Open Access Article
Open Access ArticleRemarkable synergy of borate and interfacial hole transporter on BiVO4 photoanodes for photoelectrochemical water oxidation†
Qijun
Meng
 a,
Biaobiao
Zhang
a,
Biaobiao
Zhang
 *bc,
Hao
Yang
*bc,
Hao
Yang
 a,
Chang
Liu
d,
Yingzheng
Li
d,
Alexander
Kravchenko
a,
Chang
Liu
d,
Yingzheng
Li
d,
Alexander
Kravchenko
 a,
Xia
Sheng
a,
Lizhou
Fan
a,
Xia
Sheng
a,
Lizhou
Fan
 a,
Fusheng
Li
a,
Fusheng
Li
 d and
Licheng
Sun
d and
Licheng
Sun
 *abcd
*abcd
aDepartment of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
bCenter of Artificial Photosynthesis for Solar Fuels, School of Science, Westlake University, 310024 Hangzhou, China
cInstitute of Natural Sciences, Westlake Institute for Advanced Study, Hangzhou, 310024 Zhejiang, China
dState Key Laboratory of Fine Chemicals, Institute of Artificial Photosynthesis, DUT-KTH Joint Education and Research Center on Molecular Devices, Dalian University of Technology (DUT), 116024 Dalian, China
First published on 12th May 2021
Abstract
Bismuth vanadate (BiVO4) is one of the most fascinating building blocks for the design and assembly of highly efficient artificial photosynthesis devices for solar water splitting. Our recent report has shown that borate treated BiVO4 (B-BiVO4) results in an improved water oxidation performance. In this study, further improvement of both the photoelectrochemical (PEC) activity and stability of B-BiVO4 was successfully achieved by introducing NiFeV LDHs as an oxygen evolution catalyst and interfacial hole transporter. Benefiting from the synergistic effect of co-catalyst and borate pretreatment, the as-prepared NiFeV/B-BiVO4 exhibited a high photocurrent density of 4.6 mA cm−2 at 1.23 VRHE and an outstanding onset potential of ∼0.2 VRHE with good long-term stability. More importantly, NiFeV was found to play a pivotal role in the critically efficient suppression of charge combination on the BiVO4 surface and acceleration of charge transfer rather than a mere electrocatalyst for water oxidation.
1. Introduction
Photoelectrochemical (PEC) water splitting is a promising way to produce hydrogen as a clean and renewable energy carrier using light-absorbing semiconductors.1–4 Since the photocatalytic water oxidation activity of monoclinic bismuth vanadate (BiVO4) was discovered by Kudo et al. in 1998,5 BiVO4 has drawn tremendous attention in the domain of PEC water splitting by virtue of favorable band positions and a suitable bandgap of 2.4–2.5 eV, which can allow the absorption of 11% visible light spectrum.6–8 Theoretically, the maximum photocurrent density and the solar-to-hydrogen conversion efficiency can reach values as high as 7.5 mA cm−2 and 9% under AM 1.5G illumination, respectively.9 Unfortunately, a pure BiVO4 photoelectrode still suffers from excessive electron–hole recombination (a carrier mobility of ∼4 × 10−2 cm2 V−1 s−1), poor charge transport properties (a hole diffusion length of ∼70 nm), and sluggish water oxidation kinetics, mostly resulting in disappointing photocurrent densities.10To alleviate these limitations, numerous approaches have been proposed that usually combine effective synthesis methods with multiple modification strategies, including crystal facet engineering,11–13 construction of heterojunctions,14–16 substitutional doping,17–19 oxygen evolution catalyst (OEC) loading,20–24 post-synthetic treatments,25–28etc. For example, a landmark work reported by Choi and co-workers displayed a nanoporous BiVO4 film photoanode (mean particle size ∼76 nm) via a two-step method of electrodeposition of BiOI and ensuing thermal conversion of BiOI to BiVO4 by introducing a proper vanadium source.6,29 The resulting BiVO4 exhibited a high charge separation efficiency in the bulk (ηbulk) up to 90% without routine doping or heterojunction, and after assembling a NiOOH/FeOOH OEC, a remarkable photocurrent density of 4.5 mA cm−2 at 1.23 V versus reversible hydrogen electrode (RHE) was obtained under AM 1.5G illumination.6 Inspired by the natural photosystem II (PSII), Li and co-workers successfully demonstrated a CoPO3/pGO/LDH/BiVO4 composite photoanode,30 where BiVO4, NiFe layered double hydroxide (LDH), partially oxidized graphene (pGO) and cobalt cubane molecular catalyst served as a light harvester, a hole storage layer, a charge transfer layer and an OEC, respectively. The composite photoanode possessed a remarkable photocurrent density of 4.45 mA cm−2 with an ultralow onset potential of 0.17 V and superior stability. By combining p–n heterojunction engineering, work function adjustment and co-catalyst loading,31 Yang and co-workers designed and fabricated a NiFeOx/B-C3N4/Mo-BiVO4 photoanode, achieving a photocurrent density of 5.93 mA cm−2 at 1.23 VRHE (92% IPCE) with an astonishing applied bias photon-to-current efficiency (ABPE) of 2.67% at 0.54 VRHE. In a very recent review,32 Lee and co-workers clearly elucidated the efficacy of established modification techniques on a BiVO4 photoanode by applying post-synthetic N2 treatment, Mo doping, an electron transfer layer of SnO2, and deposition of the NiFeOx electrocatalyst. The obtained NiFeOx/N, 1% Mo:BiVO4/SnO2 photoanode also exhibited outstanding PEC performance with 5.8 mA cm−2 and a maximum ABPE of 2.7%. It should be pointed out that loading with OECs contributed the most to the dramatic enhancement compared to other modifications,32 aiming at promoting the surface charge transfer at the semiconductor/electrolyte interface (ηsurface) as well as long-term photostability.
On the one hand, these recently flourished modification strategies have elevated BiVO4 to an unprecedented position as the most up-and-coming photoanode material. On the other hand, each of them developed thus far is often found to have limited efficacy in targeting an all-round improvement toward all aspects of charge carrier kinetics, catalytic kinetics of water oxidation, and even light absorption, in terms of PEC performance and fundamental understanding of the material and working principles.33,34 The elaborately modified BiVO4-based PEC devices are therefore in growing appeal toward practical applications.2
In our previous work, a facile borate modification of a BiVO4 photoanode (B-BiVO4) was reported, delivering an impressive enhancement in PEC performance with a photocurrent density of ∼3.5 mA cm−2 at 1.23 VRHE without any doping or OEC decoration.35 An analogous phenomenon engendered by borate species was also presented in several reports.36–39 However, the B-BiVO4 photoanode gradually lost its superior PEC performance within 1 h due to the photocorrosion of the adsorbed borate groups by surface charge accumulation. In this study, we solved this problem via the integration of a NiFe-based LDH, an excellent electrocatalyst for oxygen evolution reaction (OER),40–42 also recognized as the hole transfer and storage layer for photoelectrodes.30,43–45 Under the synergetic effect of molecular borate and NiFeV co-modifications, the resulting NiFeV/B-BiVO4 photoanode exhibited an outstanding photocurrent density of 4.6 mA cm−2 at 1.23 VRHE with an ultra-low onset potential of ∼0.2 VRHE and superior photostability. NiFeV OECs served as not only a sole electrocatalyst for water oxidation but also a hole reservoir that efficiently suppressed surface charge recombination and accelerated interfacial charge transfer. The results illustrated in this work provided a simple yet efficient strategy for the design of high-performance and stable planar photoanodes for PEC water splitting.
