Open Access Article
Open Access ArticleDesign guidelines for chalcogenide-based flexible thermoelectric materials
Yifan
Wang
abc,
Peijian
Lin
a,
Qing
Lou
a,
Zhongchi
Zhang
a,
Shan
Huang
*abc,
Yao
Lu
*abc and
Jiaqing
He
 *abc
*abc
aDepartment of Physics, Southern University of Science and Technology, Shenzhen 518055, China. E-mail: huangs3@sustech.edu.cn; luy8@sustech.edu.cn; hejq@sustech.edu.cn
bShenzhen Key Laboratory of Thermoelectric Materials, Southern University of Science and Technology, Shenzhen 518055, China
cGuangdong Provincial Key Laboratory of Energy Materials for Electric Power, Southern University of Science and Technology, Shenzhen 518055, China
First published on 17th March 2021
Abstract
The power source of rapidly developed flexible and wearable intelligent electronic devices should be stable and have a long working period. Flexible thermoelectric (TE) generators (TEGs) can continuously power electronic devices by harvesting heat from the human body and have become a feasible solution. They are an economical approach to energy collection and have other advantages including chemical stability and noiselessness. Here, we review recently reported chalcogenide-based flexible TE materials and divide them into three categories: flexible substrate supported films, organic-chalcogenide composites, and free-standing flexible films. We discuss recent breakthroughs for chalcogenide-based flexible TE materials with high power factors and/or figures of merit and provide detailed design guidelines for the TE properties and flexibility of the films. We further describe how these films may be assembled into device designs that take advantage of their mechanical flexibility and thermoelectric properties. Besides, the outlook and challenges of wearable inorganic-based thermoelectric materials are proposed.
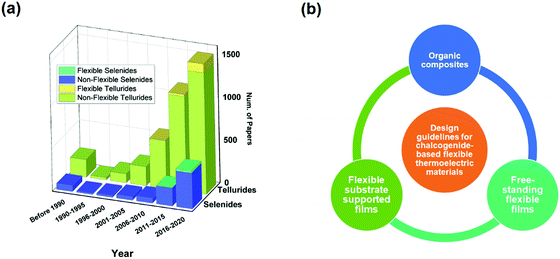
1. Introduction
Thermoelectric (TE) materials are “green” and functional materials that can realize a direct transition between heat and electricity or vice versa. TE generators (TEGs) are one of the most common applications of TE materials.1 TEGs have incomparable advantages including no noise, no moving parts, easy maintenance, long working life, and high reliability. A famous application is radioisotope batteries used in numerous NASA missions, e.g., the Voyager series.2 Therefore, a systematic review is necessary to gain a comprehensive understanding of the development of TE materials.3–5The TE effect transforms heat and electricity including the Seebeck effect and Peltier effect. The process of transforming heat into electricity is called the Seebeck effect, and the reverse is called the Peltier effect.6 A heat gradient causes a redistribution of carriers, and a concentration gradient is built, thus leading to an electrostatic potential called Seebeck voltage.7 The Seebeck coefficient (S) is used to describe such a phenomenon and is defined as 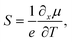 where e is the unit charge, μ is the chemical potential, and T is the temperature. The TE performance is usually denoted as a figure of merit (ZT) defined as
where e is the unit charge, μ is the chemical potential, and T is the temperature. The TE performance is usually denoted as a figure of merit (ZT) defined as 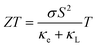 where σ, S, κe, κL, T are the electric conductivity, Seebeck coefficient, the electronic and lattice contribution to the total thermal conductivity, and temperature, respectively.8 To achieve excellent TE performance, a high ZT value is needed. Thus, the material should both have a high power factor (PF), namely, σS2, and a low thermal conductivity. However, this is not an easy task because these parameters are highly coupled.9 Other novel approaches including nanostructure engineering,10 band structure engineering,11 defect introduction,8 and increasing carrier mobility12 have also been proved effective (Fig. 1).
where σ, S, κe, κL, T are the electric conductivity, Seebeck coefficient, the electronic and lattice contribution to the total thermal conductivity, and temperature, respectively.8 To achieve excellent TE performance, a high ZT value is needed. Thus, the material should both have a high power factor (PF), namely, σS2, and a low thermal conductivity. However, this is not an easy task because these parameters are highly coupled.9 Other novel approaches including nanostructure engineering,10 band structure engineering,11 defect introduction,8 and increasing carrier mobility12 have also been proved effective (Fig. 1).
