changed to dark in 30 s due to the protonation of the pyridine nitrogen followed by PET from the excited-state probe to the ground-state PA. The Stern–Volmer constant (KSV) of the probe was calculated to be 7.69 × 104 mol L−1, and it could detect PA concentrations as low as 0.013 μM.
Pyridine-based probe 4463 (Chart 6) in THF displayed the formation of a new absorption maximum at 424 nm with PA with a color change from colorless to yellow. Probe 44 showed an 87% reduction in fluorescence intensity at 440 nm with 1 equivalent of picric acid, and with higher amounts of PA, a new emission band with weak emission appeared at 550 nm. The appearance of the new emission band could be assigned to the increased ICT upon protonation at the pyridine nitrogen, which is supported by the X-ray crystal structure in the solid state. The KSV and LOD were calculated to be 4.106 × 105 M−1 and 400 ppb, respectively.
Probe 4564 (Chart 6) in CH3CN–HEPES buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited fluorescence quenching with a gradual shift in the emission maximum from 410 nm to 460 nm as the amount of PA was increased to 2.0 equivalents. These results, along with Job's plot and DFT-DT studies, suggest that two molecules of PA bind with one molecule of probe 45 and cause fluorescence quenching through a combination of ICT and FRET mechanisms. A lightweight, convenient and portable prototype for PA detection was also fabricated.
1) exhibited fluorescence quenching with a gradual shift in the emission maximum from 410 nm to 460 nm as the amount of PA was increased to 2.0 equivalents. These results, along with Job's plot and DFT-DT studies, suggest that two molecules of PA bind with one molecule of probe 45 and cause fluorescence quenching through a combination of ICT and FRET mechanisms. A lightweight, convenient and portable prototype for PA detection was also fabricated.
Probe 4665 (Chart 6) revealed intense emission at 500 nm in THF, which vanished (94%) with 100 equivalents of PA. In the fluorescence spectrum of probe 46, the presence of the shoulder at 625 nm with higher equivalents of PA indicated the presence of charge-transfer character. Other nitro-phenols also induced significant fluorescence quenching of probe 46 at higher concentrations (Fig. 12A). Based on studies of the dynamic quenching mechanism of 46 by PA, the X-ray crystal structure of the 46 ∩ PA complex and titrations, the combined effect of the proton-induced ICT in 46 and electron transfer from the electron-rich anthracene to the electron-deficient PA moiety was assigned as the cause of the fluorescence quenching. Moreover, a human thumb impression (Fig. 12B) was clearly visible on a test strip coated with probe 46.
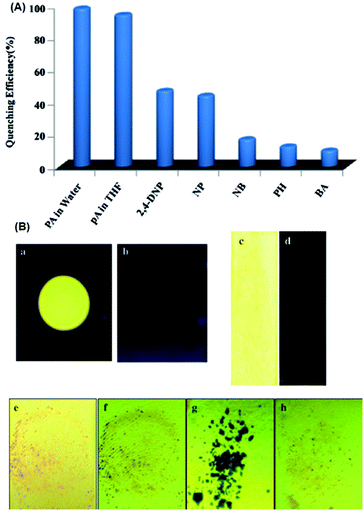 | ||
| Fig. 12 (A) Fluorescence quenching efficiency of 46 towards different analytes. (B) Images of a 46-coated TLC plate under UV light (a) before and (b) after the addition of PA. Images of test strips consisting of 46-coated Whatman Filter (c) before and (d) after being dipped into aqueous solutions of PA. Thumb impressions with PA on a test strip under (e) normal light and (f) UV light. (g) PA crystals on top of a test strip for 5 min, and the strip after the removal of the PA crystals. Reprinted from ref. 65, Copyright 2016 Wiley Online. | ||
Triphenylamine-based probe 4766 (Chart 6) having two benzothiazole moieties and one vinyl pyridine moiety at its 1, 3 and 5 positions underwent fluorescence quenching at 500 nm with a decrease in quantum yield from 0.31 to 0.08 with one equivalent of PA in a methanol–water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture. The protonation of the pyridine nitrogen associated with ICT was mainly responsible for the formation of a 1
2) mixture. The protonation of the pyridine nitrogen associated with ICT was mainly responsible for the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric non-fluorescent complex. The LOD was found to be 100.4 μM. Probe 47 was also used for the detection of Fe3+ ions.
1 stoichiometric non-fluorescent complex. The LOD was found to be 100.4 μM. Probe 47 was also used for the detection of Fe3+ ions.
N-Acylhydrazone 4867 (Chart 7) displayed an excimer emission band at 483 nm (λex = 419 nm), which underwent a 97% decrease in fluorescence intensity with 3 equivalents of PA in DMF. An ethanolic solution of 48 with 1 equivalent of PA gave vermilion precipitates (Fig. 13). None of the other tested NACs, viz. TNT, 2,6-DNT, NB, 4-NP, 1,3-DNB and NBA, caused fluorescence quenching or resulted in the formation of precipitates. The down-field shift of the protons of pyridine in the 1H NMR titration of 48 with PA pointed to proton transfer from PA to the pyridine nitrogen. The X-ray crystal structure of the 48 ∩ PA complex confirmed the proton transfer to pyridine nitrogen and the formation of a supramolecular complex through multiple hydrogen-bond and π–π interactions.
 | ||
| Fig. 13 Photographs of solid 48 and its complex 48 ∩ PA. Reprinted from ref. 67, Copyright 2011, Royal Society of Chemistry. | ||
Extending this work, these authors synthesized three additional N-acylhydrazone68-based probes, 49–51, for PA detection. Among probes 49–51, probe 50 with a pyrene moiety as the fluorophore exhibited the greatest fluorescence quenching with PA and had the highest Stern–Volmer constant (5.12 × 106 M−1) and lowest detection limit (0.102 μM). Their host–guest interactions were studied using 1H NMR titration and X-ray structural studies and revealed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes through multiple hydrogen-bond interactions in solution and in the solid state.
1 host–guest complexes through multiple hydrogen-bond interactions in solution and in the solid state.
Fluorescein-based chemosensors 52 and 5369 (Chart 7) (20 equivalents) in ethanol or water (buffer) solution with PA revealed fluorescence quenching at 545 nm and 558 nm upon excitation at 500 nm. The Ksv values and detection limits of probes 52 and 53 in ethanol were better than those observed in buffer. Probes 52 and 53 displayed fluorescence quenching with PA due to H-bonding and electron transfer. Paper strips coated with 52 and 53 could detect PA concentrations down to 10−6 M from a droplet (Fig. 14).
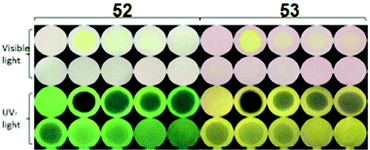 | ||
| Fig. 14 Paper strips for the visual detection of different concentrations of PA under visible and UV light. [PA] = 0 mM, 10.0 mM, 1.0 mM, 8.0 × 10−1 mM, 6.0 × 10−1 mM, 4.0 × 10−1 mM, 2.0 × 10−1 mM, 1.0 × 10−1 mM, 1.0 × 10−2 mM, and 1.0 × 10−3 mM. Reprinted from ref. 69, Copyright 2017 Royal Society of Chemistry. | ||
Pyridine-based fluorescent sensors 54 and 5570 (Chart 7) displayed fluorescence quenching at 420 nm and 450 nm with PA, respectively. Both probes 54 and 55 displayed similar fluorescence quenching efficiency (Fig. 15A) by PA (98% for 54 and 90% for 55), but probe 54 exhibited higher selectivity towards PA over other NACs than that observed for probe 55. The smaller size of the naphthalene moiety in probe 55 resulted in less steric bulk than the anthracene moiety of 54 and provided space to allow for π–π interactions with PA, 2,4-DNP and 4-NP with equal ease. Based on 1H NMR and DFT studies, hydrogen bonding between the pyridine nitrogen and OH group of PA and energy transfer via π–π interactions were proposed as mechanisms. The KSV values for PA were 5.78 × 104 for probe 54 and 3.26 × 104 for probe 55. Filter paper strips coated with aqueous suspensions of 54 and 55 detected PA concentrations as low as 10−8 M (Fig. 15B).
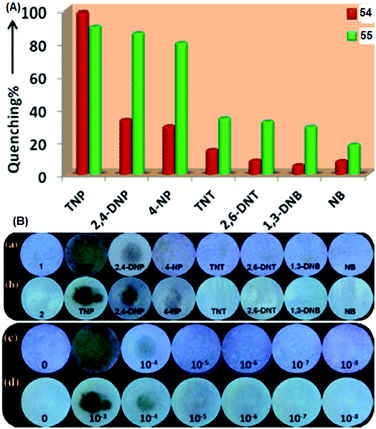 | ||
| Fig. 15 (A) A comparison of the percent quenching of 54 and 55 with different NACs. (B) Photographs of test paper strips coated with (a) 54 and (b) 55 and their responses towards NACs. Photographs of paper strips coated with (c) 54 and (d) 55 for the visual detection of increasing amounts of PA (10−8 to 10−3 M). Reprinted from ref. 70, Copyright 2018 American Chemical Society. | ||
Perylenediimide–pyridine conjugate 5671 (Chart 7) displayed red-shifts in its absorption maxima from 488/523 nm to 493/529 nm and decreased fluorescence intensity due to proton transfer from picric acid to probe 56. The KSV and LOD were found to be 6.87 × 104 M−1 and 0.0056 μM, respectively. Job's plot and 1H NMR titration analyses revealed the protonation of all four pyridine nitrogen atoms with four molecules of picric acid and the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 complex between probe 56 and picric acid.
4 complex between probe 56 and picric acid.
Probe 5772 (Chart 7) in CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and probe 5872 in CH3CN formed an organo-gel with an intertwined fiber network at critical gelation concentrations of 0.69 w/v% and 0.45 w/v%, respectively. The organo-gel prepared from probe 57 was more sensitive than that prepared from probe 58 towards PA and could also extract PA from the aqueous solution into the organogel system. The 16 nm blue-shift of the fluorescence emission band of the gel of 57 at 501 nm with PA pointed to the suppression of the TICT excited state of 57. Probe 57 displayed greater fluorescence quenching than probe 58 with PA (1 equiv.) due to the H-bonding between the pyridine nitrogen and OH of PA. The protons of the pyridine group in probe 57 shifted downfield in the 1H NMR titration with PA. Paper strips (Fig. 16) coated with probe 57 showed a change in fluorescence from blue to non-fluorescent.
1) and probe 5872 in CH3CN formed an organo-gel with an intertwined fiber network at critical gelation concentrations of 0.69 w/v% and 0.45 w/v%, respectively. The organo-gel prepared from probe 57 was more sensitive than that prepared from probe 58 towards PA and could also extract PA from the aqueous solution into the organogel system. The 16 nm blue-shift of the fluorescence emission band of the gel of 57 at 501 nm with PA pointed to the suppression of the TICT excited state of 57. Probe 57 displayed greater fluorescence quenching than probe 58 with PA (1 equiv.) due to the H-bonding between the pyridine nitrogen and OH of PA. The protons of the pyridine group in probe 57 shifted downfield in the 1H NMR titration with PA. Paper strips (Fig. 16) coated with probe 57 showed a change in fluorescence from blue to non-fluorescent.
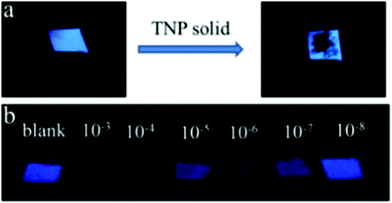 | ||
| Fig. 16 Photographs of paper strips coated with probe 57 under a UV lamp (365 nm) for the visual detection of PA: (a) solid PA and (b) dropped PA solutions with different concentrations (M L−1). Reprinted from ref. 72, Copyright 2017 American Chemical Society. | ||
Quinoline-based probes 59a–59d73 (Chart 7) showed fluorescence quenching of 86–88% upon excitation at 335 nm due to ground-state complexation between the probe and PA. The downfield shift of the quinoline protons in the 1H NMR spectra of these probes upon the addition of picric acid and Job's plot analysis confirmed the protonation of the quinoline nitrogen and the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complexes with the picrate anion.
2 stoichiometric complexes with the picrate anion.
The rhodamine–isonicotinic hydrazide-based “turn-on” fluorescent probe 6074 (Chart 7) showed selective detection of PA in CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) through a spirolactam ring-opening process. Probe 60 underwent protonation by PA at the pyridine nitrogen and displayed a new absorption maximum at 559 nm with a color change from colourless to pink and enhancement of the emission intensity at 581 nm. A Job's plot analysis revealed a 1
9) through a spirolactam ring-opening process. Probe 60 underwent protonation by PA at the pyridine nitrogen and displayed a new absorption maximum at 559 nm with a color change from colourless to pink and enhancement of the emission intensity at 581 nm. A Job's plot analysis revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe 60 and PA with a binding constant of 6.4 × 107 M−1. Probe 60 could detect PA concentrations as low as 0.0373 μM. Colourless paper strips (Fig. 17) coated with probe 60 became pink when exposed to PA.