2. Experimental section
2.1 Materials
Fluorine-doped tin oxide (FTO) coated glass substrates were purchased from Pilkington (∼8 Ω cm−2) and were successively cleaned using Milli-Q water, ethanol and acetone. Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, 98%), vanadyl acetylacetonate (VO(acac)2, 98%), nitric acid solution (HNO3, 70%), p-benzoquinone (98%), potassium iodide (KI, 99%), vanadium chloride (VCl3, 97%), nickel chloride hexahydrate (NiCl2·6H2O, 98%), iron chloride hexahydrate (FeCl3·6H2O, 97%), boric acid (H3BO3, 99.5%), sodium sulfite (Na2SO3, 98%), sodium hydroxide (NaOH, 98%), potassium hydroxide (KOH, 85%), Nafion™ 117 containing solution (5%), borax anhydrous (Na2B4O7, 98%), and deuterium oxide (D2O, 99.9%) were purchased from Sigma-Aldrich and used as received. All organic solvents including 2-propanol ethanol, absolute ethanol, and dimethyl sulfoxide (DMSO) were of analytical reagent grade and used without further purification. Ultra-pure water (18.2 MΩ cm−1) supplied by a Milli-Q system (Merck Millipore) was used in all experiments.2.2 Fabrication of BiVO4 and B-BiVO4
The detailed fabrication processes of BiVO4 and B-BiVO4 photoanodes were essentially repeated according to our previous report and an established procedure from Choi's group.6,35 In brief, 0.04 M Bi(NO3)3 and 0.4 M KI were successively added into 50 mL HNO3 aqueous solution (pH 1.7) with mild stirring for 15 min. Afterwards, 20 mL of EtOH containing 0.23 M p-benzoquinone was mixed into the above solution with constant stirring. The electrodeposition of the BiOI precursor was carried out using a standard three-electrode cell where an FTO substrate, a saturated Ag/AgCl electrode, and a platinum wire electrode (1 × 1 cm2) were used as the working electrode (WE), the reference electrode (RE), and the counter electrode (CE), respectively. The electrodeposition of BiOI precursor was carried out potentiostatically at −0.1 VAg/AgCl for 3 min at room temperature. The BiOI film was converted to BiVO4 by thermal treatment in air at 450 °C for 2 h (ramping rate: 2 °C min−1) after covering the BiOI film with 80 μL of a DMSO solution containing 0.2 M VO(acac)2. After annealing, the electrodes were soaked in 1 M NaOH solution for 30 min on a lab shaker to remove excess V2O5 from the surface of BiVO4. The final bare BiVO4 electrodes were washed thoroughly with Milli-Q water and gently dried with air stream. A 1.0 M potassium borate (KBi) buffer solution (pH = 9.3 ± 0.1, 1.0 M H3BO3 adjusted by KOH) was used for both the preparation of B-BiVO4 and the ensuing photoelectrochemical measurements.For the preparation of B-BiVO4, a bare BiVO4 photoanode was immersed into the above borate buffer in a capped brown vial and then heated at 100 °C for 30 min in an oil bath to shorten the treatment time. After cooling, the B-BiVO4 anode was taken out from the solution, rinsed with Milli-Q water and dried with gentle air stream.
2.3 Synthesis of layered double hydroxides (LDHs) (NiFeV, NiV and NiFe)
The LDH nanoparticles were synthesized according to a report from our group via a one-step hydrothermal method.46 The mole ratios of each element in NiFeV (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), NiV (3
1), NiV (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and NiFe (3
1) and NiFe (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were tuned by mixing the corresponding NiCl2, FeCl3 and VCl3 in 80 mL H2O while keeping the total amount of metal ions at 3.2 mmol. The suspension of NiV LDH in a mixed solvent of H2O, 2-propanol and Nafion (4
1) were tuned by mixing the corresponding NiCl2, FeCl3 and VCl3 in 80 mL H2O while keeping the total amount of metal ions at 3.2 mmol. The suspension of NiV LDH in a mixed solvent of H2O, 2-propanol and Nafion (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04) with a concentration of 0.5 mg mL−1 was prepared for further co-catalyst loading onto BiVO4.
0.04) with a concentration of 0.5 mg mL−1 was prepared for further co-catalyst loading onto BiVO4.
2.4 Fabrication of LDH/BiVO4 and LDH/B-BiVO4
Prior to co-catalyst loading, the above LDH nanoparticle suspension (0.5 mg mL−1) was ultrasonicated for 2 h. 5 μL of the resulting uniform suspension was then directly drop-casted onto BiVO4 or BiVO4 electrodes with an active area of 0.5 × 0.5 cm2 and was allowed to dry by evaporation under ambient conditions. In addition to LDH/B-BiVO4 electrodes, LDH/BiVO4 electrodes were also prepared for comparison using the same methods described earlier.2.5 Material characterization
The surface morphology and composition of LDH nanoparticles and the ensuing BiVO4-based photoelectrodes were determined using a field emission scanning electron microscope (FE-SEM, Hitachi, Regulus 8230) equipped with an energy-dispersive X-ray spectroscopy (EDX) detector (Oxford Ultim EXTREME). Transmission electron microscopy (TEM, JEOL JEM2100F) and high-resolution TEM (HRTEM) images were recorded at an acceleration voltage of 200 kV. The crystal structures of the samples were characterized by powder X-ray diffraction (PXRD, D8 Advance Bruker with Cu Kα (λ = 1.5406 Å) radiation. The surface chemical states and composition of the films were characterized by X-ray photoelectron spectroscopy (XPS) (ESCALAB Xi+, Thermo Fisher, UK) with a monochromated Al Kα radiation source (1486.6 eV). All binding energies were calibrated for specimen charging by referencing the C 1s peak to 284.8 eV. The optical absorption of BiVO4 photoanodes was tested using an UV-vis diffuse reflectance (UV3600, Shimadzu, Japan) spectrophotometer in the range of 300–800 nm.2.6 Photoelectrochemical measurements