TE materials have recently become a potential candidate for power supply in wearable devices. Inorganic TE materials have a key role. The most significant advantage of TE materials in this scenario is that it can harvest the heat dissipated by human bodies as a power source. According to the literature,13 the power of heat generated by the human body can reach ∼100 W. Even with a low-efficiency generator, such energy is still sufficient to drive some small devices like low-power microcontroller chips. However, traditional TE materials like selenides and tellurides are brittle inorganic semiconductors with poor flexibility.
Early approaches to wearable inorganic TE devices mainly involved unusual materials for TE like metal.13 Other novel materials like carbon nanomaterials (e.g., carbon nanotubes, graphene, carbon fibers, and fullerenes) and the carbon nanomaterial/conducting polymer composites are also possible candidates. Despite the ideal mechanical properties (flexibility) of these materials, their thermoelectric performance is still poor. Another feasible approach is to make traditional bulk thermoelectric materials flexible. Most traditional TE material systems including chalcogenides (especially selenides and tellurides) have been widely studied since the 1950s. After decades of development, the physical properties of these materials have been thoroughly studied, and a possible means for performance enhancement has been proposed. Based on a large number of studies, people can take full advantage of the thermoelectric properties of these well-studied materials while finding ways to achieve flexibility and eventually apply them to wearable devices. Despite the short time of development (flexible chalcogenides were not reported until the 2000s), this approach has a key role in the field of flexible TE materials.
This review focuses on flexible TE materials, which mainly involve flexible chalcogenide-based films. Chalcogenide-based flexible thermoelectric materials have been discussed in three design guidelines: chalcogenides deposited on flexible films, organic-chalcogenide composites, and free-standing films. In each part, we first review the fabrication methods. The properties and performance of different materials are then discussed. A brief conclusion and outlook are also provided.
2. Strategies to realize flexible chalcogenide-based films
2.1 Chalcogenide-based films on a flexible substrate
To achieve high TE performance and good flexibility of thin films, one of the most commonly used methods is to deposit inorganic nanostructures on a flexible substrate. The common flexible substrates are polyimide (PI), polyether sulfone (PES),14 and polyvinylidene fluoride (PVDF);15,16 inorganic nanostructure-based films can maintain flexibility. The common fabrication processes are magnetron sputtering,17–23 pulsed laser deposition,24–26 thermal evaporation,27,28 vacuum-assisted filtration and screen printing,29,30etc. Some additional methods like cold pressing and hot pressing5,31 can maintain the comparable bulk-like ZT value through forming a compacted film. However, the films are still rigid for wearable devices.To make Cu2Se flexible, Perez-Taborda et al.32 deposited it on a PI substrate using pulsed reactive magnetron sputtering. The Cu2Se film reached a high PF of 1100 μW m−1 K−2 at room temperature (RT). They33 also used the same method to prepare Ag2Se films, and a high PF of ∼2440 μW m−1 K−2 at RT was obtained. However, due to the weak bonding strength between the inorganic and flexible substrates, it was difficult to form a highly flexible composite film.
Aside from magnetron sputtering, another solution to making Ag2Se wearable—which itself is not flexible—is to use the hot pressing method for depositing the Ag2Se nanowires on a flexible substrate like nylon membrane.34,35 In 2019, Ding et al. developed a novel method to realize both high TE properties and excellent flexibility of the n-type Ag2Se TE material.34 They first prepared Ag2Se nanowires using selenium nanowires as templates, and then used vacuum filtration to form a film on the nylon membrane. They finally hot pressed the material at 200 °C and 1 MPa for 30 min. A PF of 987.4 μW m−1 K−2 and a ZT value of ∼0.6 were reached at RT. The film was also flexible and retained 80% of the electrical conductivity after 1500 bending cycles.