1 stoichiometry between probe 60 and PA with a binding constant of 6.4 × 107 M−1. Probe 60 could detect PA concentrations as low as 0.0373 μM. Colourless paper strips (Fig. 17) coated with probe 60 became pink when exposed to PA.
 | ||
| Fig. 17 Photographs of probe 60 on test strips, showing the colour changes for the visual detection of PA. Reprinted from ref. 74, Copyright Wiley Online. | ||
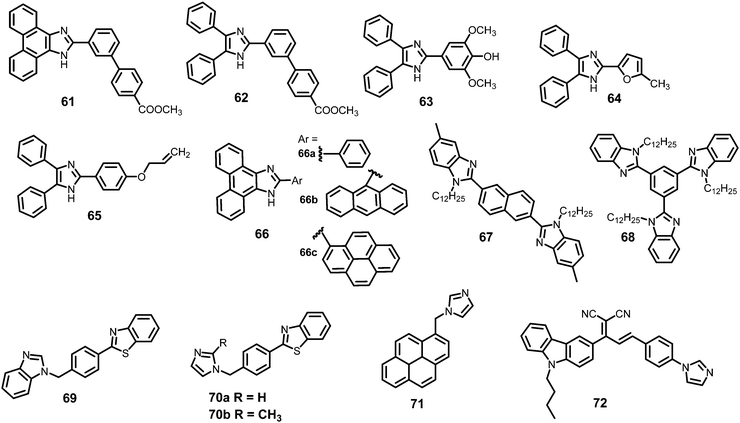
2.3 Protonation of imidazole-based fluorescent probes for picric acid
Probes 61–72 undergo protonation by picric acid at the imidazole nitrogen to form imidazolium salts, and in each case, quenching of the fluorescence intensity is observed. A comparison of the solvents, LOD, KSV values, emission maxima and applications of probes 61–72 has been given in Table 3.| Protonation of (benz)imidazole-based fluorescent probes for picric acid | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. no. | Probe | Solvent | LOD (μM) | K SV (M−1) | λ ex (nm) | λ em (nm) | Applications | Ref. |
| 1 | 61 | DMSO–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.17 | 3.75 × 104 | 270 | 392 | Water samples | 75 |
| 2 | 62 | EtOH–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.49 | 2.4 × 104 | 280 | 345 | Water samples | 75 |
| 3 | 63 | DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
3.223 | 1.168 × 104 | 312 | 413 | Paper strips | 76 |
| 4 | 64 | DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
2.987 | 2.65 × 104 | 309 | 409 | Paper strips | 76 |
| 5 | 65 | DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
2.996 | 2.05 × 104 | 309 | 401 | Paper strips | 76 |
| 6 | 66a | EtOH | 2.8 | 8.62 × 104 | 340 | 386 | — | 77 |
| 7 | 66b | EtOH | 2.3 | 1.45 × 105 | 340 | 486 | — | 77 |
| 8 | 66c | EtOH | 4.6 | 8.53 × 104 | 340 | 456 | — | 77 |
| 9 | 67 | H2O–THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.0025 | 2.54 × 105 | 335 | 422 | Paper strips, water samples | 78 |
| 10 | 68 | THF | 0.218 | 1.115 × 105 | 300 | 376 | TLC plate | 79 |
| Paper strip | ||||||||
| 11 | 69 | CHCl3 | 21.6 | 5.1 × 104 | 307 | 367 to 381 | — | 80 |
| 12 | 70b | CHCl3 | 19.0 | 9.9 × 106 | 307 | 367 to 383 | Paper strips | 80 |
Acetone–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||||||||
| 13 | 71 | Toluene | 2.515 | 6.58 × 104 | 345 | 378, 397 | — | 81 |
| CH3CN | 1.065 | 1.85 × 106 | ||||||
| 14 | 72 | H2O–DMF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.55 | 1.3 × 104 | 400 | 574 | Mechanochromic behaviour | 82 |
| Protonation of imine-based fluorescent probes for picric acid | ||||||||
| 15 | 73 | CH3CN | 0.01 | 6.814 × 106 | 368 to 344 | 425 to 462 | Paper strips | 83 |
| 16 | 74 | CH3CN–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
— | — | 390 | 467 | Paper strips | 84 |
| 17 | 75 | THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
0.010 | 2.03 × 104 | 375 | 469 | Test strips | 85 |
| 18 | 76 | THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
0.0726 | 70 × 10−6 | 430 | 585, 620 | TLC plate, test strip | 86 |
| Protonation of amide-based fluorescent probes for picric acid | ||||||||
| 19 | 77 | H2O–THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.47 | 7.0 × 104 | 371 | 558 | Filter paper | 87 |
| 20 | 78 | DMSO | 0.011 | 7.40 × 104 | 370 | 435 | Paper strips | 88 |
| 21 | 79 | PBS buffer | 0.0000256 | 2.20 × 107 | 338 | 484 | Paper strips, TLC plate | 89 |
| 22 | 80 | THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
3.93 | 9.6 × 104 | 350 | 590 | TLC plate | 90 |
| 23 | 81 | THF | 2.98 | 6.02 × 103 | 455 | 529 | — | 91 |
| 24 | 82 | EtOH | 0.820 | — | 314 | 420 to 460, 586 | Paper strips | 92 |
| 25 | 83a | MeOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.00002 | 7.05 × 107 | 480 | 539 to 581 | Water sample | 93 |
| 26 | 83b | MeOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
— | — | — | — | — | 93 |
| 27 | 84 | MeOH | 0.035 | — | 314 | 430 to 578 | TLC strip-solid PA | 94 |
Imidazole-based probe 6175 (Chart 8) displayed 95.3% fluorescence quenching at 392 nm (Fig. 18) in DMSO–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and probe 62 underwent 92.4% fluorescence quenching at 345 nm in EtOH–H2O (7
3), and probe 62 underwent 92.4% fluorescence quenching at 345 nm in EtOH–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with 12 equivalents of PA, whereas the other NACs induced insignificant changes in the fluorescence intensity of probes 61 and 62. In their 1H NMR spectra, the imidazole NH signals of 61 and 62 disappeared upon the interaction of PA with the probe. Probes 61 and 62 formed 1
3) with 12 equivalents of PA, whereas the other NACs induced insignificant changes in the fluorescence intensity of probes 61 and 62. In their 1H NMR spectra, the imidazole NH signals of 61 and 62 disappeared upon the interaction of PA with the probe. Probes 61 and 62 formed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexes with PA, respectively. Probes 61 and 62 detected PA concentrations as low as 0.17 μM and 0.49 μM.
1 stoichiometric complexes with PA, respectively. Probes 61 and 62 detected PA concentrations as low as 0.17 μM and 0.49 μM.
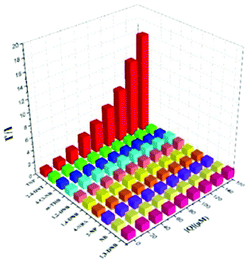 | ||
| Fig. 18 Change in the fluorescence intensity of probe 61 with various NACs. Reprinted from ref. 75, Copyright 2019 Elsevier. | ||
Probes 63–6576 (Chart 8) displayed blue emission maxima between 400–413 nm, which were selectively quenched by PA in a DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) mixture. These derivatives formed GSCT complexes with PA owing to the protonation of the imidazole nitrogen, and the increased efficiency of PET and IFE caused faster fluorescence quenching. Probe 63, due to the additional hydrogen bonding interaction of the OH of the probe with picric acid, exhibited higher-efficiency fluorescence quenching by PA than that observed for probes 64 and 65. Cyclic voltammetry studies showed electron transfer from the high-energy LUMO of the photo-excited probe to the low-energy LUMO of PA. These probes were able to detect PA from real samples. Paper strips coated with these probes could detect PA via fluorescence quenching.
9) mixture. These derivatives formed GSCT complexes with PA owing to the protonation of the imidazole nitrogen, and the increased efficiency of PET and IFE caused faster fluorescence quenching. Probe 63, due to the additional hydrogen bonding interaction of the OH of the probe with picric acid, exhibited higher-efficiency fluorescence quenching by PA than that observed for probes 64 and 65. Cyclic voltammetry studies showed electron transfer from the high-energy LUMO of the photo-excited probe to the low-energy LUMO of PA. These probes were able to detect PA from real samples. Paper strips coated with these probes could detect PA via fluorescence quenching.
The phenanthroline based probes 66a–66c77 (Chart 8) exhibited emission maxima at 386 nm, 486 nm and 456 nm, respectively. Solutions of these probes in ethanol showed fluorescence quenching with picric acid with respective limits of detection of 2.8 μM, 2.3 μM and 4.6 μM. The X-ray crystal structures of complexes of 66a and 66c with picric acid clearly show the transfer of the proton from picric acid to the phenanthroline nitrogen to form an ionic complex. The lack of change in the fluorescence lifetimes of these probes with picric acid further indicated that the existence of electrostatic interactions between the protonated probe and the picrate ion followed by PET was mainly responsible for the fluorescence quenching of probes 66 with picric acid.
Probe 6778 (Chart 8) with two benzimidazole moieties linked at the 2- and 6-positions of the naphthalene and long hydrophobic alkyl chains at the benzimidazole nitrogen self-assembled in water to form rod-like structures (Fig. 19), which were disrupted by PA. A solution of probe 67 displayed an emission band at 422 nm, which was completely quenched by 5 equivalents of PA. GSCT complex formation via the picric-acid-mediated protonation of the benzimidazole nitrogen followed by efficient electron transfer from the fluorophore to picric acid are responsible for the fluorescence quenching. Probe 67 can detect PA from both the solution phase (LOD 0.0025 μM) and the solid-state via a contact mode (11.46 ag cm2).
 | ||
| Fig. 19 The rod-like self-assemblies of probe 67 undergo a morphological change in the presence of two equivalents of PA (SEM images). Reprinted from ref. 78, Copyright 2017 Elsevier. | ||
Among a series of benzimadazole-based tripodal derivatives, probe 6879 (Chart 8) with a C12H25 alkyl chain exhibited detection of picric acid (Fig. 20) both in solution and the solid phase with a detection limit of 0.218 μM. Chain lengths longer or shorter than C12 reduced the sensitivity of the probe towards picric acid. The fluorescence intensity enhancement of probe 68 was due to the protonation of the benzimidazole nitrogen with benzoic acid. In the case of picric acid and 2,4-DNP, the electrostatic interactions between protonated 68 and the picrate/2,4-dinitrophenolate anions caused efficient energy transfer from the photoexcited π-electron-rich 68 to the ground state of electron-deficient PA, resulting in fluorescence quenching.
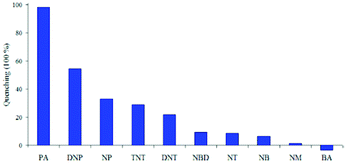 | ||
| Fig. 20 The effects of different NACs on the fluorescence quenching of probe 68. Reprinted from ref. 79, Copyright 2014 American Chemical Society. | ||
Probe 6980 (CHCl3) with one equivalent PA and probe 7080 (Chart 8) with two equivalents of PA displayed >90% fluorescence quenching of the fluorescence at ∼367 nm, along with a red-shift of the maxima to 381 nm. Probe 69 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (probe
1 (probe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) picric acid) stoichiometric complex, while probe 70 formed a 1
picric acid) stoichiometric complex, while probe 70 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complex. 1H NMR, Job's plot and absorbance studies revealed that in the case of 70, both the imidazole and benzothiazole nitrogen atoms are protonated by two molecules of picric acid to form a GSCT complex. Other NACs, such as DNT and 2,4-DNP, also induced significant quenching of the fluorescence of probes 69 and 70 (Fig. 21).
2 stoichiometric complex. 1H NMR, Job's plot and absorbance studies revealed that in the case of 70, both the imidazole and benzothiazole nitrogen atoms are protonated by two molecules of picric acid to form a GSCT complex. Other NACs, such as DNT and 2,4-DNP, also induced significant quenching of the fluorescence of probes 69 and 70 (Fig. 21).
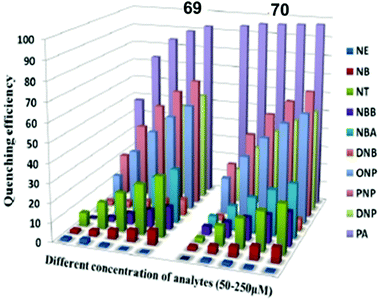 | ||
| Fig. 21 The quenching efficiencies of different NACs toward probes 69 and 70. Reprinted from ref. 80, Copyright 2019 Wiley Online. | ||
Paper strips coated with 70 turned from fluorescent blue to non-fluorescent (under 365 nm light) with 1 mM PA.
Probe 7181 (Chart 8) displayed both ON–OFF and OFF–ON fluorescence behaviour with picric acid, depending on the solvent. In CH3CN, 71 displayed OFF–ON behaviour, but exhibited ON–OFF fluorescence quenching upon PA binding in toluene solution. In toluene, the fluorescence of probe 71 at 378 and 397 nm was quenched owing to GSCT complex formation and FRET from the fluorophore to picrate anion, and the detection limit for PA was 2.515 μM. However, in CH3CN, the fluorescence intensity of 71 increased only 1.25-fold with a LOD of 1.065 μM for PA. The down-field shift of the imidazole and pyrene protons in the 1H NMR spectrum of 71 pointed to protonation of the imidazole nitrogen and face-to-face π–π stacking interaction between the pyrene and picrate rings. Probe 71 formed two types (Fig. 22) of crystals with PA (space groups P21/n and P21/c), and strong electrostatic interactions between the picrate anion and imidazolium moiety and π–π interactions between the pyrene ring and picrate anion were observed in the X-ray structures of both crystals.
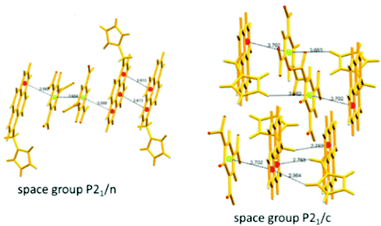 | ||
| Fig. 22 X-ray crystal structures of complexes of probe 71 with PA showing π–π interactions between pyrene and the picrate anion. Reprinted from ref. 81, Copyright 2017 Elsevier. | ||
Probe 7282 (Chart 8) gave an emission maximum of 460 nm in DMF, which was red-shifted to 574 nm in 90% water due to the formation of spherical aggregates with a diameter of 233 nm. These aggregates showed 96% fluorescence quenching with 10 equivalents of picric acid and an LOD of 3.5 μM for picric acid. In the 1H NMR spectrum of 72, the C2–H of the imidazole moiety was shifted downfield by 1.39 ppm, which pointed to the formation of an imidazolium moiety via proton transfer from picric acid, and the resulting electrostatic interactions caused efficient PET from the fluorophore to the picrate anion to form a non-fluorescent complex.
2.4 Protonation of imine-based fluorescent probes for picric acid
The nitrogen in Schiff bases is basic in nature and is well known to undergo protonation with general acids, and accordingly, forms a salt with the nitrophenolate ions upon protonation by phenolic nitroaromatic compounds and facilitates electron transfer/energy transfer processes for efficient fluorescence quenching. A comparison of solvents, LOD, KSV values, emission maxima and applications of probes 73–76 has been presented in Table 3.Fluorescent probe 7383 (Chart 9) in CH3CN with PA exhibited a blue-shift in its absorption band from 368 nm to 344 nm, and fluorescence quenching with a red-shift of the emission band from 425 nm to 462 nm and a fluorescence color change from blue to dark. A Job's plot analysis showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with a binding constant of 6.814 × 106 M−1. The protonation of the imine nitrogen followed by energy transfer from the probe to the picrate was responsible for the quenching of the emission intensity. The LOD and KSV values are 0.01 μM and 2.29 × 107 M−1 respectively. Probe 73 detected 10−9 M PA using paper strips (Fig. 23).
1 stoichiometry with a binding constant of 6.814 × 106 M−1. The protonation of the imine nitrogen followed by energy transfer from the probe to the picrate was responsible for the quenching of the emission intensity. The LOD and KSV values are 0.01 μM and 2.29 × 107 M−1 respectively. Probe 73 detected 10−9 M PA using paper strips (Fig. 23).
 | ||
| Fig. 23 Response of probe-73-coated paper strips to PA: (a) 10 μM, (b) 1.0 μM, (c) 0.1 μM, (d) 0.01 μM, (e) 8 nM, (f) 4 nM, (g) 1 nM, (h) 0.5 nM, (i) 0.1 nM, and (j) 0. Reprinted from ref. 83, Copyright 2019 Royal Society of Chemistry. | ||
Probe 7484 (Chart 9) is AIEE-active, and upon the addition of 80% water to its solution in CH3CN, displayed an 833-fold enchantment in fluorescence intensity and achieved a 0.79 quantum yield. Probe 74 with 24 equivalents of PA exhibited >98% fluorescence quenching at 476 nm with a change from a cyan-blue colour to non-fluorescence. Other phenolic NACs, such as 2,4-DNP and 4-NP (Fig. 24), also caused significant fluorescence quenching of probe 74. Along with the probable protonation of amine nitrogen, the intercalation of the picric acid between two pyrene rings held together by π–π stacking interactions in the aggregated state contributed to a CT process from pyrene to picric acid.