All photoelectrochemical tests were performed using a CHI 660E potentiostat at room temperature in the same three-electrode configuration, except the use of the BiVO4 photoanode as the WE. A NEWPORT LCS-100 solar simulator (type 94011A-ES, a 100 W Xenon arc lamp with an AM 1.5G filter) was used as the illumination source. 1.0 M Potassium borate buffer solution (pH 9.3) was used as the electrolyte for all PEC measurements. For all cases, light is irradiated from the back side of the FTO substrate and the illuminated areas were fixed at 0.5 × 0.5 cm2. For sulfite oxidation, 0.2 M Na2SO3 was added into the electrolyte as a hole scavenger. J–V curves both under AM1.5 illumination and in the dark were obtained by linear sweep voltammetry (LSV) with a scan rate of 10 mV s−1. Electrochemical impedance spectroscopy (EIS) spectra of the electrodes were measured at 0.6 VRHE at frequencies ranging from 100 kHz to 0.01 Hz. Mott–Schottky (MS) spectra were obtained in the voltage window of 0–0.4 VRHE in the dark with a 10 mV increment and 1 kHz frequency. The incident photon-to-current conversion efficiency (IPCE) was measured at 1.23 VRHE using a Zahner CIMP-QE/IPCE system. Intensity modulated photocurrent spectroscopy (IMPS) was performed on Zahner IMPS electrochemical workstation setup with the scanning frequency in the range of 0.1 Hz to 10 kHz (amplitude: 5 mV).20,47 The modulated light source (10 mW cm−2) for IMPS characterization was a calibrated light-emitting diode (LED). H/D kinetic isotope effect (KIE) measurements were performed according to a previous report20 by comparing photocurrent densities at different overpotentials (η) in 0.1 M anhydrous borax (Na2B4O7) H2O and D2O solutions, with pH values of 9.303 and 9.445 (measured using a 781 pH/Ion Meter, Metrohm), respectively. The long-term electrolysis was carried out with a constant potential at 0.6 VRHE. The actual amount of oxygen evolution was determined by gas chromatography (GC-2014 SHIMADZU). The amount of O2 produced was calculated theoretically by converting the charge passed to μmol gas according to Faraday's law.2.7 Relative equations
The recorded potential versus Ag/AgCl (EAg/AgCl) was converted against reversible hydrogen electrode (RHE) according to the Nernst equation:| ERHE = EAg/AgCl + 0.197 + 0.059 × pH | (1) |
The applied bias photon-current efficiency (ABPE) was calculated from the LSV curves of BiVO4 photoanodes:
 | (2) |
The light harvesting efficiency (LHE) of the BiVO4 photoanode was calculated from the UV-Vis diffuse reflectance spectra:
| LHE (%) = (1 − 10−A) × 100% | (3) |
The photocurrent density arising from PEC performance (JPEC) can be described as follows:
| JPEC = Jabs × ηbulk × ηsurface | (4) |
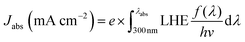 | (5) |
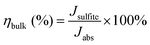 | (6) |
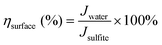 | (7) |
The H/D KIE values can be defined as
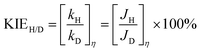 | (8) |
3. Results and discussion
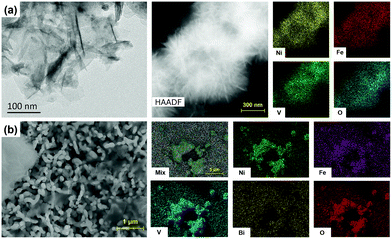
The bare BiVO4 film photoanode was fabricated according to a previously employed procedure,6 while the NiFeV LDH nanoparticles were synthesized by a typical one-pot hydrothermal method.46 Then the preparation of NiFeV/B-BiVO4 was a simple two-step process. The borate pretreatment was performed according to our previous report.35 Subsequently, a diluted suspension of NiFeV LDH was drop-casted onto the surface of the B-BiVO4 electrode, instead of the in situ hydrothermal/solvothermal growth30,50–52 and electrodeposition.53–55 In the two abovementioned methods, LDHs either grow homogenously in situ and completely cover the surface of BiVO4 as oriented nanosheets or form a layer wrapped around the BiVO4 particles. However, BiVO4 is sensitive to the pH of the electroplating solution and the applied potential during electrolysis,56–58 and the relatively high temperature of the hydrothermal process might cause detrimental surface etching on BiVO4.59 Considering the instability of borate treated BiVO4,35 it is clearly challenging to maintain the pre-enhanced PEC activity of B-BiVO4 when loading OECs. In this case, drop-casting of OEC co-catalysts was expected to keep the B-BiVO4 electrode surface intact to the maximum extent. Prior to physical characterization and PEC testing, the mass loading of NiFeV LDH was optimized to 10 μg cm−2 using bare BiVO4 as the substrate (Fig. S1, ESI†). Hence, all LDH/BiVO4-based photoanodes were prepared with the same mass loading of 10 μg cm−2 for the subsequent experiments.The UV/Vis absorption spectrum of B-BiVO4 (Fig. S2, ESI†) was almost unchanged after NiFeV LDH loading, indicating that the band gaps of B-BiVO4 and NiFeV/B-BiVO4 were the same (2.54 eV). The X-ray diffraction (XRD) patterns of the NiFeV LDH, B-BiVO4 and NiFeV/B-BiVO4 electrodes are shown in Fig. S3 (ESI†). Apart from the FTO signals, all XRD peaks can be attributed to monoclinic BiVO4. After drop-casting of NiFeV LDH on the B-BiVO4 electrode, a discernable reflection peak at 2θ = 11.4° can be observed in the pattern of NiFeV/B-BiVO4 compared to that of bare BiVO4, which could be attributed to the (003) lattice plane of NiFeV.40–42 However, the additional main diffraction peaks of NiFeV LDH were too weak to identify due to the very low loading amount on the surface of B-BiVO4.60,61
The morphologies of the NiFeV LDHs were first studied by transmission electron microscopy (TEM). NiFeV LDHs display rippled nanosheets with a size of several hundred nanometers and a thickness of ∼3–5 nm, which is in accordance with previous reports in spite of the small differences in the ratio of each metal element.40–42 The aggregates with laminations were observed by field emission scanning electron microscopy (FE-SEM), and the atomic ratios of Ni, Fe, and V were determined by EDX analysis (Fig. S4, ESI†), showing that the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V molar ratio (2.83
V molar ratio (2.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.22
0.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was close to the ratio of the starting materials (3
1) was close to the ratio of the starting materials (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The SEM images in Fig. 1b demonstrated that the NiFeV nanosheets were distributed over the surface of BiVO4 with aggregate phases (which occurred during the drying stage of drop-casting), which was also confirmed by the corresponding elemental mapping images. Drop-casting of NiFeV LDH on BiVO4 did not alter the quintessential worm-like morphology of the latter, which was characterized by a dendritic diameter of 300–400 nm and an average thickness of about ∼650 nm (Fig. S5, ESI†).
1). The SEM images in Fig. 1b demonstrated that the NiFeV nanosheets were distributed over the surface of BiVO4 with aggregate phases (which occurred during the drying stage of drop-casting), which was also confirmed by the corresponding elemental mapping images. Drop-casting of NiFeV LDH on BiVO4 did not alter the quintessential worm-like morphology of the latter, which was characterized by a dendritic diameter of 300–400 nm and an average thickness of about ∼650 nm (Fig. S5, ESI†).