The high flexibility comes from the following three aspects. First, the substrate (nylon) is highly flexible. Furthermore, Ag2Se nanowires after hot pressing form a web-like porous structure. Such a structure gives certain flexibility to the film.36 There are also Ag and Se atoms that diffuse into the porous nylon membrane forming a strong bond between the film and substrate. Despite the excellent flexibility, the film still suffers from low PF versus Ag2Se films on glass.37 The main reason is that the growth orientation (00l) does not agree with the orientation (0l0) that has the highest PF.
Alternating the composition of the film can also have positive effects on TE properties. Lu et al. studied the influence of Cu addition on the TE properties of the Ag2Se film and prepared a flexible n-type Ag2Se/Ag/CuAgSe film on the nylon membrane substrate (Fig. 2a).35 The film reached a PF of 1593.9 μW m−1 K−2 at 300 K with a molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag
Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se = 1
Se = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.35 It had superior flexibility because the PF only dropped by 10% after 1000 bending cycles. The composition of the film was Ag nanoparticles, CuAgSe nanoparticles, and Ag2Se nanograins. The electron conductivity is enhanced by Ag, which ejects electrons into the conduction band of the other two composites.38 The energy filtering effect at the heterogeneous interfaces makes the composite film maintain a medium Seebeck coefficient. Furthermore, the defects in Ag2Se grain and heterointerfaces of the composites scatter the short-wavelength phonons, which suppress the thermal conductivity of the film.39 Besides, a flexible TEG is assembled using the optimum hybrid film, which generates a voltage and a maximum power density of 4.9 mV and 1.38 W m−2 at a temperature difference of 18 K. However, the enhancement of PF is mainly due to the greatly enhanced electrical conductivity. Hence, the corresponding electronic thermal conductivity is also high, leading to a lower ZT value (ZT ∼ 0.4) at RT.
3.35 It had superior flexibility because the PF only dropped by 10% after 1000 bending cycles. The composition of the film was Ag nanoparticles, CuAgSe nanoparticles, and Ag2Se nanograins. The electron conductivity is enhanced by Ag, which ejects electrons into the conduction band of the other two composites.38 The energy filtering effect at the heterogeneous interfaces makes the composite film maintain a medium Seebeck coefficient. Furthermore, the defects in Ag2Se grain and heterointerfaces of the composites scatter the short-wavelength phonons, which suppress the thermal conductivity of the film.39 Besides, a flexible TEG is assembled using the optimum hybrid film, which generates a voltage and a maximum power density of 4.9 mV and 1.38 W m−2 at a temperature difference of 18 K. However, the enhancement of PF is mainly due to the greatly enhanced electrical conductivity. Hence, the corresponding electronic thermal conductivity is also high, leading to a lower ZT value (ZT ∼ 0.4) at RT.