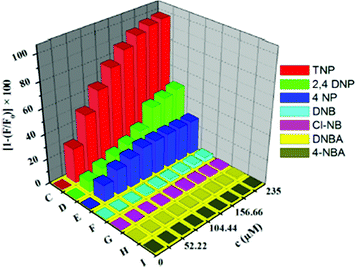 | ||
Fig. 24 Quenching efficiency (%) of the excimer fluorescence (at λem = 467 nm with λex = 390 nm) of probe 74 (10 μM) in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H2O/CH3CN upon the addition of different NACs. Reprinted from ref. 84, Copyright 2018 American Chemical Society. 2 H2O/CH3CN upon the addition of different NACs. Reprinted from ref. 84, Copyright 2018 American Chemical Society. | ||
Another pyrene-Schiff-base AIE fluorescent probe, 7585 (Chart 9), exhibited fluorescence quenching at 469 nm in THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) with the gradual addition of PA due to ground-state energy transfer via π–π and coulombic interaction between the fluorescent probe and the picrate anion. The KSV and detection limit were calculated to be 2.03 × 104 M−1 and 0.01 μM, respectively. Probe 75 showed fluorescence quenching by NACs, which followed the order PA > 2,4-DNP > 4-NP > TNB > NM, DNT, NT.
9) with the gradual addition of PA due to ground-state energy transfer via π–π and coulombic interaction between the fluorescent probe and the picrate anion. The KSV and detection limit were calculated to be 2.03 × 104 M−1 and 0.01 μM, respectively. Probe 75 showed fluorescence quenching by NACs, which followed the order PA > 2,4-DNP > 4-NP > TNB > NM, DNT, NT.
AIE-based probe 7686 (Chart 9) showed the formation of J aggregates with an emission maximum at 585 nm in THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). These aggregates underwent >98% fluorescence quenching with 4 equivalents of picric acid, with a change in fluorescence colour from orange to dark (Fig. 25), and could detect PA concentrations as low as 0.072 μM in solution. The other NACs caused only a slight change in the fluorescence intensity of 76. TLC strips coated with aggregates of 76 could detect ∼0.3 pg of PA.
9). These aggregates underwent >98% fluorescence quenching with 4 equivalents of picric acid, with a change in fluorescence colour from orange to dark (Fig. 25), and could detect PA concentrations as low as 0.072 μM in solution. The other NACs caused only a slight change in the fluorescence intensity of 76. TLC strips coated with aggregates of 76 could detect ∼0.3 pg of PA.
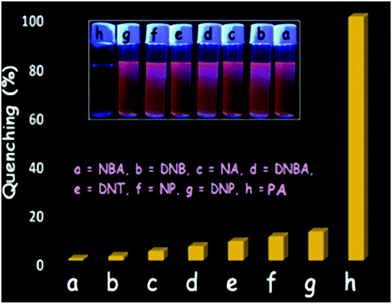 | ||
| Fig. 25 Quenching efficiency (%) (λem = 585 nm) of aggregates of 76 with NACs (50 equivalents). Inset: Visual colour change observed with only PA under illumination at 365 nm. Reprinted from ref. 86, Copyright 2019 Elsevier. | ||
2.5 Protonation of amide-based fluorescent probes for picric acid
Due to the resonance of the nitrogen lone pair with the amide oxygen, the amide oxygen is very electron-rich and easily undergoes protonation. The amide-based probes 77 to 84 exhibit fluorescence quenching upon protonation by picric acid. A comparison of the solvents, LODs, KSV values, emission maxima and applications of probes 77–84 is given in Table 3.The fluorescent probe 7787 (Chart 10) with anthracene and 1,8-naphthalimide units displayed fluorescence enhancement upon the addition of >50% water to its THF solution, which pointed to the formation of aggregates. These aggregates showed ∼88% and ∼30% fluorescence quenching at 508 nm upon the addition of 50 equivalents of picric acid or TNT, respectively. The effect of DNP on the fluorescence of 77 was not evaluated. TD-DFT studies revealed that in the excited state, the intermolecular proton transfer from picric acid to the oxygen of 1,8-naphthalimide is strengthened, which facilitates the non-irradiative PET process to induce the fluorescence quenching.
When the concentration of the tripodal probe 7888 was increased to >10 μM in DMSO, a sudden increase in blue fluorescence (λem = 435 nm) was observed due to the formation of self-assembled aggregates. In 1H NMR studies, the down-field shift of the amide NH protons and up-field shift of the aromatic protons with increasing amounts of the probe were highly consistent with the formation of aggregates through π–π and hydrogen bond interactions. These aggregates displayed 99.2% fluorescence quenching with 10 equivalents of picric acid owing to protonation at the amide oxygen, which resulted in the de-aggregation of the molecules of probe 78. The addition of cyanide ions removed the proton and caused re-aggregation of the molecules of probe 78 to restore the blue emission. Paper strips coated with 78 could detect 10−8 M PA.
Probe 7989 (Chart 10) showed an AIEE phenomenon at 484 nm in a mixture of DMSO–H2O with increasing amounts of water, and this emission was quenched by 2 equivalents of PA. Picric acid caused much more efficient fluorescence quenching than that observed for 2,4-DNP and 4-NP. Probe 79 formed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (probe 79
2 (probe 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NAC) stoichiometric complexes with picric acid, 2,4-DNP and 4-NP, but the binding constant for picric acid (35 × 107 M−1) was much higher than those for 2,4-DNP (8.71 × 105 M−1) and 4-NP (3.83 × 105 M−1). The partial de-aggregation of the aggregates and π–π interactions with energy transfer from the probe to PA were responsible for the fluorescence quenching. Paper strips coated with probe 79 solution allowed the detection of 10 nM PA by the naked eye (Fig. 26). The paper strips coated with 79 exhibited fluorescence quenching in the presence of PA vapor.
NAC) stoichiometric complexes with picric acid, 2,4-DNP and 4-NP, but the binding constant for picric acid (35 × 107 M−1) was much higher than those for 2,4-DNP (8.71 × 105 M−1) and 4-NP (3.83 × 105 M−1). The partial de-aggregation of the aggregates and π–π interactions with energy transfer from the probe to PA were responsible for the fluorescence quenching. Paper strips coated with probe 79 solution allowed the detection of 10 nM PA by the naked eye (Fig. 26). The paper strips coated with 79 exhibited fluorescence quenching in the presence of PA vapor.
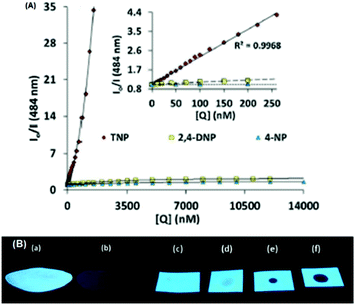 | ||
| Fig. 26 (A) Fluorescence quenching of probe 79 at 484 nm with PA, DNP, and NP. (B) Fluorescence changes of 79-coated paper strips (a) before and (b) after being dipped into PA solution (1 μM) or after exposure to (c) H2O or PA solutions with different concentrations: (d) 1 nM, (e) 3 nM, and (f) 10 nM (4 μL), as seen under 365 nm illumination. Reprinted from ref. 89, Copyright 2017 Elsevier. | ||
Naphthalene-1,8-diimide derivative 8090 (Chart 10) showed excimer emission centred at 600 nm in THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) due to the formation of aggregates. The formation of these aggregates was confirmed by SEM and DLS studies. Probe 80 displayed 99% fluorescence quenching at 600 nm with 30 equivalents of PA with a fluorescence colour change from orange to dark in THF–water. Probe 80 formed a GSCT complex with PA via protonation of the amide oxygen, which was responsible for the fluorescence quenching. The non-phenolic nitroaromatic compounds TNT, ONT, 2,4-DNT and NB induced insignificant changes in the fluorescence of the aggregates. Paper strips coated with probe 80 showed 50% fluorescence quenching with 60 μL of PA solution.
9) due to the formation of aggregates. The formation of these aggregates was confirmed by SEM and DLS studies. Probe 80 displayed 99% fluorescence quenching at 600 nm with 30 equivalents of PA with a fluorescence colour change from orange to dark in THF–water. Probe 80 formed a GSCT complex with PA via protonation of the amide oxygen, which was responsible for the fluorescence quenching. The non-phenolic nitroaromatic compounds TNT, ONT, 2,4-DNT and NB induced insignificant changes in the fluorescence of the aggregates. Paper strips coated with probe 80 showed 50% fluorescence quenching with 60 μL of PA solution.
Perylene-based probe 8191 (Chart 10) displayed respective 70% and 22% reductions in emission intensity in the presence of picric acid and 2,4-DNP in THF. Non-phenolic NACs caused much smaller fluorescence quenching. Protonation of the amide oxygen of 81 by picric acid and 2,4-DNP formed a non-fluorescent ground state complex. The KSV and detection limit for PA are 6.02 × 103 M−1 and 2.98 μM, respectively.
Rhodamine-based fluorescent and colorimetric probe 8292 (Chart 10) revealed a new absorption maximum at 560 nm in 5 min with 200 μM PA, with a colour change from colourless to pink and a maximum increase in fluorescence intensity at 586 nm in 80 min due to spiro-lactam ring-opening. The lowest detection limit for PA was found to be 0.82 μM. A Benesi–Hildebrand plot revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe 82 and PA with an association constant of 5834 L mol−1. Paper strips coated with probe 82 solution detected 50 μM PA with color change to pink (naked eye) and orange fluorescence under 365 light (Fig. 27).
1 stoichiometry between probe 82 and PA with an association constant of 5834 L mol−1. Paper strips coated with probe 82 solution detected 50 μM PA with color change to pink (naked eye) and orange fluorescence under 365 light (Fig. 27).
 | ||
| Fig. 27 Paper strips coated with probe 82 for the recognition of PA (50 μM to 10 mM) under (a) sunlight and (b) 365 nm UV light. Reprinted from ref. 92, Copyright 2018 Royal Society of Chemistry. | ||
Rhodamine 83a93 (Chart 10) displayed selective detection of PA in MeOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with an increase in quantum yield from 0.065 to 0.611. Upon the addition of PA, the appearance of a new absorption maximum at 554 nm and a 99-fold enhancement in emission intensity at 581 nm were observed for probe 83a due to spirolactam ring opening. Probe 83a could detect PA concentrations as low as 2 × 10−11 M. Probe 83b lacking an OH moiety on the Schiff-base was only weakly sensitive to picric acid.
4) with an increase in quantum yield from 0.065 to 0.611. Upon the addition of PA, the appearance of a new absorption maximum at 554 nm and a 99-fold enhancement in emission intensity at 581 nm were observed for probe 83a due to spirolactam ring opening. Probe 83a could detect PA concentrations as low as 2 × 10−11 M. Probe 83b lacking an OH moiety on the Schiff-base was only weakly sensitive to picric acid.
Rhodamine-appended hexaphenylbenzene-based probe 8494 (Chart 10) with PA in methanol showed a colour change from colourless to pink (naked eye) and non-fluorescent to red (under 365 nm light). Probe 84 did not exhibit any emission band, but the addition of 30 equivalents of PA resulted in a 147-fold enhancement at 578 nm (Φ = 0.82). Other NACs, such as 4-NP, 2,4-DNP, 2-NT, 2,4-DNT, 1,4-BQ, NM, 1,4-DNB, benzoic acid and 4-NBA did not cause any fluorescence or daylight colour change. The colour/fluorescence change was reversed with tetrabutylammonium hydroxide. Probe 84 could detect PA concentrations as low as 0.035 μM. Proton transfer from picric acid to the spiro-lactam ring resulted in opening of the spiro-lactam ring of rhodamine to the amide and activated the TBET process in 84via the conjugated linker from the donor to the acceptor. TLC strips (Fig. 28) coated with the probe displayed a color change from colourless to pink and could detect 10−8 M PA.