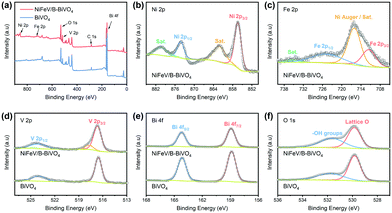
The X-ray photoelectron spectroscopy (XPS) measurement was carried out to examine the elemental composition of the NiFeV/B-BiVO4 surface by taking the C 1s peak at 284.8 eV as a standard reference (Fig. 2a). As expected, the noticeable Ni (Fig. 2b) and Fe signals (Fig. 2c) in the XPS spectra derived from LDHs (Fig. S6, ESI†) and a mixed V environment composed of NiFeV and BiVO4 were observed in the V 2p spectrum of Fig. 2d. As can be seen from Fig. 2e, the binding energies of 158.9 eV and 164.2 eV can be ascribed to Bi 4f7/2 and Bi 4f5/2,27 respectively. For the O 1s core-level spectra (Fig. 2f), two peaks can be clearly identified.47,62 In particular, the peak at 529.8 eV belongs to lattice oxygen, clearly originating from BiVO4. While the O 1s peak at 531.7 eV is associated with surface hydroxy species, in our case, it was closely related to both the chemisorption of [B(OH)4]− upon borate treatment35,63 and NiFeV modification (Fig. S6, ESI†). Although no obvious B 1s signals were detected,35 overall the XPS spectra indicated that NiFeV LDHs were successfully loaded onto the surface of the B-BiVO4 photoanode.
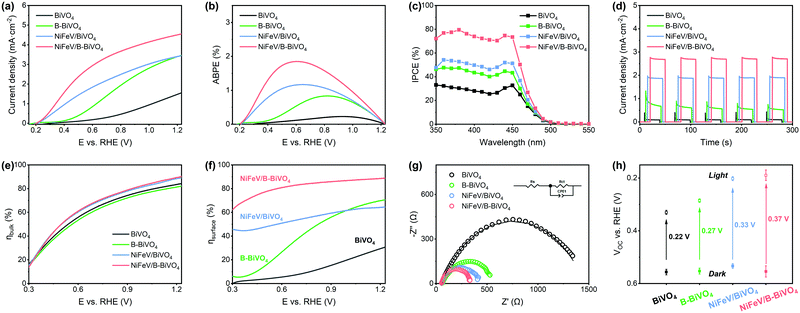
The PEC water oxidation activities of NiFeV/B-BiVO4 and other relevant photoanodes were measured in 1.0 M potassium borate buffer solution at pH 9.3 under AM 1.5 G simulated illumination. As shown in Fig. 3a, the photocurrent density of bare BiVO4 at 1.23 VRHE was about 1.6 mA cm−2. NiFeV/BiVO4 and NiFeV/B-BiVO4 showed much earlier photocurrent onsets (defined at 0.1 mA cm−2 photocurrent density) of 0.22 VRHE and 0.21 VRHE, respectively, and generated higher photocurrent in the low bias region (E < 0.6 VRHE), which is also corroborated by the dark current density results (Fig. S7, ESI†). In the high bias region (E > 0.9 VRHE), the PEC performance of B-BiVO4 was parallel to that of NiFeV/BiVO4. After the borate and NiFeV LDH co-modification, the photocurrent density of NiFeV/B-BiVO4 reached 4.6 mA cm−2, significantly outperforming either of the singly modified photoanodes (∼3.5 mA cm−2). Such a synergistic effect was also observed in the applied bias photon-to-current efficiency (ABPE) and incident photon-to-current conversion efficiency (IPCE) measurements (Fig. 3b and c). The maximum ABPE of NiFeV/B-BiVO4 was 1.85% at 0.62 VRHE, which is 8 times as high as that of unmodified BiVO4 (0.23% at 0.91 VRHE), and the IPCE of NiFeV/B-BiVO4 reached a maximum of ∼80% at 1.23 VRHE at a wavelength of ∼380 nm. It is important to note here that the photocurrent increase in the low bias region for NiFeV/B-BiVO4 goes beyond the simple accumulation of effects from NiFeV LDH and borate species, displaying a truly synergistic behavior (Fig. 3a and b) and indicating the positive cooperation between two co-modifications. Compared with previously reported BiVO4 photoanodes modified with the LDH co-catalyst, the PEC performance of NiFeV/B-BiVO4 is ranked among the best (Table S1, ESI†). For example, Wang and co-workers deposited a ternary NiFeY LDH on BiVO4 and the resulting photoanode exhibited remarkable PEC performance (∼5.2 mA cm−2 at 1.23 VRHE) with outstanding stability at 0.8 VRHE over 25 h,64 while its onset potential (0.31 VRHE) remained reasonably high. The CoPO3/pGO/LDH/BiVO4 composite photoanode,30 reported by Li and co-workers, had an unprecedentedly low onset potential (0.17 V) and a high photocurrent (4.45 mA cm−2); however, it required laborious multistep procedures to obtain an integrated photoanode using four components.
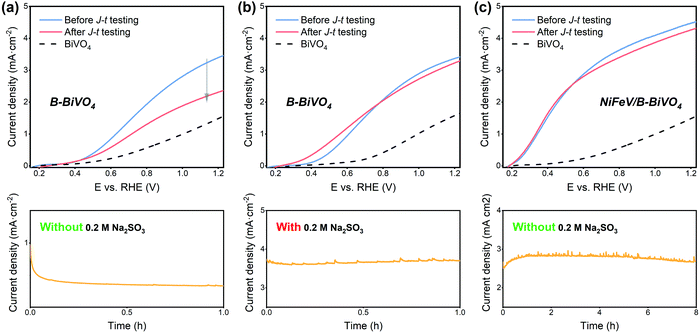
Under chopped light illumination (Fig. 3d), the unmodified BiVO4 showed large photocurrent transient spikes for each light on–off cycle. On the contrary, these transient spikes were eliminated to a large extent in the NiFeV/B-BiVO4 photoanode, implying that the severe surface recombination on BiVO4 was significantly decreased after borate and NiFeV co-modification. Theoretically, the measured photocurrent density (JPEC) is governed by the relation: JPEC = Jabs × ηbulk × ηsurface,6,32 where Jabs is the photon absorption rate expressed as the current density at 100% internal quantum efficiency, ηbulk refers to the yield of the photogenerated holes that reach the electrode/electrolyte interface, and ηsurface indicates the charge injection efficiency of surface-reaching holes into the electrolyte for water oxidation. To gain an in-depth understanding of synergistic effects on carrier kinetics, ηbulk and ηsurface of BiVO4 photoanodes were investigated carefully. As can be seen from Fig. 3e, the ηbulk values of all photoanodes were over 80% at 1.23 VRHE, benefiting from the well-established synthesis method of BiVO4 by Choi and co-workers.6 There was almost no difference in ηbulk between BiVO4 and B-BiVO4, whereas the ηbulk of BiVO4 was somewhat improved after loading with NiFeV LDHs. This small increment of ηbulk, however, was not expected to contribute much to the remarkable activity of the NiFeV/B-BiVO4 photoanode. Additionally, the Mott–Schottky (MS) plots (Fig. S8, ESI†) showed that, at each frequency, the slopes of both B-BiVO4 and NiFeV/BiVO4 were marginally lower than that of bare BiVO4, suggesting that the donor density of BiVO4 just slightly increased. Overall, these results fully indicated that both borate and NiFeV modifications had little effect on bulk properties within the BiVO4 electrode. By comparing the photocurrent densities of photoanodes with and without Na2SO3 as a hole scavenger (Fig. 3f and Fig. S9, ESI†), a superior ηsurface of NiFeV/B-BiVO4 was about 90% at 1.23 VRHE, which was 3 times higher than that of bare BiVO4 (31%). In particular, it is worth noting that the ηsurface of NiFeV/BiVO4 was much better than that of B-BiVO4 at a lower applied bias; when the bias was further increased to >1.0 VRHE, the ηsurface of B-BiVO4 became comparable to that of NiFeV/BiVO4. The trends of the ηsurface were consistent with their PEC performances (Fig. 3a) and evinced that both borate and LDH modifications were efficient in accelerating the surface charge carrier kinetics for OER.
The interfacial carrier kinetics was further investigated by using electrochemical impedance spectroscopy (EIS). As can be seen from Fig. 3g, the semicircles of Nyquist plots for all photoanodes fitted well with an equivalent circuit model (inset in Fig. 3g) composed of a series resistance (Rs), an interfacial charge transfer resistance (Rct) and a constant phase angle element (CPE).20,65,66 All modified photoanodes featured noticeably smaller semicircles in EIS plots compared to bare BiVO4. In particular, the Rct value of NiFeV/B-BiVO4 was only 289.2 Ω (Table S2, ESI†) at 0.6 VRHE, implying the fastest charge transfer at the electrode/electrolyte interface by borate and NiFeV co-modification, which was demonstrated in the trends of ηsurface as shown in Fig. 3f. The band bending was further investigated by open-circuit voltage (Voc) measurements. The photovoltage of a photoanode arises from the splitting of the electron and hole quasi-Fermi level under steady-state light illumination, which acts as a driving force for injecting the photogenerated holes into the electrolyte for OER.65,67 According to several previous studies,30,43,49,62,68 the simple integration of OECs on the surface of photoanodes could enlarge the difference in photovoltage between the dark and light conditions, resulting in greater band bending.44 Smith and co-workers also reported that the photocharged BiVO4 photoanodes in a borate buffer solution achieved favorable band bending, which is responsible for the strong suppression of surface recombination.37 From Fig. 3h, the photovoltages of BiVO4, B-BiVO4, NiFeV/BiVO4, and NiFeV/B-BiVO4 were 0.22 V, 0.27 V, 0.33 V and 0.37 V, respectively. After NiFeV and borate co-modification, a greater driving force for water oxidation with more efficient charge separation in the photoanode was obtained, which resulted in a more negative onset potential for OER.30
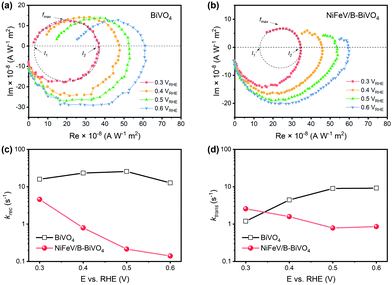
It has been reported that both reduced charge surface recombination and enhanced water oxidation kinetics could contribute to the improvement of surface charge transfer efficiency.69–72 In order to understand the main reason for the synergistically enhanced PEC performance, intensity modulated photocurrent spectroscopy (IMPS) was performed in the low bias region (0.3–0.6 VRHE) to measure charge transfer rate constant (ktrans) and surface recombination rate constant (krec), respectively.47,73,74 According to the generalized theory of IMPS, as the frequency increases, the relaxation in the concentration of photogenerated holes at the semiconductor surface is characterized by fmax (at the apex of the upper semicircle), where 2πfmax = ktrans + krec.75,76 The charge transfer efficiency, in terms of ktrans/(ktrans + krec), can be derived from the intersections of the semicircle with the real axis at low and high frequencies (i.e., I1 and I2, respectively), where I1/I2 = ktrans/(ktrans + krec).
Therefore, the absolute values of the phenomenological rate constants ktrans and krec were readily obtained. Typical IMPS responses of bare BiVO4 and NiFeV/B-BiVO4 are shown in Fig. 4a and b, respectively. The upper semicircle of BiVO4 did not change noticeably upon the increase of applied bias, implying the severe charge recombination existing on bare BiVO4;54,77 conversely, the upper semicircle of the NiFeV/B-BiVO4 photoanode became smaller when the applied bias was increased. The krec of bare BiVO4 did not change significantly over the entire potential range (Fig. 4c). In sharp contrast, significantly lower krec values were observed for NiFeV/B-BiVO4. Even at the lowest applied bias of 0.3 VRHE, the krec of bare BiVO4 was up to 15.9 s−1, which was larger than that of NiFeV/B-BiVO4 (4.6 s−1) by a factor of 3.5. Most notably, at the point of maximum ABPE value of NiFeV/B-BiVO4 (i.e., 0.6 VRHE), this ratio approached a value of 90, indicating that the charge recombination was greatly suppressed over the entire potential range. These results coincided with the enhancement (inter alia E < 0.6 VRHE) of photocurrent density of NiFeV/B-BiVO4 (Fig. 3a) and further confirmed the dominating role of NiFeV co-catalysts within the synergy.
Generally, loading an OEC catalyst on a photoanode should result in an increase in water oxidation rates.16,20,43 However, the ktrans value of bare BiVO4 appeared to be higher than that of NiFeV/B-BiVO4 to some extent at most potentials (Fig. 4d). This abnormal phenomenon has also been analogously observed in CoPi/BiVO4,70 NiFeOx/Fe2O3,78 Co-LaFeO3/BiVO4,16 and so on. It should be noted that the smaller ktrans measured here on NiFeV/B-BiVO4 does not certainly suggest slower water oxidation. This is because the ktrans derived from IMPS more likely corresponds to the rate-determining steps (RDS) of the complex charge transfer processes from the photoelectrode to water.78 Moreover, NiFeV LDH has been confirmed as an efficient water oxidation catalyst according to our electrochemical testing (Fig. S10, ESI†) and related literature.40–42 Furthermore, the actual charge transfer efficiency ktrans/(ktrans + krec) of NiFeV/B-BiVO4 was evidently higher than that of bare BiVO4 (Fig. S11, ESI†) owing to minimized recombination, displaying the same trend as the ηsurface results in Fig. 3f. To further support this experimentally, the H/D kinetic isotope effect (KIE) was assessed by comparing photocurrent densities at different overpotentials (after corrections) in 0.1 M anhydrous borax (Na2B4O7) H2O and D2O solutions (Fig. S12, ESI†).20 Herein, H/D KIE studies were performed to investigate the proton transfer kinetics and probe the RDS in the water oxidation process on the surface of BiVO4.79,80 The KIE of the bare BiVO4 photoanode approached 1.5, in agreement with a previous report,20 indicating that the RDS of PEC water oxidation on BiVO4 involved the proton transfer process. B-BiVO4 showed a moderate KIE value of ∼1.2, which indicated slight acceleration of proton transfer. After loading LDH co-catalysts, both NiFeV/BiVO4 and NiFeV/B-BiVO4 displayed negligible H/D kinetic isotope effects (KIE ≈ 1.0) at all applied potentials, suggesting that the proton transfer is no longer involved in the RDS.80 Moreover, it revealed that hole transfer into the LDH co-catalysts (instead of catalytic processes of water oxidation on the BiVO4 surface) is rate-limiting,79 indicative of very fast subsequent injection of photogenerated holes into the electrolyte. It might be therefore concluded that the synergistic effects of borate and NiFeV co-modifications not only increased the overall rate of water oxidation, but also promoted charge transfer and reduced charge recombination.