 | ||
| Fig. 28 Probe-84-coated paper strips upon interaction with different concentrations of PA [(a) 1.049 mg, (b) 0.1049 mg, (c) 1.049 μg, (d) 10.49 ng, and (e) 104.9 pg]. Reprinted from ref. 92, Copyright 2015 Royal Society of Chemistry. | ||
3. Positively charged probes for the detection of picric acid
Due to the ease of the dissociation of picric acid to the picrate anion, it can form a neutral electrostatic complex with positively charged fluorophores, thus leading to the proximity of the fluorophore to the picrate anion and efficient electron/energy transfer processes. Positively charged probes bearing dialkylamino moieties can undergo protonation with picric acid and restrict the ICT from the dialkylamino moiety to the rest of the molecule. This restriction of ICT leads to a non-fluorescent complex in the presence of picric acid. These probes have been classified as mono-, di- and tripodal fluorescent derivatives, and are discussed in this order. A comparison of the solvents, LODs, KSV values, emission maxima and applications of probes 85–128 has been presented in Table 4.| Monopodal positively charged probes for picric acid | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. no. | Probe | Solvent | LOD (μM) | K SV (M−1) | λ ex (nm) | λ em (nm) | Applications | Ref. |
| 1 | 85 | MeOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.7 | 2.38 × 104 | 430 to 350 | 580 | — | 95 |
| 2 | 86 | MeOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) 1)) |
3.6 | 1.08 × 104 | 430 to 350 | 593 | — | 95 |
| 3 | 87 | MeOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 430 | 599 | — | 95 |
| 4 | 88 | H2O | 1.98 | — | 365 | 390 to 455 | Water sample | 96 |
| 5 | 89 | H2O–THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.0082 | 1.67 × 109 | 550 | 620 | Paper strips | 97 |
| 610 | 730 | |||||||
| 6 | 90 | H2O–THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.0088 | — | 460 | ∼710 | Paper strips | 97 |
| 7 | 91 | H2O | 0.107 | 2.2 × 104 | 325 to 340 | 480 | TLC plate | 98 |
| 8 | 92 | H2O | 0.087 | 5 × 104 | 325 to 340 | 410 | TLC plate | 98 |
| 9 | 93 | H2O | 1.878 | — | 292 | 402 | — | 99 |
| 10 | 94 | PBS–DMSO (0.5%) | — | 5.52 × 103 | 336 | 378 | — | 100 |
| 11 | 95 | PBS–DMSO (0.5%) | — | 6.55 × 104 | 336 | 383 | — | 100 |
| 12 | 96 | PBS–DMSO (0.5%) | 0.00029 | 8.34 × 106 | 336 | 494 | Paper strips, water sample | 100 |
| 13 | 97 | CH3CN | 0.070 | — | 620 | 684 to 644 | Paper strips | 101 |
| Dipodal positively charged probes for picric acid | ||||||||
| 14 | 98 | SDS–H2O | 1 | — | 345 | 480 | — | 102 |
| 15 | 99 | Aqueous media, 95% | 0.00056 | 0.1 × 108 | 325 | 406 | Paper strips | 103 |
| 16 | 100 | H2O | — | 1.75 × 104 | 365 | 513 | — | 104 |
| 17 | 101 | H2O | — | 6.04 × 104 | 365 | 485 | — | 104 |
| 18 | 102 | NMP | 1.0 | 16.8 × 106 | 337 | ∼470 | — | 105 |
| 19 | 103 | DMF and H2O | 1.0 | — | 370 | 545, 580 | — | 106 |
| 20 | 104 | DMF | 1.0 | — | 370 | 543 | — | 106 |
| 21 | 105 | DMF | 1.0 | — | 370 | 543 | — | 106 |
| Tripodal positively charged probes for picric acid | ||||||||
| 22 | 106 | DMSO | 2.039 | 3.8 × 104 | 371 | 399, 422, 446 | Water samples | 107 |
| 23 | 107 | DMSO | 1.545 | 3.3 × 104 | 371 | 399, 422, 446 | Water samples | 107 |
| 24 | 108 | HEPES–DMSO (2%) | — | 2.67 × 105 | 290 | 402 | — | 108 |
| 25 | 109 | HEPES–DMSO (2%) | 0.001 | 3.57 × 105 | 290 | 470 | Paper strips | 108 |
| 26 | 110 | HEPES–DMSO (2%) | — | — | 290 | 450 to 485 | — | 108 |
| 27 | 111 | H2O–DMSO (2%) | 0.000005 | 1.20 × 1011 | 290 | 402 | Paper strips | 109 |
| 1.10 × 108 | ||||||||
| 4.78 × 105 | ||||||||
| 28 | 112 | HEPES–DMSO (2%) | 0.00001 | 1.57 × 109 | 335 | 520 | Paper strips | 110 |
| 29 | 113 | H2O–DMSO (2%) | 0.025 | 6.25 × 105 | 290 | 454 | Paper strips | 111 |
| 30 | 114 | H2O–DMSO (2%) | 0.0000001 | 2.56 × 1011 | 290 | 448 | Paper strips | 111 |
| 31 | 115 | H2O–DMSO (2%) | 0.0000001 | 5.04 × 1011 | 290 | 412 | Paper strips | 111 |
| 32 | 116 | H2O–DMSO (2%) | 0.0000001 | 6.73 × 108 | 335 | 480 | Paper strips | 112 |
| 33 | 117 | H2O | 15.2 | 3.43 × 103 | 340 | ∼480 | Paper strips | 113 |
| 34 | 118 | HEPES buffer | 0.0001 (UV) | — | 490 | 615 | Soil sample | 114 |
| 0.003 (FL) | ||||||||
| 35 | 119 | HEPES buffer | 0.020 (UV) | — | 490 | 615 | Turn-ON | 115 |
| 0.002 (FL) | Paper strips | |||||||
| Metal-complex-based probes for picric acid | ||||||||
| 36 | 120 | DMSO–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) 95) |
0.0121 | 1.165 × 106 | 383 | 485 | Paper strips | 116 |
| 37 | 121 | H2O | 0.99 | 5.29 × 104 | 400 | 470 | Thin film | 117 |
| 38 | 122 | H2O–THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
0.03 | 1.92 × 105 | 380 | 460 | Paper strips | 118 |
| 39 | 123 | THF–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.170 | 9 × 104 | 380 | 458 | — | 119 |
| 40 | 124 | DMSO | 3.056 | 1.6 × 105 | 630 | 684, 752 | Thin film | 120 |
| 41 | 125 | DMSO | 4.803 | 2.02 × 105 | 630 | 696, 761 | Thin film | 120 |
| Miscellaneous | ||||||||
| 42 | 126 | Toluene | 0.131 | 2.1 × 106 | 500 | 600 | Paper strips | 121 |
| 43 | 127 | CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.707 ± (0.06) | 9.2 × 106 | 504 to 516 | 518 to 535 | Thin film | 122 |
| 44 | 128 | H2O | 0.0172 | 1.65 × 106 | 279 | 348 | — | 123 |
3.1 Monopodal cationic fluorescent probes for picric acid
The quinoline derivatives 85 and 8695 (Chart 11) having electron-donor groups on the quinoline moiety exhibited emission maxima at 580 nm and 593 nm, respectively, in MeOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which revealed 86% and 87% fluorescence quenching with 100 ppm PA, whereas derivative 8795 was silent to the presence of PA. In the 1H NMR spectrum of 85, the lack of change in the chemical shift values even after the addition of 5 equivalents of picric acid suggested no protonation of the fluorophore, and DFT calculations indicated that electron transfer from the LUMO of the fluorophore to the picrate anion was the cause of the fluorescence quenching. In probe 86, a change in the chemical shifts in its 1H NMR spectrum upon the addition of PA pointed to restriction of ICT and the formation of a non-fluorescent complex. Therefore, probes 85 and 86 followed different mechanisms for fluorescence quenching by picric acid. Thin films of probes 85–87 emitted in the orange-to-red region. Upon exposure to TFA vapor, the fluorescence colour of the film of probe 85 changed from orange to red, and the fluorescence of films of 86 and 87 was completely quenched without any appreciable shift in the emission maxima. These effects were reversed upon interaction with TEA vapor.
1), which revealed 86% and 87% fluorescence quenching with 100 ppm PA, whereas derivative 8795 was silent to the presence of PA. In the 1H NMR spectrum of 85, the lack of change in the chemical shift values even after the addition of 5 equivalents of picric acid suggested no protonation of the fluorophore, and DFT calculations indicated that electron transfer from the LUMO of the fluorophore to the picrate anion was the cause of the fluorescence quenching. In probe 86, a change in the chemical shifts in its 1H NMR spectrum upon the addition of PA pointed to restriction of ICT and the formation of a non-fluorescent complex. Therefore, probes 85 and 86 followed different mechanisms for fluorescence quenching by picric acid. Thin films of probes 85–87 emitted in the orange-to-red region. Upon exposure to TFA vapor, the fluorescence colour of the film of probe 85 changed from orange to red, and the fluorescence of films of 86 and 87 was completely quenched without any appreciable shift in the emission maxima. These effects were reversed upon interaction with TEA vapor.
The ratiometric probe 8896 (Chart 11) in a 100% aqueous medium displayed a red shift in fluorescence maximum from 390 to 440 nm after the addition of PA with a fluorescence colour change from blue to cyan (Fig. 29). The formation of a new emission band at 440 nm was assigned to the protonation of 88 at the quinoline nitrogen. A plot of the I455nm/I390nm ratio with PA concentration showed a good linear relationship between 0.5 μM and 20 μM. This probe was able to detect PA from real samples.
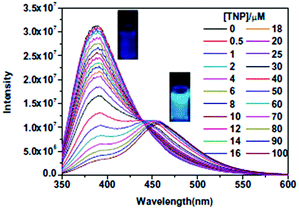 | ||
| Fig. 29 Changes in the emission spectrum and emission colour of 88 (1.0 × 10−5 M) with increasing amounts of PA. Reprinted from ref. 96, Copyright 2019 Elsevier. | ||
Indolium-based probes 89 and 9097 (Chart 11) in a THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture exhibited 92% and 93% fluorescence quenching at their maxima of 510 nm and 620 nm for 89 and 720 nm for 90 with the incremental addition of PA solution. The protonation of the N,N-dialkylamino moiety by PA restricted ICT and was responsible for the fluorescence quenching. Paper strips coated with 89 and 90 could be used for detecting PA from real samples.
1) mixture exhibited 92% and 93% fluorescence quenching at their maxima of 510 nm and 620 nm for 89 and 720 nm for 90 with the incremental addition of PA solution. The protonation of the N,N-dialkylamino moiety by PA restricted ICT and was responsible for the fluorescence quenching. Paper strips coated with 89 and 90 could be used for detecting PA from real samples.
Coumaryl-linked imidazolium salts 91 and 9298 (Chart 11) showed decreased fluorescence intensity at 480 nm and 410 nm, respectively, with increasing concentration of PA due to the formation a GSCT complex, as evidenced by 1H NMR, UV-Vis and fluorescence lifetime studies. Among the other NACs, only 4-NP exhibited a fairly strong interaction with 91 and 92. These probes were able to detect PA using probe-coated TLC plates. Ionic liquid-based fluorophore 9399 (Chart 11) demonstrated 91% and 93% fluorescence quenching with PA and 4-NP, respectively, caused by the formation of a GSCT complex. The information for 2,4-DNP is not available.
Pyrene-appended imidazolium salts 94–96100 (Chart 11) could detect PA with little interference from 2,4-DNP and 4-NP in PBS–DMSO (99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) solution. The green emission of probe 96 was completely quenched with PA, and it formed a 1
0.5) solution. The green emission of probe 96 was completely quenched with PA, and it formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complex. The loss of π–π interactions between the two pyrene rings and electron transfer from pyrene to PA were responsible for the fluorescence quenching. Probes 94 and 95 also showed fluorescence quenching of their monomer emission bands. Paper strips (Fig. 30) coated with 96 were able to detect PA.
2 stoichiometric complex. The loss of π–π interactions between the two pyrene rings and electron transfer from pyrene to PA were responsible for the fluorescence quenching. Probes 94 and 95 also showed fluorescence quenching of their monomer emission bands. Paper strips (Fig. 30) coated with 96 were able to detect PA.
 | ||
| Fig. 30 Fluorescence changes of probe-96-coated paper strips with PA; (a) a 96-coated paper strip, (b) a 96-coated strip dipped in 1 μM PA solution (in 100% water), and a 96-coated strip upon exposure to 5 μL of aqueous solutions with the following concentrations of PA: (c) 0, (d) 5 nM, (e) 10 nM, (f) 20 nM, (g) 50 nM, and (h) 100 nM. Observed under 365 nm light. Reprinted from ref. 100. Copyright 2016 Elsevier. | ||
Squaraine–dansyl conjugate 97101 (Chart 12) displayed a ratiometric change in absorbance and fluorescence upon titration with PA in CH3CN. With increasing amounts of PA, the solution of 97 exhibited reduced absorbance at 663 nm with the appearance of a new absorption maximum at 627 nm. Upon excitation at 620 nm, probe 97 displayed a blue-shift in fluorescence maximum from 684 nm to 644 nm. The plot of FI644nm/FI684nm with PA shows good linearity in the range 5–100 μM, and the lowest detection limit was 70 nM. In probe 97, protonation of the dimethylamine nitrogen restricted ICT and the twisted dimethylamino phenyl unit prevented π–π interactions of the squaraine plane with PA, reducing the efficiency of electron and energy transfer. Paper strips coated with probe 97 could detect PA with a color change from a blue to a brownish color (Fig. 31).
 | ||
| Fig. 31 Visual color changes of probe-97-coated paper strips with different amounts of PA; the [PA] values are (A) 10−2 M, (B) 10−4 M, (C) 10−6 M, (D) 10−9 M, (E) 10−11 M, and (F) no PA. Reprinted from ref. 101, Copyright 2013 Royal Society of Chemistry. | ||
3.2 Dipodal dicationic fluorescent probes for picric acid
The fluorescent probe 98102 (Chart 12) exhibited a varied distribution in differently charged micelles, which further influenced the sensing behaviour of the obtained micellar sensor systems towards explosives. The UV-Vis, steady-state and time-resolved emission studies revealed that probe 98 is encapsulated by SDS, but not by the cationic surfactant DTAB. The solution of 98 in SDS showed preferential fluorescence quenching with PA (Fig. 32), but in DTAB solution, the most selective quenching was observed with PYX. The quenching mechanism of the two micellar sensor systems by explosives was static in nature.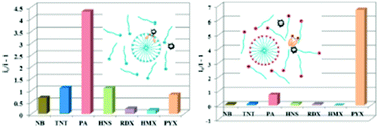 | ||
| Fig. 32 Fluorescence quenching efficiency of explosives. (left) Probe 98 (1 μM)–SDS (8 mM) sensor system; (right) Probe 98 (1 μM)–DTAB (14 mM) sensor system. [explosives] = 100 μM. Reprinted from ref. 102, Copyright 2014 American Chemical Society. | ||
Imidazolium-based conjugated polyelectrolyte 99103 (Chart 12) revealed fluorescence quenching efficiency following the order PA > 2,4-DNP > 4-NP, but neutral NACs did not cause fluorescence quenching. The ease of the dissociation of PA in water accelerated the electrostatic interaction with cationic probe 99 and resulted in a decrease in emission intensity at 406 nm with a colour change from blue to dark under UV light. Paper strips coated with 99 could detect 2.29 pg cm−2 PA. A transparent fluorescent film of commercial chitosan with 5% of probe 99 was also used for the detection of PA (Fig. 33).
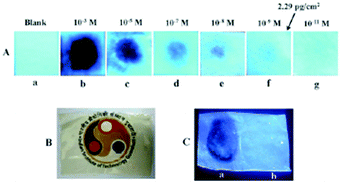 | ||
| Fig. 33 (A) The colour of fluorescent test strips under UV light (a) before and after (b–g) treatment with 10 μL of solutions of various concentrations of PA. (B) Probe-99-doped transparent chitosan film in daylight. (C) Visualization of 99-doped CS fluorescent film under UV light: (a) dark spot of a residual PA thumbprint and (b) a thumb impression as a control (at 365 nm). Reprinted from ref. 103, Copyright 2015 Royal Society of Chemistry. | ||
Pyrenoviologen cationic derivatives 100 and 101104 (Chart 12) possessing high quantum yields of 64% and 95%, respectively, displayed electrostatic interactions with picrate anions in water, and electron transfer between them lead to the fluorescence response. The fluorescence emission spectra of 100 (10 μM) at 513 nm and 101 (10 μM) at 482 nm were completely quenched upon the addition of PA (55 μM).
Dodecylbenzene-sulfonic acid (DBSA)-doped polyaniline 102105 (Chart 12) in the solvent NMP underwent fluorescence quenching at 475 nm in the order PA > 1,3-DNB ≈ NB. The results with 2,4-DNP and 4-NP were not discussed. The FE-SEM and AFM images of 102 displayed changes in morphology after the addition of PA (Fig. 34).
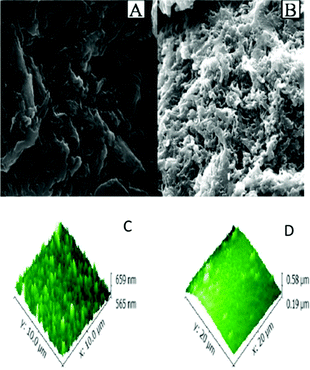 | ||
| Fig. 34 (a) FESEM images of probe 102 and (b) probe 102 + PA. (c) AFM images of 102 and (d) 102 + PA. Reprinted from ref. 105, Copyright 2018 Wiley Online. | ||
Perylene diimide probes 103–105106 (Chart 12) displayed quenching of their yellow fluorescence upon the addition of PA and 4-nitroaniline (4-NA) with equal ease in DMF and water (pH range 1.0 to 10.0). The probes showed stronger fluorescence in DMF than in water.
3.3 Tripodal tricationic fluorescent probes for picric acid
Anthracene-functionalized tris-imidazolium salts 106–107107 (Chart 13) found applications for the detection of picric acid in organic and aqueous media. Both 106 and 107 exhibited characteristic anthracene monomer emission in DMSO, with 92% and 91% quenching, respectively, upon the addition of 100 equivalents of PA. Other NACs displayed much smaller fluorescence quenching, but the study with 2,4-DNP – the most interfering nitrophenol – is not available. Probes 106 and 107 showed static quenching due to the ground-state charge-transfer complex formation. X-ray diffraction and computational studies reveal that from among several possibilities, 106 and 107 adopt a cis,cis,cis conformation and exhibit a bowl-shaped structure in the solid state (Fig. 35). The 1H NMR and DFT studies of 106 and 107 with PA and the X-ray crystal structure of complex of 106 with PA showed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric probe ∩ 2PA complex via the replacement of two bromide ions with picrate anions.
2 stoichiometric probe ∩ 2PA complex via the replacement of two bromide ions with picrate anions.
 | ||
| Fig. 35 The X-ray crystal structure of 106 (left) and the energy-optimized structure of 107 (right). Reprinted from ref. 107, Copyright 2013 American Chemical Society. | ||
The X-ray crystal structures and DFT optimization studies of tripodal probes 108 and 109108 (Chart 13) show that the three biphenyl substituents are oriented on the same side of the triethylbenzene to form a pseudo-cavity, which is more pre-organised for the encapsulation of PA in comparison to 106 and 107. Upon excitation at 290 nm, the solution of 108 displayed a decrease in the emission intensity at 402 nm due to a combination of electrostatic, hydrogen-bonding and π–π interactions with the picrate anion (Fig. 36). The Job's plot of 108 revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with PA with a log
1 stoichiometric complex with PA with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β11 value of 5.82 ± 0.06. The solution of 109 in HEPES buffer displayed a decrease in emission intensity at 470 nm with a colour change from blue to dark in the presence of PA. The poor efficiency of monopod 110 towards PA further highlighted the role of pre-organization in 108 and 109 for the highly sensitive and selective recognition of PA. The significant up-field shift (Δδ = 0.8 ppm) of the PA protons in 1H NMR titration studies confirmed that PA was encapsulated in the cavity of 108 and 109. Paper strips coated with probe 109 detected 137 ag cm−2 PA in solution. The paper strips of 109 could also detect PA vapor in 120 s.
β11 value of 5.82 ± 0.06. The solution of 109 in HEPES buffer displayed a decrease in emission intensity at 470 nm with a colour change from blue to dark in the presence of PA. The poor efficiency of monopod 110 towards PA further highlighted the role of pre-organization in 108 and 109 for the highly sensitive and selective recognition of PA. The significant up-field shift (Δδ = 0.8 ppm) of the PA protons in 1H NMR titration studies confirmed that PA was encapsulated in the cavity of 108 and 109. Paper strips coated with probe 109 detected 137 ag cm−2 PA in solution. The paper strips of 109 could also detect PA vapor in 120 s.
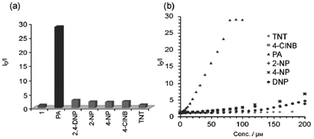 | ||
Fig. 36 (a) Quenching efficiency of 108 with NACs. (b) Stern–Volmer plots of the effects of various NACs on the fluorescence intensity of 108 (HEPES buffer/DMSO (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)). Reprinted from ref. 108, Copyright 2014 Wiley Online. 2)). Reprinted from ref. 108, Copyright 2014 Wiley Online. | ||
Tripod 111109 (Chart 13), with an extended hydrophobic pseudo-cavity in comparison to the biphenyl in 108 due to the presence of the triphenylene moiety, could detect 5 × 10−13 M PA in water (2% DMSO) and 2.29 × 10−20 g cm−2 PA using coated paper strips. The molecules of 111 self-assembled to form a rod-like morphology in an aqueous medium, as observed through field-emission SEM, TEM and dynamic light scattering studies. With the gradual addition of PA, these aggregates underwent an aggregation–disaggregation process to form complex morphological structures (10−12–10−10 M PA) and then spherical aggregates at 10−9–10−8 M PA (Fig. 37). Further increasing the amount of PA to 10−7–10−6 M resulted in the dissolution of these spherical aggregates to give well-dispersed spheres. During fluorescence studies, these aggregates demonstrated super-amplified fluorescence quenching (>97%) by 10−5 to 0.2 equivalents of PA. Thin films of tripod 111 exhibited fluorescence quenching within 6 min in the presence of PA.
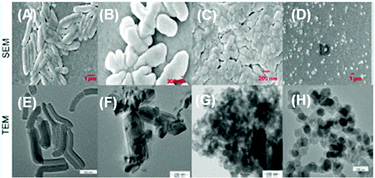 | ||
| Fig. 37 Morphological (upper panels: SEM images, lower panels: TEM images) changes in TRIPOD 111 upon the addition of various amounts of picric acid: (A and E) [PA] = 0 M, (B, F) [PA] = 10−12 M, (C, G) [PA] = 10−8 M, and (D, H) [PA] = 10−7 M. Reprinted from ref. 109, Copyright 2015 American Chemical Society. | ||
Dansyl-conjugated probe 112110 (Chart 13) in HEPES buffer (2% DMSO) showed amplified quenching at 540 nm in the presence of PA, with an associated change in the fluorescence color from yellow to dark (Fig. 38A). The morphology of probe 112 (Fig. 38B) changed after the addition of 1 equivalent of PA (Fig. 38C). Paper strips coated with 112 detected 13.7 ag cm−2 PA using the contact mode method (Fig. 38D). Probe 112 exhibited a combination of electrostatic and hydrophobic π–π interactions with the picrate anion.
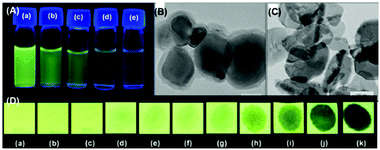 | ||
| Fig. 38 (A) Visual fluorescence changes of probe 112 in blank solution (a) and with increasing concentrations of PA: (b) 50 pM, (c) 1 nM, (d) 2 nM, and (e) 4 nM. (B and C) TEM images of (B) probe 111 (1 μM) and (C) probe 111 (1 μM) + PA (10 μM). (D) Photographs of probe-112-coated paper strips (under 365 nm UV light): (a) a virgin test strip, (b) a paper strip with a drop of water, and strips with 6 μl of PA solutions with concentrations of (c) 10−14 M, (d) 10−13 M, (e) 10−12 M, (f) 10−11 M, (g) 10−10 M, and (h) 10−9 M. Reprinted from ref. 110, Copyright 2016 Elsevier. | ||
Imidazolium-based fluorescent probes 113–115111 (Chart 13) were used for the detection of nitroaromatics such as PA, 2,4-DNP, TNT and ClDNB in water–DMSO (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Among the three probes, probe 113 did not undergo aggregation and remained in the molecularly dissolved state due to its short alkyl chains, but the other two probes (114 and 115) self-assembled in water.
2). Among the three probes, probe 113 did not undergo aggregation and remained in the molecularly dissolved state due to its short alkyl chains, but the other two probes (114 and 115) self-assembled in water.
Upon excitation at 290 nm, probes 113–115 displayed emission maxima at 454 nm, 448 nm and 412 nm, respectively. Upon the addition of 0.1 equivalents of nitroaromatic derivatives such as PA, 2,4-DNP, TNT and Cl-DNB to a solution of 114, 92%, 89%, 73% and 51% quenching of the emission intensity was observed, respectively. Similarly, probe 115 also showed 94%, 93%, 73% and 66% fluorescence quenching with PA, 2,4-DNP, TNT and Cl-DNB, respectively. Probe 114 is 10, 6000, and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times more selective towards PA in comparison to 2,4-DNP, TNT and Cl-DNB due to the RET process. Paper strips coated with probe 114 detected 2.29 × 10−20 g cm−2 PA (Fig. 39A–Q) by the contact mode, whereas probes 113 and 115 were less sensitive to PA. Probe 114 showed morphological changes after the addition of PA (Fig. 39b–e). Probes 114 and 115 could detect PA in the vapor phase in 30 s.
000 times more selective towards PA in comparison to 2,4-DNP, TNT and Cl-DNB due to the RET process. Paper strips coated with probe 114 detected 2.29 × 10−20 g cm−2 PA (Fig. 39A–Q) by the contact mode, whereas probes 113 and 115 were less sensitive to PA. Probe 114 showed morphological changes after the addition of PA (Fig. 39b–e). Probes 114 and 115 could detect PA in the vapor phase in 30 s.
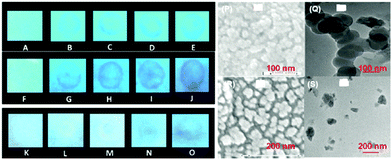 | ||
| Fig. 39 Fluorescence of probe-coated paper strips upon interaction with 10 μl of solutions with different concentrations of PA. (A–E) Probe 113 with (A) water; (B) 10−13 M PA; (C) 10−11 M PA; (D) 10−9 M PA; and (E) 10−7 M PA. (F–J) Probe 114 with (F) water; (G) 10−17 M PA; (H) 10−15 M PA; (I) 10−13 M PA; and (J) 10−11 M PA. (K–O) Probe 115 with (K) water; (L) 10−17 M PA; (M) 10−15 M PA; (N) 10−13 M PA; and (O) 10−11 M PA. SEM (P) and TEM (Q) images of probe 114 alone, and (R) and (S) probe 114 with PA (1 equiv.). Reprinted from ref. 111, Copyright 2016 Royal Society of Chemistry. | ||
Pyridinium–dansyl conjugate-based tripod 116112 (Chart 13) underwent aggregation in water (2% DMSO) with emission at 480 nm (Φ = 0.71), and these aggregates displayed amplified fluorescence quenching with only PA owing to the excited state energy transfer from 116 to PA. Other NACs, including the nitrophenols 2,4-DNP and 4-NP, had minimal effect on the fluorescence of the aggregates of 116. Probe 116 exhibited a linear change in fluorescence over a large range of PA concentration from 10−13 to 10−5 M. SEM and TEM images revealed changes in the morphology of probe 116 from leaf-like structures to a spherical shape with PA (Fig. 40a–f). Paper strips coated with probe 116 could detect 137 fg cm−2 PA in the solid state (Fig. 40g). The fluorescence lifetime of 116 increased from 0.73 ns to 1.45 ns in the presence of PA, which pointed to dynamic fluorescence quenching of aggregates of 116 with sub-micromolar amounts of PA.
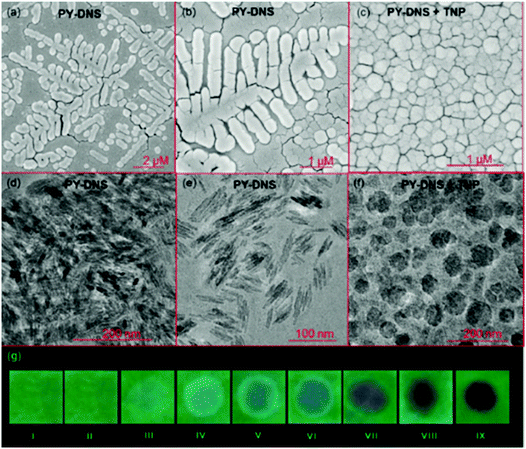 | ||
Fig. 40 (a and b) SEM images of thin films of 116 alone and (c) a 116 and PA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution obtained on a glass plate. (d and e) TEM images of two different sections of thin film of 116. (f) A TEM image of thin film from the 1 1) solution obtained on a glass plate. (d and e) TEM images of two different sections of thin film of 116. (f) A TEM image of thin film from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of 116 and PA. (g) Photographs of probe 116-coated paper strips: (i) a virgin test strip; (ii) a strip with water; (iii–ix) strips with 6 μL of solutions of PA with different concentrations: (iii) 10−13 M; (iv) 10−12 M; (v) 10−11 M; (vi) 10−10 M; (vii) 10−9 M; (viii) 10−8 M; and (ix) 10−7 M. Reprinted from ref. 112, Copyright 2017 Royal Society of Chemistry. 1 solution of 116 and PA. (g) Photographs of probe 116-coated paper strips: (i) a virgin test strip; (ii) a strip with water; (iii–ix) strips with 6 μL of solutions of PA with different concentrations: (iii) 10−13 M; (iv) 10−12 M; (v) 10−11 M; (vi) 10−10 M; (vii) 10−9 M; (viii) 10−8 M; and (ix) 10−7 M. Reprinted from ref. 112, Copyright 2017 Royal Society of Chemistry. | ||
The tripodal Schiff base 117113 (Chart 13) with H-bonding donor triaminoguanidinium moieties, the requisite hydrophobicity and π-electron-rich naphthalene moieties formed π-stacked crystals. When dispersed in water, these crystals113 displayed fluorescence quenching at 480 nm in accordance with the acidity of the phenolic protons in the order PA > 2,4-DNP > 4-NP, but neutral NACs caused minimal fluorescence quenching.
The stilbazolium-based dipodal114 probe 118 (Chart 14) with both absorption and emission in the visible region formed aggregates with an absorption maximum at 475 nm (red colour), which demonstrated a highly selective and sensitive change in colour from red to light-yellow with picric acid. The LOD for picric acid is 100 pM, which is the lowest in the literature using UV-Visible spectroscopy. These aggregates with fluorescence in the red region (615 nm) exhibited fluorescence quenching selectively with picric acid. TNT and other NACs induced inconsequential changes in the visible spectrum of the aggregates of 118, even when present in excess.