In our previous report,35 the adsorption of borate on the surface of BiVO4 resulted in a molecular level modification, which reduced surface charge trapping, thereby causing a significant increase in the photocurrent of B-BiVO4. However, this improvement gradually degenerated within short-term photolysis (Fig. 5a and Fig. S13, ESI†). The degradation of efficiency was likely caused by the accumulation of photogenerated holes at the B-BiVO4 surface, where they easily recombined with electrons and could not be consumed for water oxidation quickly.29,72,81 When using the electrolyte with the Na2SO3 hole scavenger (Fig. 5b), the photocurrent of B-BiVO4 was maintained after 1 h photolysis at 0.6 VRHE in that electrolyte. After NiFeV LDH modification, as shown in Fig. 5c, the NiFeV/B-BiVO4 photoanode exhibited good stability and about 95% of the initial photocurrent density was retained after 8 h photolysis. A long-term stability test (24 h at 0.6 VRHE) of NiFeV/B-BiVO4 showed that over 80% of the initial photocurrent density was maintained (Fig. S14, ESI†). Besides, a high faradaic efficiency of 95% was obtained for NiFeV/B-BiVO4 (Fig. S15, ESI†); both the morphology of BiVO4 and the nanostructures of the catalysts did not show obvious changes after stability tests (Fig. S16, ESI†). The improvement of photostability could be attributed to the improved interfacial charge transfer efficiency by the NiFeV catalyst, which can reduce surface recombination and kinetically suppress photocorrosion.29,36,82,83 Besides, it has been reported that the injection of surface-reaching holes into OECs was thermodynamically favorable compared to direct transfer to the solution and the catalysts were typically permeable for the electrolyte and redox-active.44 Therefore, holes can accumulate throughout the LDHs, thereby diminishing surface recombination.30
These results clearly demonstrated that loading LDH co-catalysts onto B-BiVO4 did not only further enhance the photocurrent density and lower the onset potential, but also significantly improved the stability of B-BiVO4. This synergetic effect of borate treatment and LDH modification can also be realized on NiFe/B-BiVO4 (Fig. S17, ESI†) and NiV/B-BiVO4 photoanodes (Fig. S18, ESI†), which can achieve photocurrents of ∼3.7 mA cm−2 and ∼4.3 mA cm−2 at 1.23 VRHE, respectively, with relatively low onset potentials (∼0.25 VRHE) and good stability.
From the above discussions, the overall effects of charge transfer and surface recombination are schematically summarized in Scheme 1. For bare BiVO4, the photogenerated holes can be directly consumed for water oxidation or trapped by surface state and recombined with electrons. The co-modification showed slight improvement in the intrinsically decent charge separation of bare BiVO4. The self-anchored borate species serve as a passivator contributing to the decrease of surface charge recombination as well as a ligand in modifying the catalytic site for water oxidation.35,36 Furthermore, the main role of NiFeV LDH is to efficiently suppress surface recombination and promote interfacial charge transfer efficiency, thereby improving PEC performance and stability simultaneously. The high intrinsic catalytic activity of NiFeV LDH is also conducive to a greater driving force for water oxidation and a lower onset potential.
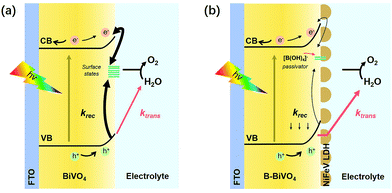 | ||
| Scheme 1 Schematic illustration of the working principles of (a) bare BiVO4 and (b) NiFeV/B-BiVO4 photoanodes. | ||
4. Conclusions
In conclusion, our work provided insights into the rational design of a BiVO4 based photoanode for highly efficient PEC water splitting combined with post-synthetic borate treatment and NiFeV co-catalyst loading. The optimized NiFeV/B-BiVO4 photoanode achieved a remarkable photocurrent density of 4.6 mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 with an ultra-low onset potential (0.2 VRHE) with a high ABPE of 1.85% at 0.6 VRHE. It is worth noting that NiFeV/B-BiVO4 exhibited a strong photocurrent increase over a low applied bias range (<0.6 VRHE). More importantly, studies on NiFeV/B-BiVO4 surface kinetics further demonstrated the outstanding contribution of NiFeV co-modification to the suppression of charge recombination and the promotion of charge transfer efficiency. Moreover, loading with the NiFeV co-catalyst significantly improves the stability of borate-treated BiVO4. This work has revealed and emphasized the significance of the synergy of co-modifications in photoelectrochemical devices.
cm−2 with an ultra-low onset potential (0.2 VRHE) with a high ABPE of 1.85% at 0.6 VRHE. It is worth noting that NiFeV/B-BiVO4 exhibited a strong photocurrent increase over a low applied bias range (<0.6 VRHE). More importantly, studies on NiFeV/B-BiVO4 surface kinetics further demonstrated the outstanding contribution of NiFeV co-modification to the suppression of charge recombination and the promotion of charge transfer efficiency. Moreover, loading with the NiFeV co-catalyst significantly improves the stability of borate-treated BiVO4. This work has revealed and emphasized the significance of the synergy of co-modifications in photoelectrochemical devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Swedish Research Council (2017-00935), Knut and Alice Wallenberg Foundation (KAW 2016.0072), and the Foundation of Westlake University. The authors thank the Instrumentation and Service Center for Physical Sciences (ISCPS) at Westlake University (Hangzhou, China) for the assistance in measurement and data interpretation. Q. Meng is thankful to the China Scholarship Council for a special scholarship award.References
- M. Grätzel, Nature, 2001, 414, 338–344 Search PubMed.
- J. H. Kim, D. Hansora, P. Sharma, J.-W. Jang and J. S. Lee, Chem. Soc. Rev., 2019, 48, 1908–1971 Search PubMed.
- M. A. Lumley, A. Radmilovic, Y. J. Jang, A. E. Lindberg and K.-S. Choi, J. Am. Chem. Soc., 2019, 141, 18358–18369 Search PubMed.
- X. Ding, L. Zhang, Y. Wang, A. Liu and Y. Gao, Coord. Chem. Rev., 2018, 357, 130–143 Search PubMed.
- A. Kudo, K. Ueda, H. Kato and I. Mikami, Catal. Lett., 1998, 53, 229–230 Search PubMed.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 Search PubMed.
- D. K. Lee, D. Lee, M. A. Lumley and K.-S. Choi, Chem. Soc. Rev., 2019, 48, 2126–2157 Search PubMed.
- J. Seo, H. Nishiyama, T. Yamada and K. Domen, Angew. Chem., Int. Ed., 2018, 57, 8396–8415 Search PubMed.
- Y. Park, K. J. McDonald and K.-S. Choi, Chem. Soc. Rev., 2013, 42, 2321–2337 Search PubMed.
- F. F. Abdi, T. J. Savenije, M. M. May, B. Dam and R. van de Krol, J. Phys. Chem. Lett., 2013, 4, 2752–2757 Search PubMed.
- H. S. Han, S. Shin, D. H. Kim, I. J. Park, J. S. Kim, P.-S. Huang, J.-K. Lee, I. S. Cho and X. Zheng, Energy Environ. Sci., 2018, 11, 1299–1306 Search PubMed.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 Search PubMed.
- S. Wang, G. Liu and L. Wang, Chem. Rev., 2019, 119, 5192–5247 Search PubMed.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 Search PubMed.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 Search PubMed.
- Y. Gao, G. Yang, Y. Dai, X. Li, J. Gao, N. Li, P. Qiu and L. Ge, ACS Appl. Mater. Interfaces, 2020, 12, 17364–17375 Search PubMed.
- W. J. Jo, J.-W. Jang, K.-j. Kong, H. J. Kang, J. Y. Kim, H. Jun, K. P. S. Parmar and J. S. Lee, Angew. Chem., Int. Ed., 2012, 51, 3147–3151 Search PubMed.
- J. M. Lee, J. H. Baek, T. M. Gill, X. Shi, S. Lee, I. S. Cho, H. S. Jung and X. Zheng, J. Mater. Chem. A, 2019, 7, 9019–9024 Search PubMed.
- D. K. Zhong, S. Choi and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370–18377 Search PubMed.
- F. Li, H. Yang, Q. Zhuo, D. Zhou, X. Wu, P. Zhang, Z. Yao and L. Sun, Angew. Chem., Int. Ed., 2021, 60, 1976–1985 Search PubMed.
- J.-B. Pan, B.-H. Wang, J.-B. Wang, H.-Z. Ding, W. Zhou, X. Liu, J.-R. Zhang, S. Shen, J.-K. Guo, L. Chen, C.-T. Au, L.-L. Jiang and S.-F. Yin, Angew. Chem., Int. Ed., 2021, 60, 1433–1440 Search PubMed.
- W. Yong, L. Fei, Z. Xu, Y. Fengshou, D. Jian, B. Lichen and S. Licheng, Angew. Chem., Int. Ed., 2017, 56, 6911–6915 Search PubMed.
- C. Xu, W. Sun, Y. Dong, C. Dong, Q. Hu, B. Ma and Y. Ding, J. Mater. Chem. A, 2020, 8, 4062–4072 Search PubMed.
- X. Cao, C. Xu, X. Liang, J. Ma, M. Yue and Y. Ding, Appl. Catal., B, 2020, 260, 118136 Search PubMed.
- B. Lamm, B. J. Trześniewski, H. Döscher, W. A. Smith and M. Stefik, ACS Energy Lett., 2017, 3, 112–124 Search PubMed.
- B. Zhang, L. Chou and Y. Bi, Appl. Catal., B, 2020, 262, 118267 Search PubMed.
- T. W. Kim, Y. Ping, G. A. Galli and K.-S. Choi, Nat. Commun., 2015, 6, 8769 Search PubMed.
- Q. Zhang, M. Liu, W. Zhou, Y. Zhang, W. Hao, Y. Kuang, H. Liu, D. Wang, L. Liu and J. Ye, Nano Energy, 2021, 81, 105651 Search PubMed.
- J. A. Seabold and K.-S. Choi, J. Am. Chem. Soc., 2012, 134, 2186–2192 Search PubMed.
- S. Ye, C. Ding, R. Chen, F. Fan, P. Fu, H. Yin, X. Wang, Z. Wang, P. Du and C. Li, J. Am. Chem. Soc., 2018, 140, 3250–3256 Search PubMed.
- K.-H. Ye, H. Li, D. Huang, S. Xiao, W. Qiu, M. Li, Y. Hu, W. Mai, H. Ji and S. Yang, Nat. Commun., 2019, 10, 1–9 Search PubMed.
- J. H. Kim and J. S. Lee, Adv. Mater., 2019, 1806938 Search PubMed.
- H. L. Tan, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2017, 5, 16498–16521 Search PubMed.
- D. Lee, W. Wang, C. Zhou, X. Tong, M. Liu, G. Galli and K.-S. Choi, Nat. Energy, 2021, 6, 287–294 Search PubMed.
- Q. Meng, B. Zhang, L. Fan, H. Liu, M. Valvo, K. Edström, M. Cuartero, R. de Marco, G. A. Crespo and L. Sun, Angew. Chem., Int. Ed., 2019, 58, 19027–19033 Search PubMed.
- R.-T. Gao and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 23094–23099 Search PubMed.
- N. J. Firet, A. Venugopal, M. A. Blommaert, C. Cavallari, C. J. Sahle, A. Longo and W. A. Smith, Chem. Mater., 2019, 31, 7453–7462 Search PubMed.
- B. J. Trześniewski, I. A. Digdaya, T. Nagaki, S. Ravishankar, I. Herraiz-Cardona, D. A. Vermaas, A. Longo, S. Gimenez and W. A. Smith, Energy Environ. Sci., 2017, 10, 1517–1529 Search PubMed.
- B. J. Trześniewski and W. A. Smith, J. Mater. Chem. A, 2016, 4, 2919–2926 Search PubMed.
- K. N. Dinh, P. Zheng, Z. Dai, Y. Zhang, R. Dangol, Y. Zheng, B. Li, Y. Zong and Q. Yan, Small, 2018, 14, 1703257 Search PubMed.
- J. Jiang, F. Sun, S. Zhou, W. Hu, H. Zhang, J. Dong, Z. Jiang, J. Zhao, J. Li, W. Yan and M. Wang, Nat. Commun., 2018, 9, 2885 Search PubMed.
- P. Li, X. Duan, Y. Kuang, Y. Li, G. Zhang, W. Liu and X. Sun, Adv. Energy Mater., 2018, 8, 1703341 Search PubMed.
- X. Ning, P. Du, Z. Han, J. Chen and X. Lu, Angew. Chem., Int. Ed., 2021, 60, 3504–3509 Search PubMed.
- F. A. Laskowski, M. R. Nellist, J. Qiu and S. W. Boettcher, J. Am. Chem. Soc., 2018, 141, 1394–1405 Search PubMed.