Upon the addition of water to its DMSO solution, tripod 119115 (Chart 14) displayed aggregation-based fluorescence quenching with a blue-shift in emission maximum from 635 nm to 615 nm, pointing to the formation of H-aggregates. These aggregates with picric acid exhibited simultaneous fluorescence reduction at 615 nm and an increase in fluorescence intensity between 510–540 nm, leading to ratiometric behaviour for picric acid. Paper strips coated with aggregates of 119 differentiated PA from other NACs through a fluorescence colour change from pink-red to blue with picric acid only (Fig. 41) and provided unprecedented discrimination of TNP from other NACs. Silica nanoparticles with probe 119 adsorbed on them highlighted latent fingerprints on a variety of substrates including aluminium foil, metal sheets, coins, ceramic tiles, etc., with a high resolution with possible applications in modern forensics.
 | ||
| Fig. 41 Paper strips coated with probe 119 (DMAS-TP) (1 mM) demonstrated changes in their fluorescence upon treatment with solutions of varying concentrations of picric acid and 10−6 M solutions of other NACs (10 μl each). Reprinted from ref. 115, Copyright 2021 Royal Society of Chemistry. | ||
TD-DFT studies of 118 and 119 demonstrated that restriction in ICT owing to proton transfer from picric acid to the dimethylamino group was responsible for the depletion of the colour of the probes with picric acid and that the proximity of the picrate anion to the probe resulted in charge transfer from the picrate to the DMAS moiety, leading to the non-emissive/poorly emissive excited state.
4. Metal complexes for the detection of picric acid
The weak fluorescence of the hydrazone-based probe 120116 (Chart 15) in a 95% aqueous medium (5% DMSO) was enhanced with a blue-shift of the emission maximum from 529 nm to 485 nm upon the addition of Al3+ ions. The increased fluorescence was assigned to the formation of a stable chelated system with high rigidity. The high fluorescence of the in situ prepared 120–Al(III) ensemble was quenched by the addition of PA, with a detection limit of 12.15 nM. An INHIBIT logic gate was also elaborated using this probe. Furthermore, test strips of 120 were also able to detect the presence of PA. Similarly, another AIEE-active Al(III) complex, 121,117 was used for the detection of picric acid in a pure aqueous system and a detection limit of 1.67 μM was calculated. However, the 121–Al3+ ensemble exhibited slightly more quenching with DNP as compared to PA.The AIEE-based hexaphenylbenzene derivative 122118 (Chart 15) exhibited an increase in quantum yield from 0.48 to 0.72 and a 7.14-fold enhancement in emission intensity at 460 nm with 30 equivalents of Hg2+ in H2O–THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). The 1
6). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric 122–Hg2+ ensemble was further used for the detection of PA through the fluorescence quenching process. The quenching in FL by PA was assigned to electrostatic interaction between the host and guest and electron transfer from the excited 122–Hg2+ complex to PA, as confirmed by pH and 1H NMR titration studies. Other nitro derivatives, such as TNT, 2,4-DNT, 1,3-DNB, etc., did not show any effect on the fluorescence intensity of this ensemble because of the lack of tendency to protonate the N,N-dimethylamino group. Furthermore, paper strips (Fig. 42) coated with 122–Hg2+ could detect PA concentrations as low as 0.03 μM.
1 stoichiometric 122–Hg2+ ensemble was further used for the detection of PA through the fluorescence quenching process. The quenching in FL by PA was assigned to electrostatic interaction between the host and guest and electron transfer from the excited 122–Hg2+ complex to PA, as confirmed by pH and 1H NMR titration studies. Other nitro derivatives, such as TNT, 2,4-DNT, 1,3-DNB, etc., did not show any effect on the fluorescence intensity of this ensemble because of the lack of tendency to protonate the N,N-dimethylamino group. Furthermore, paper strips (Fig. 42) coated with 122–Hg2+ could detect PA concentrations as low as 0.03 μM.
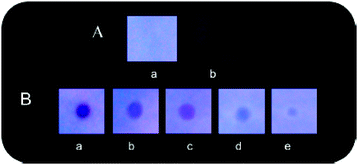 | ||
| Fig. 42 (A) Paper strips of the 122–Hg2+ ensemble (a) before and (b) after being dipped into PA solution (10−3 M in THF). (B) Demonstration of the effect of different concentrations of PA on test strips of the 122–Hg2+ ensemble [(a) 229 × 103 ppm, (b) 229 ppm, (c) 2.29 ppm, (d) 22.9 ppb, and (e) 0.22 ppb]. All images were taken under 365 nm UV illumination. Reprinted from ref. 118, Copyright 2013 American Chemical Society. | ||
Hg2+ interacts with the sulphur and the imino nitrogen of probe 123119 (Chart 15) to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complex 123 ∩ (Hg2+)2 with a nearly 290-fold increase in quantum yield from 0.001 to 0.290. The formation of nanoaggregates of 123 ∩ (Hg2+)2 was confirmed by the appearance of level-off long wavelength tails in its absorbance spectra. This highly blue-fluorescent 123 ∩ (Hg2+)2 complex (λem = 458 nm) became weakly fluorescent (λem = 00 nm) with PA, whereas other nitroaromatics such as TNT, 2,4-DNT, 1,3-DNB, nitromethane, NT, NB, and DMDNB did not show any effect on the fluorescence intensity. The protonation of the amine nitrogen followed by electron transfer from 123 (Hg2+)2 to PA was responsible for the fluorescence quenching mechanism. NOR and INHIBIT logic gates were also constructed using PA and H+ as inputs, and emission at 458 and 404 nm as outputs.
2 stoichiometric complex 123 ∩ (Hg2+)2 with a nearly 290-fold increase in quantum yield from 0.001 to 0.290. The formation of nanoaggregates of 123 ∩ (Hg2+)2 was confirmed by the appearance of level-off long wavelength tails in its absorbance spectra. This highly blue-fluorescent 123 ∩ (Hg2+)2 complex (λem = 458 nm) became weakly fluorescent (λem = 00 nm) with PA, whereas other nitroaromatics such as TNT, 2,4-DNT, 1,3-DNB, nitromethane, NT, NB, and DMDNB did not show any effect on the fluorescence intensity. The protonation of the amine nitrogen followed by electron transfer from 123 (Hg2+)2 to PA was responsible for the fluorescence quenching mechanism. NOR and INHIBIT logic gates were also constructed using PA and H+ as inputs, and emission at 458 and 404 nm as outputs.
The water-soluble ZnPc derivatives 124 and 125120 (Chart 15) with four and eight 4-dimethylaminopyridines gave quantum yields of 0.11 and 0.16, respectively, in DMSO. Probes 124 and 125 showed 87% and 89% fluorescence quenching at 680 nm and 695 nm, respectively, with 10 equivalents of PA due to the protonation of the N,N-dimethyl amino units, which prevented intramolecular charge transfer from the dimethylamino groups to the macrocycle core. Both probe 124 and 125 followed the quenching order NM < NB < NT < 4-NP < 2,4-DNT < 2,4-DNP < TNT < PA and could detect PA concentrations as low as 0.7 ± 0.1 and 1.1 ± 0.1 ppm, respectively. The thin films of 124 and 125 showed 28.6, 9.8, 9.2, and 7.5% fluorescence quenching in 240 s by the vapor of PA, TNT, 2,4-DNP, and 2,4-DNT, respectively.
5. Miscellaneous
BODIPY derivative 126121 (Chart 16) displayed 95% quenching of the emission intensity at 600 nm in the presence of 500 equivalents of PA due to the enhancement in intermolecular PET. The down-field shift of the 19F NMR signal in the presence of PA supported hydrogen bonding between fluorine and the phenolic hydrogen of PA. All other NACs resulted in less than 30% fluorescence quenching. Furthermore, 126 can be used for instant visualization of traces of PA.BODIPY-based turn-on chemodosimeter 127122 (Chart 16) exploited the strong acidic character of picric acid for highly selective hydrolysis of the acetal to the aldehyde in CH3CN–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Upon the addition of >20 equivalents of picric acid, the absorption maximum was red-shifted from 504 to 516 nm and the emission maxima from 518 to 535 nm, along with a six-fold enhancement in the emission intensity (Fig. 43). The fluorescence colour of the solution changed from pale green to bright yellow-orange due to the inhibition of PET. In the solid state, thin films of 127 displayed a red-shifted emission band at 612 nm, which exhibited 83% quenching of its fluorescence intensity upon exposure to saturated PA vapour for 500 s. Other NACs did not cause fluorescence quenching in these films.
1). Upon the addition of >20 equivalents of picric acid, the absorption maximum was red-shifted from 504 to 516 nm and the emission maxima from 518 to 535 nm, along with a six-fold enhancement in the emission intensity (Fig. 43). The fluorescence colour of the solution changed from pale green to bright yellow-orange due to the inhibition of PET. In the solid state, thin films of 127 displayed a red-shifted emission band at 612 nm, which exhibited 83% quenching of its fluorescence intensity upon exposure to saturated PA vapour for 500 s. Other NACs did not cause fluorescence quenching in these films.
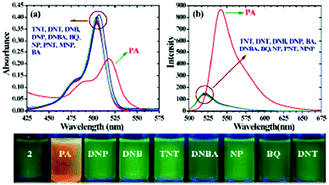 | ||
Fig. 43 Changes in the (a) absorption and (b) emission intensity of BODIPY 126 (5 μM) with nitroaromatics (50 equiv.) in CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) solution. Bottom panel: Color changes observed upon the addition of various nitroaromatic compounds to BODIPY 126 under a UV lamp. Reprinted from ref. 121, Copyright 2015 Royal Society of Chemistry. 1; v/v) solution. Bottom panel: Color changes observed upon the addition of various nitroaromatic compounds to BODIPY 126 under a UV lamp. Reprinted from ref. 121, Copyright 2015 Royal Society of Chemistry. | ||
BSA protein probe 128123 in an aqueous medium displayed 80.1% fluorescence quenching at 348 nm with 20 μM PA due to electron transfer and Förster resonance energy transfer mechanisms, as well as acid–base pairing interactions between the amino groups of 128 and PA. The response and equilibrium time was found to be less than 1 min. The α-helix content of BSA decreased with the addition of PA.
6. Conclusions
Picric acid (pKa 0.42), one of the most acidic nitroaromatic explosive materials, has the advantage of being able to selectively protonate basic sites such as amines (both aromatic and aliphatic), imines, aza-heterocycles (pyridine, imidazole), amides, etc., that are present in fluorophores. Other closely related nitrophenol derivatives, such as 2,4-DNP (pKa 4.04) and 4-nitrophenol (pKa 7.07), have poor capacity to protonate aromatic amines and other basic sites in comparison to picric acid. The protonation of basic nitrogen/oxygen in the fluorophore activates electrostatic interactions between the protonated fluorophore and the picrate anion. Alternately, the picrate anion can undergo electrostatic interactions with positively charged fluorescent probes. The proximity between the fluorophore and picrate anion that arises due to the strong electrostatic interactions facilitates electron/energy transfer processes and thus leads to selective fluorescence quenching with picric acid over other phenolic and non-phenolic nitroaromatic derivatives. This has defined a distinct feature for the design of fluorescent probes for the selective detection of picric acid over other nitroaromatic compounds.In general, picric acid is a natural fluorescence quencher via either the formation of non-fluorescent ground state charge transfer complexes due to the protonation of the basic site in the fluorophore or due to electron/energy transfer from the fluorophore in the excited state to the ground state of picric acid. However, fluorescence quenching is inherently a negative signal. Molecular probes that show an increase in fluorescence or a shift in the wavelength of the fluorescence maximum upon interaction with an analyte are always preferred, as these provide a positive signal for analysis.
However, FE and ratiometric fluorescence changes in response to PA have only been observed in a limited number of examples. Probe 97,101 which is a conjugate of two fluorophores, namely, squaraine and dansyl moieties, displayed a blue-shift in its fluorescence maximum from 684 nm to 644 nm in CH3CN and CH3CN–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In this case, the protonation of the dimethylamine nitrogen inhibited PET from the dansyl moiety and released the fluorescence of the squaraine moiety. In another case, the BODIPY probe 2552 displayed a 300-fold enhancement in emission intensity with PA in ethanol. Another ICT-based probe, 119,115 showed the distinct appearance of blue fluorescence upon the treatment of probe-coated paper strips with picric acid. In all these examples, the picric-acid-mediated protonation at the amine nitrogen is the key mechanistic feature. In the curcumin-based probes 27 and 28,54 the protonation of the amine by picric acid induced the aggregation of the resulting salts, and thus aggregation-induced emission enhancement was observed.
1). In this case, the protonation of the dimethylamine nitrogen inhibited PET from the dansyl moiety and released the fluorescence of the squaraine moiety. In another case, the BODIPY probe 2552 displayed a 300-fold enhancement in emission intensity with PA in ethanol. Another ICT-based probe, 119,115 showed the distinct appearance of blue fluorescence upon the treatment of probe-coated paper strips with picric acid. In all these examples, the picric-acid-mediated protonation at the amine nitrogen is the key mechanistic feature. In the curcumin-based probes 27 and 28,54 the protonation of the amine by picric acid induced the aggregation of the resulting salts, and thus aggregation-induced emission enhancement was observed.
Furthermore, the detection of picric acid vapor using fluorescent probes can provide an advantage for device formation. However, only a few cases in which thin films108,109,111,120 of a probe exhibit fluorescence quenching with picric acid within a few minutes have been reported, along with other cases in which a probe deposited on a TLC plate53,89 or THF–water33 solutions of a probe detect picric acid vapor.
In future, we anticipate the development of fluorescent probes for picric acid with two or more fluorescent moieties whose signals before and after interaction with picric acid differ, thus providing an increase or ratiometric change in the fluorescence. Fluorescence probes with increased ICT upon protonation with picric acid will appear in the literature, which are expected to display a shift in the fluorescence maximum to longer wavelengths with or without ratiometric changes. Fluorescence probes exhibiting a decrease in ICT upon protonation with a blue-shift in the fluorescence maximum will be developed.
Most of the fluorescent probes applied for the detection of picric acid emit in the blue or green region of the visible light spectrum and are plagued by interference from the background fluorescence of natural samples. There are only a few examples of red-emitting probes that have found application in the detection of picric acid. Increasing emphasis will be placed on the development of red- or NIR-emitting probes in future. Such probes are expected to open up new vistas for investigating the effects of PA in live cells and tissues. The development of fluorescent probes for the selective detection of PA vapor is another noteworthy area for future research.