- B. Zhang, X. Huang, Y. Zhang, G. Lu, L. Chou and Y. Bi, Angew. Chem., Int. Ed., 2020, 59, 18990–18995 Search PubMed.
- K. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo and L. Sun, Nat. Commun., 2016, 7, 11981 Search PubMed.
- D. Zhou, K. Fan, Q. Zhuo, Y. Zhao and L. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 2723–2733 Search PubMed.
- K. Zhang, B. Jin, C. Park, Y. Cho, X. Song, X. Shi, S. Zhang, W. Kim, H. Zeng and J. H. Park, Nat. Commun., 2019, 10, 2001 Search PubMed.
- F. Tang, W. Cheng, H. Su, X. Zhao and Q. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 6228–6234 Search PubMed.
- Y. Tang, R. Wang, Y. Yang, D. Yan and X. Xiang, ACS Appl. Mater. Interfaces, 2016, 8, 19446–19455 Search PubMed.
- W. He, R. Wang, L. Zhang, J. Zhu, X. Xiang and F. Li, J. Mater. Chem. A, 2015, 3, 17977–17982 Search PubMed.
- L. Wang, F. Dionigi, N. T. Nguyen, R. Kirchgeorg, M. Gliech, S. Grigorescu, P. Strasser and P. Schmuki, Chem. Mater., 2015, 27, 2360–2366 Search PubMed.
- H. She, P. Yue, X. Ma, J. Huang, L. Wang and Q. Wang, Appl. Catal., B, 2020, 263, 118280 Search PubMed.
- X. Lv, X. Xiao, M. Cao, Y. Bu, C. Wang, M. Wang and Y. Shen, Appl. Surf. Sci., 2018, 439, 1065–1071 Search PubMed.
- P. Yue, H. She, L. Zhang, B. Niu, R. Lian, J. Huang, L. Wang and Q. Wang, Appl. Catal., B, 2021, 286, 119875 Search PubMed.
- H. Chen, S. Wang, J. Wu, X. Zhang, J. Zhang, M. Lyu, B. Luo, G. Qian and L. Wang, J. Mater. Chem. A, 2020, 8, 13231–13240 Search PubMed.
- S. Wang, P. Chen, J. H. Yun, Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2017, 56, 8500–8504 Search PubMed.
- D. Lee, A. Kvit and K.-S. Choi, Chem. Mater., 2018, 30, 4704–4712 Search PubMed.
- T. S. Sinclair, H. B. Gray and A. M. Müller, Eur. J. Inorg. Chem., 2018, 1060–1067 Search PubMed.
- Y. He, C. Zhang, J. Hu and M. K. Leung, Appl. Energy, 2019, 255, 113770 Search PubMed.
- Q. Wang, T. Niu, L. Wang, J. Huang and H. She, Chinese J. Catal., 2018, 39, 613–618 Search PubMed.
- S. Kumar and A. K. Satpati, Electrochim. Acta, 2020, 137565 Search PubMed.
- L. Shan, G.-l. Wang, J. Suriyaprakash, D. Li, L.-z. Liu and L.-m. Dong, J. Alloys Compd., 2015, 636, 131–137 Search PubMed.
- D. He, R.-T. Gao, S. Liu, M. Sun, X. Liu, K. Hu, Y. Su and L. Wang, ACS Catal., 2020, 10, 10570–10576 Search PubMed.
- S. Wang, T. He, P. Chen, A. Du, K. Ostrikov, W. Huang and L. Wang, Adv. Mater., 2020, 2001385 Search PubMed.
- J. Jian, Y. Xu, X. Yang, W. Liu, M. Fu, H. Yu, F. Xu, F. Feng, L. Jia, D. Friedrich, R. van de Krol and H. Wang, Nat. Commun., 2019, 10, 2609 Search PubMed.
- M. R. Nellist, F. A. Laskowski, F. Lin, T. J. Mills and S. W. Boettcher, Acc. Chem. Res., 2016, 49, 733–740 Search PubMed.
- C. Du, X. Yang, M. T. Mayer, H. Hoyt, J. Xie, G. McMahon, G. Bischoping and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 12692–12695 Search PubMed.
- D. R. Gamelin, Nat. Chem., 2012, 4, 965–967 Search PubMed.
- C. Zachäus, F. F. Abdi, L. M. Peter and R. van de Krol, Chem. Sci., 2017, 8, 3712–3719 Search PubMed.
- M. Barroso, C. A. Mesa, S. R. Pendlebury, A. J. Cowan, T. Hisatomi, K. Sivula, M. Grätzel, D. R. Klug and J. R. Durrant, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15640–15645 Search PubMed.
- R. Liu, Z. Zheng, J. Spurgeon and X. Yang, Energy Environ. Sci., 2014, 7, 2504–2517 Search PubMed.
- F. Li, Y. Li, Q. Zhuo, D. Zhou, Y. Zhao, Z. Zhao, X. Wu, Y. Shan and L. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 11479–11488 Search PubMed.
- F. Yu, F. Li, T. Yao, J. Du, Y. Liang, Y. Wang, H. Han and L. Sun, ACS Catal., 2017, 7, 1868–1874 Search PubMed.
- R. Peat and L. Peter, J. Electroanal. Chem., 1987, 228, 351–364 Search PubMed.
- M. G. Ahmed, I. E. Kretschmer, T. A. Kandiel, A. Y. Ahmed, F. A. Rashwan and D. W. Bahnemann, ACS Appl. Mater. Interfaces, 2015, 7, 24053–24062 Search PubMed.
- J. Xie, C. Guo, P. Yang, X. Wang, D. Liu and C. M. Li, Nano Energy, 2017, 31, 28–36 Search PubMed.
- J. E. Thorne, J.-W. Jang, E. Y. Liu and D. Wang, Chem. Sci., 2016, 7, 3347–3354 Search PubMed.
- W. Li, D. He, S. W. Sheehan, Y. He, J. E. Thorne, X. Yao, G. W. Brudvig and D. Wang, Energy Environ. Sci., 2016, 9, 1794–1802 Search PubMed.
- Y. Zhang, H. Zhang, H. Ji, W. Ma, C. Chen and J. Zhao, J. Am. Chem. Soc., 2016, 138, 2705–2711 Search PubMed.
- F. M. Toma, J. K. Cooper, V. Kunzelmann, M. T. McDowell, J. Yu, D. M. Larson, N. J. Borys, C. Abelyan, J. W. Beeman, K. M. Yu, J. Yang, L. Chen, M. R. Shaner, J. Spurgeon, F. A. Houle, K. A. Persson and I. D. Sharp, Nat. Commun., 2016, 7, 12012 Search PubMed.
- D. K. Lee and K.-S. Choi, Nat. Energy, 2018, 3, 53–60 Search PubMed.
- R.-T. Gao, D. He, L. Wu, K. Hu, X. Liu, Y. Su and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 6213–6218 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM, EDS, XRD, and UV-vis absorption spectra, XPS, Mott–Schottky, and KIE results. See DOI: 10.1039/d1ma00344e |
| This journal is © The Royal Society of Chemistry 2021 |