Abbreviations
| ACN | Acetonitrile |
| ACQ | Aggregation-caused quenching |
| AFM | Atomic force microscopy |
| AIE | Aggregation-induced enhancement |
| AIEE | Aggregation-induced emission enhancement |
| BODIPY | Boron-dipyrromethene |
| BQ | 1,4-Benzoquinone |
| ClDNB | Chlorodinitrobenzene |
| CT | Charge transfer |
| DBSA | Dodecylbenzene-sulfonic acid |
| DFT | Density functional theory |
| DLS | Dynamic light scattering |
| DMF | N,N-Dimethylformamide |
| DMNB | Dimethylnitrobenzene |
| DMSO | Dimethyl sulfoxide |
| DNB | Dinitrobenzene |
| DNBA | 1,4-Dinitrobenzoic acid |
| 2,4- DNP | 2,4-Dinitrophenol |
| 2,4-DNT | 2,4-Dinitrotoulene |
| DTAB | Dodecyltrimethylammonium bromide. |
| ESIPT | Excited-state intramolecular proton transfer |
| FE | Fluorescence enhancement |
| FI | Fluorescence intensity |
| FRET | Förster resonance energy transfer |
| FQ | Fluorescence quenching |
| GSCT | Ground-state charge transfer |
| HCHO | Formaldehyde |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| HOMO | Highest occupied molecular orbital |
| ICT | Internal charge transfer |
| IFE | Inner filter effect |
| LOD | Limit of detection |
| LUMO | Lowest unoccupied molecular orbital |
| MeOH | Methanol |
| MOF | Metal organic framework |
| NA | Nitroaniline |
| NACs | Nitroaromatics |
| NB | Nitrobenzene |
| NM | Nitromethane |
| NMR | Nuclear magnetic resonance |
| NMP | N-Methyl-2-pyrrolidone |
| 4-NP | 4-Nitrophenol |
| NT | Nitrotoulene |
| PA | Picric acid |
| PBS | Phosphate buffer saline |
| PET | Photo-induced energy transfer |
| pKa | Acid dissociation constant |
| PYX | 2,6-Bis(picrylamino)-3,5-dinitropyridine |
| RDX | Hexahydro-1,3,5-trinitro-1,3,5-triazine |
| RET | Resonance energy transfer |
| RIR | Restricted intramolecular rotation |
| SDS | Sodium dodecylsulfate |
| SEM | Scanning electron microscopy |
| TBET | Through-bond energy transfer |
| TEA | Triethylamine |
| TEM | Transmission electron microscopy |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TNP | Trinitrophenol |
| TNT | Trinitrotoulene |
| TPE | Tetraphenylethylene |
| V.P. | Vapour pressure |
Author contributions
Sukhvinder Dhiman, Nancy Singla, Manzoor Ahmad and Prabhpreet Singh: review writing; Subodh Kumar: conceptualization, review writing and fund raising.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Department of Science and Technology, New Delhi, SERB grant EMR/2016/001535. We thank UGC for the UPE and PURSE programmes to the university and DST for the FIST program. S. Dhiman is thankful to the CSIR, India for a Senior Research Fellowship (Grant no. 09/254(0304)/2020-EMR-I). SK thanks UGC for a UGC-BSR faculty F.4-5(11)/2019 (BSR) fellowship.References
- J. Akhavan, The chemistry of explosives, Royal Society of Chemistry, 2011 Search PubMed.
- S. Shanmugaraju and P. S. Mukherjee, Chem. Commun., 2015, 51, 16014–16032 RSC.
- P. Wexler, Encyclopedia of Toxicology, 2nd edn, 2005 Search PubMed.
- P. Wexler, Encyclopedia of Toxicology, 3rd edn, 2014 Search PubMed.
- M. Russel, The Chemistry of Fireworks: Edition 2, 2009, p. 138 Search PubMed.
- P. G. Thorne and T. F. Jenkins, Special Report, USA Cold Regions Research and Engineering Laboratory, Hanover, NH, 1995, pp. 95–120 Search PubMed.
- P. G. Thorne and T. F. Jenkins, Field Anal. Chem. Technol., 1997, 1, 165–170 CrossRef.
- S. H. Yalkowsky, Y. He and P. Jain, Handbook of Aqueous Solubility Data, 2nd edn, CRC Press, Boca Raton, FL, 2010, p. 200 Search PubMed.
- R. M. Hebert and A. M. Jackovitz, Wildlife Toxicity Assessment for Picric Acid (2,4,6-Trinitrophenol), 2015, pp. 271–277 Search PubMed.
- M. Takahashi, H. Ogata, H. Izumi, K. Yamashita, M. Takechi, M. Hirata-Koizumi, E. Kamata, R. Hasegawa and M. Ema, Congenit. Anom., 2004, 44, 204–214 CrossRef CAS PubMed.
- J. F. Wyman, M. P. Serve, D. W. Hobson, L. H. Lee and D. E. Uddin, J. Toxicol. Environ. Health, 1992, 37, 313–327 CrossRef CAS PubMed.
- (a) K. R. Cooper, D. T. Burton, W. L. Goodfellow and D. H. Rosenblatt, J. Toxicol. Environ. Health, 1984, 14, 731–747 CrossRef CAS PubMed; (b) K.-M. Wollin and H. H. Dieter, Arch. Environ. Contam. Toxicol., 2005, 49, 18–26 CrossRef CAS PubMed.
- J. Y. Shen, J. F. Zhang, Y. Zuo, L. J. Wang, X. Y. Sun, J. S. Li, W. Q. Han and R. He, J. Hazard. Mater., 2009, 163, 1199–1206 CrossRef CAS.
- (a) M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543–2555 RSC; (b) Y. Salinas, R. Martınez-Manez, M. D. Marcos, F. Sancenon, A. M. Costero, M. Parraad and S. Gilad, Chem. Soc. Rev., 2012, 41, 1261–1296 RSC; (c) G. V. Zyryanov, D. S. Kopchuk, I. S. Kovalev, E. V. Nosova, V. L. Rusinov and O. N. Chupakhin, Russ. Chem. Rev., 2014, 83, 783–819 CrossRef.
- (a) K. K. Kartha, A. Sandeep, V. K. Praveen and A. Ajayaghosh, Chem. Rec., 2015, 15, 252–265 CrossRef CAS PubMed; (b) X. Sun, Y. Wang and Y. Lei, Chem. Soc. Rev., 2015, 44, 8019–8061 RSC; (c) S. Shanmugaraju and P. S. Mukherjee, Chem. – Eur. J., 2015, 21, 6656–6666 CrossRef CAS PubMed; (d) M. Chhatwal, R. Mittal, R. D. Gupta and S. K. Awasthi, J. Mater. Chem. C, 2018, 6, 12142–12158 RSC; (e) E. V. Verbitskiy, G. L. Rusinov, O. N. Chupakhin and V. N. Charushin, Dyes Pigm., 2020, 180, 108414 CrossRef CAS; (f) A. S. Tanwar, N. Meher, L. R. Adil and P. K. Iyer, Analyst, 2020, 145, 4753–4767 RSC.
- M. E. Walsh, Talanta, 2001, 54, 427–438 CrossRef CAS PubMed.
- P. T. Sukhanov, A. A. Kushnir, E. V. Churilina, N. V. Maslova and G. V. Shatalov, Anal. Chem., 2017, 72, 468–472 CrossRef CAS.
- (a) J. Yinon, Mass Spectrom. Rev., 1982, 1, 257–307 CrossRef CAS; (b) J. C. Mathurin, T. Faye, A. Brunot and J. C. Tabet, Anal. Chem., 2000, 72, 5055–5062 CrossRef CAS PubMed; (c) A. Prakash, S. Chandra and D. Bahadur, Carbon, 2012, 50, 4209–4219 CrossRef CAS; (d) D. Lin, J. Wu, M. Wang, F. Yan and H. Ju, Anal. Chem., 2012, 84, 3662–3668 CrossRef CAS PubMed.
- (a) M. Krausa and K. Schorb, J. Electroanal. Chem., 1999, 461, 10–13 CrossRef CAS; (b) C. L. M. Palenzuela, F. Novotný, P. Krupička, Z. Sofer and M. Pumera, Anal. Chem., 2018, 90, 5753–5757 CrossRef PubMed; (c) A. Ramachandran, J. S. Arya Nair and S. K. Yesodha, ACS Sustainable Chem. Eng., 2019, 7, 6732–6743 CrossRef CAS.
- (a) J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834–5840 CrossRef CAS PubMed; (b) A. Hakonen, P. O. Andersson, M. S. Schmidt, T. Rindzevicius and M. Kall, Anal. Chim. Acta, 2015, 893, 1–13 CrossRef CAS PubMed.
- (a) F. Qiu, Y.-H. Huang, Q. M. Ge, M. Liu, H. Cong and Z. Tao, Spectrochim. Acta, Part A, 2020, 226, 117583 CrossRef CAS PubMed; (b) V. Bharadwaj, J. E. Park, S. K. Sahoo and H.-J. Choi, ChemistrySelect, 2019, 4, 10895–10901 CrossRef CAS.
- (a) A. Kumar, A. Kumar and D. S. Pandey, Dalton Trans., 2016, 45, 8475–8484 RSC; (b) N. Goel and N. Kumar, Inorg. Chim. Acta, 2017, 463, 14–19 CrossRef CAS.
- (a) S. Senthilkumar, R. Goswami, N. L. Obasi and S. Neogi, ACS Sustainable Chem. Eng., 2017, 5, 11307–11315 CrossRef CAS; (b) H.-R. Fu, L.-B. Yan, N.-T. Wu, L.-F. Ma and S.-Q. Zang, J. Mater. Chem. A, 2018, 6, 9183–9191 RSC; (c) S. Xing, Q. Bing, H. Qi, J. Liu, T. Bai, G. Li, Z. Shi, S. Feng and R. Xu, ACS Appl. Mater. Interfaces, 2017, 9, 23828–23835 CrossRef CAS PubMed.
- (a) Y. Wang, X. Chang, N. Jing and Y. Zhang, Anal. Methods, 2018, 10, 2775–2784 RSC; (b) J. Li, L. Zhang, P. Li, Y. Zhang and C. Dong, Sens. Actuators, B, 2018, 258, 580–588 CrossRef CAS.
- (a) K. Shanmugaraj and S. Abraham John, New J. Chem., 2018, 42, 7223–7229 RSC; (b) W. J. Zhang, S. G. Liu, L. Han, Y. Ling, L. L. Liao, S. Mo, H. Q. Luo and N. B. Li, Anal. Methods, 2018, 10, 4251–4256 RSC.
- (a) X.-D. Zhang, J.-A. Hua, J.-H. Guo, Y. Zhao and W.-Y. Sun, J. Mater. Chem. C, 2018, 6, 12623–12630 RSC; (b) X. Yin, S. Meng and J. Xie, Polyhedron, 2018, 139, 262–266 CrossRef CAS; (c) L. Li, J. Cheng, Z. Liu, L. Song, Y. You, X. Zhou and W. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 44109–44115 CrossRef CAS PubMed.
- CRC Handbook of Chemistry and Physics, 95th edn, ed. W. M. Haynes, CRC Press LLC, Boca Raton, FL, 2014–2015, pp. 5–97 Search PubMed.
- (a) R. Sun, X. Huo, H. Lu, S. Feng, D. Wang and H. Liu, Sens. Actuators, B, 2018, 265, 476–487 CrossRef CAS; (b) A. Biswas, D. Giri, D. Das, A. De, S. K. Patra and R. Samanta, J. Org. Chem., 2017, 82, 10989–10996 CrossRef CAS PubMed; (c) S. Kumar, N. Venkatramaiah and S. Patil, J. Phys. Chem. C, 2013, 117, 7236–7245 CrossRef CAS; (d) N. Wang, J.-C. Yang, L.-D. Chen, J. Li, Y. An, C.-W. Lu and Y.-Q. Tian, New J. Chem., 2017, 41, 2786–2792 RSC; (e) W. Xue, Y. Zhang, J. Duan, D. Liu, Y. Ma, N. Shi, S. Chen, L. Xie, Y. Qian and W. Huang, J. Mater. Chem. C, 2015, 3, 8193–8199 RSC.
- (a) N. Venkatramaiah, A. D. G. Firmino, F. A. Almeida Paz and J. P. C. Tome, Chem. Commun., 2014, 50, 9683–9686 RSC; (b) V. Bhalla, H. Arora, H. Singh and M. Kumar, Dalton Trans., 2013, 42, 969–974 RSC; (c) S. Shanmugaraju, H. Jadhav, R. Karthik and P. S. Mukherjee, RSC Adv., 2013, 3, 4940–4950 RSC; (d) H. Ma, F. Li, L. Yao, Y. Feng, Z. Zhang and M. Zhang, Sens. Actuators, B, 2018, 259, 380–386 CrossRef CAS; (e) E. Hussain, Y. Li, C. Cheng, H. Zhuo, S. A. Shahzad, S. Ali, M. Ismail, H. Qi and C. Yu, Talanta, 2020, 207, 120316 CrossRef CAS PubMed.
- (a) N. Venkatramaiah, S. Kumar and S. Patil, Chem. – Eur. J., 2012, 18, 14745–14751 CrossRef CAS PubMed; (b) S. Shanmugaraju, S. A. Joshi and P. S. Mukherjee, J. Mater. Chem., 2011, 21, 9130–9138 RSC; (c) S. S. Babu and S. K. Shanmugam, J. Mater. Chem. C, 2017, 5, 4788–4796 RSC; (d) Y. Chandrasekaran, N. Venkatramaiah and S. Patil, Chem. – Eur. J., 2016, 22, 5288–5294 CrossRef CAS PubMed.
- (a) R. Hu, J. L. Maldonado, M. Rodriguez, C. Deng, C. K. W. Jim, J. W. Y. Lam, M. M. F. Yuen, G. Ramos-Ortiz and B. Z. Tang, J. Mater. Chem., 2012, 22, 232–240 RSC; (b) H. Li, H. Wu, E. Zhao, J. Li, J. Z. Sun, A. Qin and B. Z. Tang, Macromolecules, 2013, 46, 3907–3914 CrossRef CAS.
- (a) C. Y. K. Chan, J. W. Y. Lam, C. Deng, X. Chen, K. S. Wong and B. Z. Tang, Macromolecules, 2015, 48, 1038–1047 CrossRef CAS; (b) J. Li, J. Liu, J. W. Y. Lama and B. Z. Tang, RSC Adv., 2013, 3, 8193–8196 Search PubMed.
- V. Vij, V. Bhalla and M. Kumar, ACS Appl. Mater. Interfaces, 2013, 5, 5373–5380 CrossRef CAS PubMed.
- M. K. Chahal and M. Sankar, Anal. Methods, 2015, 7, 10272–10279 RSC.
- A. Pandith, A. Kumar, J. Y. Lee and H. S. Kim, Tetrahedron Lett., 2015, 56, 7094–7099 CrossRef CAS.
- A. K. Bandela, S. Bandaru and C. P. Rao, Chem. – Eur. J., 2015, 21, 13364–13374 CrossRef CAS PubMed.
- V. Mahendran and S. Shanmugam, RSC Adv., 2015, 5, 92473–92479 RSC.
- V. Bhalla, H. Arora and M. Kumar, RSC Adv., 2015, 5, 32637–32642 RSC.
- P. Vishnoi, M. G. Walawalkar, S. Sen, A. Datta, G. N. Patwari and R. Murugavel, Phys. Chem. Chem. Phys., 2014, 16, 10651–10658 RSC.
- P. Vishnoi, M. G. Walawalkar and R. Murugavel, Cryst. Growth Des., 2014, 14, 5668 CrossRef CAS.
- P. Vishnoi, S. Sen, G. N. Patwari and R. Murugavel, New J. Chem., 2015, 39, 886 RSC.
- S. Nagendran, P. Vishnoi and R. Murugavel, J. Fluoresc., 2017, 27, 1299–1305 CrossRef CAS PubMed.
- K. Ponnuvel, G. Banuppriya and V. Padmini, Sens. Actuators, B, 2016, 234, 34–45 CrossRef CAS.
- S. S. Babu and S. Shanmugam, ChemistrySelect, 2018, 3, 4075–4081 CrossRef CAS.
- W. Fang, W. Zhao, P. Pei, R. Liu, Y. Zhanga, L. Konga and J. Yang, J. Mater. Chem. C, 2018, 6, 9269–9276 RSC.
- X. Lu, G. Zhanga, D. Li, X. Tian, W. Ma, S. Li, Q. Zhang, H. Zhou, J. Wu and Y. Tian, Dyes Pigm., 2019, 170, 107641 CrossRef CAS.
- A. Shylaja, S. R. Rubina, S. S. Roja and R. R. Kumar, Dyes Pigm., 2020, 174, 108062 CrossRef CAS.
- A. S. Tanwar, S. Hussain, A. H. Malik, M. A. Afro and P. K. Iyer, ACS Sens., 2016, 1, 1070–1077 CrossRef CAS.
- M. Sathiyaraj, K. Pavithra and V. Thiagarajan, New J. Chem., 2020, 44, 8402–8411 RSC.
- P. Kumar, D. Arya, D. Nain, A. Singh, A. Ghosh and D. Amilan Jose, Dyes Pigm., 2019, 166, 443–450 CrossRef CAS.
- J. Gao, X. Chen, S. Chen, H. Meng, Y. Wang, C. Li and L. Feng, Anal. Chem., 2019, 91, 13675–13680 CrossRef CAS PubMed.
- Y. Erande, S. Chemate, A. More and N. Sekar, RSC Adv., 2015, 5, 89482–89487 RSC.
- K. Maiti, A. K. Mahapatra, A. Gangopadhyay, R. Maji, S. Mondal, S. S. Ali, S. Das, R. Sarkar, P. Datta and D. Mandal, ACS Omega, 2017, 2, 1583–1593 CrossRef CAS PubMed.
- B. Gogoi and N. S. Sarma, ACS Appl. Mater. Interfaces, 2015, 7, 11195–11202 CrossRef CAS PubMed.
- S. Kaur, A. Gupta, V. Bhalla and M. Kumar, J. Mater. Chem. C, 2014, 2, 7356–7363 RSC.
- S. Kaur, V. Bhalla, V. Vij and M. Kumar, J. Mater. Chem. C, 2014, 2, 3936–3941 RSC.
- A. Sil, D. Giri and S. K. Patra, J. Mater. Chem. C, 2017, 5, 11100–11110 RSC.
- S. Maity, M. Shyamal, D. Das, A. Maity, S. Dey and A. Misra, New J. Chem., 2018, 42, 1879–1891 RSC.
- V. Kachwal, P. Alam, H. R. Yadav, S. S. Pasha, A. R. Choudhury and I. R. Laskar, New J. Chem., 2018, 42, 1133–1140 RSC.
- A. Kathiravan, A. Gowri, T. Khamrang, M. D. Kumar, N. Dhenadhayalan, K.-C. Lin, M. Velusamy and M. Jaccob, Anal. Chem., 2019, 91, 13244–13250 CrossRef CAS PubMed.
- Y. Ma, Y. Zhang, X. Liu, Q. Zhang, L. Kong, Y. Tian, G. Li, X. Zhang and J. Yang, Dyes Pigm., 2019, 163, 1–8 CrossRef CAS.
- H. Li, R. Jia and Y. Wang, Spectrochim. Acta, Part A, 2020, 228, 117793 CrossRef CAS PubMed.
- J. Pan, F. Tang, A. Ding, L. Kong, L. Yang, X. Tao, Y. Tian and J. Yang, RSC Adv., 2015, 5, 191–195 RSC.
- P. Ghosh and P. Banerjee, Anal. Chim. Acta, 2017, 965, 111–122 CrossRef CAS PubMed.
- D. C. Santra, M. K. Bera, P. K. Sukul and S. Malik, Chem. – Eur. J., 2016, 22, 2012–2019 CrossRef CAS PubMed.
- J. B. Arockiam and S. Ayyanar, Sens. Actuators, B, 2017, 242, 535–544 CrossRef CAS.
- Y. Peng, A.-J. Zhang, M. Dong and Y.-W. Wang, Chem. Commun., 2011, 47, 4505–4507 RSC.
- M. Dong, Y.-W. Wang, A.-J. Zhang and Y. Peng, Chem. – Asian J., 2013, 8, 1321–1330 CrossRef CAS PubMed.
- Z.-H. Fu, Y.-W. Wang and Y. Peng, Chem. Commun., 2017, 53, 10524–10527 RSC.
- G. Chakraborty and S. K. Mandal, ACS Omega, 2018, 3, 3248–3256 CrossRef CAS PubMed.
- B. Pramanik, S. Das and D. Das, Chem. – Asian J., 2020, 15, 4291–4296 CrossRef CAS PubMed.
- X. Cao, N. Zhao, H. Lv, Q. Ding, A. Gao, Q. Jing and T. Yi, Langmuir, 2017, 33, 7788–7798 CrossRef CAS PubMed.
- S. S. Harmalkar, A. V. Naik, M. K. Nilajakar and S. N. Dhuri, ChemistrySelect, 2020, 5, 8447–8454 CrossRef CAS.
- P. Sakthivel, K. Sekar, S. Singaravadivel and G. Sivaraman, ChemistrySelect, 2019, 4, 3817–3822 CrossRef CAS.
- H.-L. Ding, L.-D. Chen, N. Wang, K. Li, Y. An and C.-W. Lü, Talanta, 2019, 195, 345–353 CrossRef CAS PubMed.
- Jigyasa and J. K. Rajput, Sens. Actuators, B, 2018, 259, 990–1005 CrossRef CAS.
- R. Ahmed, A. Ali, M. Ahmad, A. Alsalme, R. A. Khan and F. Ali, New J. Chem., 2020, 44, 20092–20100 RSC.
- Y.-C. Wu, S.-H. Luo, L. Cao, K. Jiang, L.-Y. Wang, J.-C. Xie and Z.-Y. Wang, Anal. Chim. Acta, 2017, 976, 74–83 CrossRef CAS PubMed.
- J.-F. Xiong, J.-X. Li, G.-Z. Mo, J.-P. Huo, J.-Y. Liu, X.-Y. Chen and Z.-Y. Wang, J. Org. Chem., 2014, 79, 11619–11630 CrossRef CAS PubMed.
- S. Chaudhary, H. Sharma and M. D. Milton, ChemistrySelect, 2018, 3, 4598–4608 CrossRef CAS.
- R. Sodkhomkhum, M. Masik, S. Watchasit, C. Suksai, J. Boonmak, S. Youngme, N. Wanichacheva and V. Ervithayasuporn, Sens. Actuators, B, 2017, 245, 665–673 CrossRef CAS.
- S. Zhang, H. Zhu, J. Huang, L. Kong, Y. Tian and J. Yang, ChemistrySelect, 2019, 4, 7380–7387 CrossRef CAS.
- B. K. Kundu, R. Pragti, S. M. Mobin and S. Mukhopadhyay, New J. Chem., 2019, 43, 11483–11492 RSC.
- A. S. M. Islam, M. Sasmal, D. Maiti, A. Dutta, B. Show and M. Ali, ACS Omega, 2018, 3, 10306–10316 CrossRef CAS PubMed.
- A. Panigrahi, B. P. Sahu, S. Mandani, D. Nayak, S. Giri and T. K. Sarma, J. Photochem. Photobiol., A, 2019, 374, 194–205 CrossRef CAS.
- M. Shyamal, D. Das, P. K. Giri, S. Maity and A. Misra, Mater. Today Chem., 2019, 14, 100193 CrossRef CAS.
- (a) H. Ma, C. He, X. Li, O. Ablikim, S. Zhang and M. Zhang, Sens. Actuators, B, 2016, 230, 746–752 CrossRef CAS; (b) Y. Ma, L. Zhao, Y. Li, J. Liu, Y. Yang and T. Chu, Tetrahedron, 2018, 74, 2684–2691 CrossRef CAS.
- Q. Lin, X.-W. Guan, Y.-Q. Fan, J. Wang, L. Liu, J. Liu, H. Yao, Y.-M. Zhang and T.-B. Wei, New J. Chem., 2019, 43, 2030–2036 RSC.
- A. Kumar and P. S. Chae, Sens. Actuators, B, 2017, 240, 1–9 CrossRef CAS.
- P. Lasitha and E. Prasad, RSC Adv., 2015, 5, 41420–41427 RSC.
- S. Maity, M. Shyamal, P. Mazumdar, G. P. Sahoo, R. Maity, G. S. Morán and A. Misra, J. Mol. Liq., 2016, 224, 255–264 CrossRef CAS.
- E. Zhang, P. Ju, P. Guo, X. Hou, X. Hou, H. Lv, J. Wang and Y. Zhang, RSC Adv., 2018, 8, 31658–31665 RSC.
- M. Ghosh, S. Ta, M. Banerjee, M. Mahiuddin and D. Das, ChemistrySelect, 2018, 3, 6145–6151 CrossRef CAS.
- R. Chopra, V. Bhalla, M. Kumar and S. Kaur, RSC Adv., 2015, 5, 24336–24341 RSC.
- Y. Ma, Y. Zhang, L. Kong and J. Yang, CrystEngComm, 2019, 21, 94–101 RSC.
- J. Du, J. Liu, Y. Ren, C. Wang, F. Bai and H. Hao, Spectrochim. Acta, Part A, 2019, 211, 287–290 CrossRef CAS PubMed.
- N. Nagamani, S. Lakshmanan, D. Govindaraj, C. Ramamoorthy, N. Ramalakshmi and S. A. Antony, Spectrochim. Acta, Part A, 2019, 207, 321–327 CrossRef CAS PubMed.
- S. Kumari, S. Joshi, T. C. Cordova-Sintjago, D. D. Pant and R. Sakhuja, Sens. Actuators, B, 2016, 229, 599–608 CrossRef CAS.
- S. K. Patil, D. V. Awale, M. M. Vadiyar, S. A. Patil, S. C. Bhise, A. H. Gore, G. B. Kolekar, J. H. Kim and S. S. Kolekar, ChemistrySelect, 2017, 2, 4124–4130 CrossRef CAS.
- A. Kumar, A. Pandith and H.-S. Kim, Sens. Actuators, B, 2016, 231, 293–301 CrossRef CAS.
- Y. Xu, B. Li, W. Li, J. Zhao, S. Sun and Y. Pang, Chem. Commun., 2013, 49, 4764–4766 RSC.
- L. Ding, Y. Bai, Y. Cao, G. Ren, G. J. Blanchard and Y. Fang, Langmuir, 2014, 30, 7645–7653 CrossRef CAS PubMed.
- S. Hussain, A. H. Malik, M. A. Afroza and P. K. Iyer, Chem. Commun., 2015, 51, 7207–7210 RSC.
- N. Yan, J. Song, F. Wang, L. Kan, J. Song, W. Wang, W. Ma, W. Zhang and G. He, Chin. Chem. Lett., 2019, 30, 1984–1988 CrossRef CAS.
- V. Lakshmidevi, C. V. Yelamaggad and A. Venkataraman, ChemistrySelect, 2018, 3, 2655–2664 CrossRef CAS.
- P. S. Hariharan, J. Pitchaimani, V. Madhu and S. P. Anthony, J. Fluoresc., 2016, 26, 395–401 CrossRef CAS PubMed.
- B. Roy, A. K. Bar, B. Gole and P. S. Mukherjee, J. Org. Chem., 2013, 78, 1306–1310 CrossRef CAS PubMed.
- R. Kumar, S. Sandhu, P. Singh, G. Hundal, M. S. Hundal and S. Kumar, Asian J. Org. Chem., 2014, 3, 805–813 CrossRef CAS.
- S. Sandhu, R. Kumar, P. Singh, A. Mahajan, M. Kaur and S. Kumar, ACS Appl. Mater. Interfaces, 2015, 7, 10491–10500 CrossRef CAS PubMed.
- N. Tripathi, S. Sandhu, P. Singh, A. Mahajan, M. Kaur and S. Kumar, Sens. Actuators, B, 2016, 231, 79–87 CrossRef CAS.
- S. Sandhu, R. Kumar, P. Singh and S. Kumar, J. Mater. Chem. C, 2016, 4, 3209–3216 RSC.
- N. Tripathi, P. Singh and S. Kumar, New J. Chem., 2017, 41, 8739–8747 RSC.
- S. Mukherjee, A. V. Desai, A. I. Inamdar, B. Manna and S. K. Ghosh, Cryst. Growth Des., 2015, 15, 3493–3497 CrossRef CAS.
- S. Dhiman, G. Kumar, V. Luxami, P. Singh and S. Kumar, New J. Chem., 2020, 44, 10870–10877 RSC.
- S. Dhiman, M. Ahmad, G. Kumar, V. Luxami, P. Singh and S. Kumar, J. Mater. Chem. C, 2021, 9, 1097–1106 RSC.
- S. Sharma, G. Dubey, B. S. Sran, P. V. Bharatam and G. Hundal, ACS Omega, 2019, 4, 18520–18529 CrossRef CAS PubMed.
- R. Purkait, A. Dey, S. Deya, P. P. Ray and C. Sinha, New J. Chem., 2019, 43, 14979–14990 RSC.
- V. Bhalla, S. Kaur, V. Vij and M. Kumar, Inorg. Chem., 2013, 52, 4860–4865 CrossRef CAS PubMed.
- M. Kumar, S. I. Reja and V. Bhalla, Org. Lett., 2012, 14, 6084–6087 CrossRef CAS PubMed.
- S. Kasthuri, P. Gawas, S. Maji, N. Veeraiah and N. Venkatramaiah, ACS Omega, 2019, 4, 6218–6228 CrossRef CAS PubMed.
- M. Hengchang, Z. Zhongwei, J. Yuanyuan, Z. Lajia, Q. Chunxuan, C. Haiying, Y. Zengming, Y. Zhiwang and Z. Lei, RSC Adv., 2015, 5, 87157–87167 RSC.
- S. Madhu, A. K. Bandela and M. Ravikanth, RSC Adv., 2014, 4, 7120–7123 RSC.
- X. Sun, X. Ma, C. V. Kumar and Y. Lei, Anal. Methods, 2014, 6, 8464–8468 RSC.
| This journal is © The Royal Society of Chemistry 2021 |