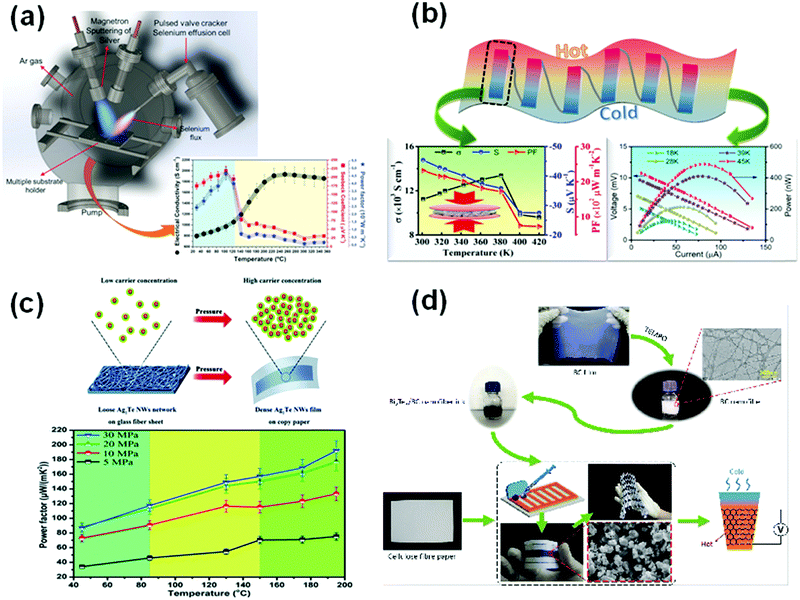 | ||
| Fig. 2 (a) A cross-sectional diagram of the new method of manufacturing PHRMS deposition system is presented. The dependence on temperature for the Seebeck coefficient, electrical conductivity, and PF. (Reproduced and adapted with permission from ref. 33, copyright (2017) John Wiley & Sons.) (b) A highly flexible Ag2Se-based composite film on nylon with a record PF is prepared for thermoelectric generator (reproduced with permission from ref. 35, copyright (2020) Royal Society of Chemistry). (c) A simulated diagram of carrier concentration and morphology of the Ag2Te film before and after being compressed, and PF of the Ag2Te film (reproduced with permission from ref. 47, copyright (2017) Royal Society of Chemistry). (d) Honeycomb-like TE generator based on paper (reproduced with permission from ref. 74, copyright (2019) Royal Society of Chemistry). | ||
Jiang et al. studied Ag2Se and tried to improve the processing procedure by suppressing the orientation of (00l) of the Ag2Se film and therefore enhancing the TE performance. A high PF of 1882 μW m−1 K−2 at RT was obtained.40 They also assembled a flexible TEG, which created an output voltage of 4.3 mV under the 6.7 K temperature difference. By increasing the reaction temperature of Se nanowires and AgNO3, Ag2Se nanostructures are formed instead of Ag2Se nanowires due to the reduction in surface energy. The formation of nanostructures suppresses the orientation of (00l), thus increasing the TE performance (ZT ∼ 0.8 at RT).
Besides using the nylon membrane as the substrate, Gao et al. demonstrated a set of annealed paper-supported silver selenide films.41 The PF of the Ag2.3Se film reached up to 2450.9 ± 364.4 μW m−1 K−2 at RT. An optimized length device was built using this film, which had an output power of 0.67 μW with a temperature difference of 25 K. Silver selenide showed tight bonding to the paper.
Besides Ag2Se, other chalcogenides have also been proposed as possible candidates for flexible TE materials. Lin et al. proposed a novel way to produce copper selenide films.42 An ink solution of Cu2Se was formed and deposited on the substrate via spin coating. Such a process secured a smooth and highly crystalline thin film (∼55 nm), which was also void-free. Therefore, the high TE properties of copper selenides were secured. High flexibility was reached when the film is coated on a flexible substrate, e.g., plastic.
In 2016, Yun et al. reported a mixture of HgSe and Ag nanoparticles deposited on flexible plastic films.43 They confirmed that the electrical conductivity could be tuned by changing the ratio of Ag and HgSe NPs; an optimized ZT value and PF were reached. They presumed that such an optimization was achieved through adjustment of the carrier concentration. They further proposed that the energy barriers were lowered by Ag2Se NPs attached to the surface of the HgSe NPs. The plastic film with NPs deposited showed high flexibility. The composite film showed a slight decrease in electric conductivity and an increase in the Seebeck coefficient.
By controlling the conditions of the reaction, Rongione et al. successfully synthesized SnSe nanosheets with a controlled growth direction.44 The nanosheet has low thermal conductivity versus bulk SnSe. The boundary contributes most of the thermal resistivity because the thickness of the flakes is only about 150 nm. In other words, the thermal conductivity is suppressed, thus reaching a high TE performance. They also showed high flexibility by depositing the nanosheets on a polyethylene terephthalate substrate and performing bending tests. The thermal properties and electrical properties did not change much after 1000 cycles.
Attention has also been paid to the Ag2Te nanostructure to see whether there is a possible way for flexible applications. The conducting type of Ag2Te can be converted between n-type and p-type by adjusting the composition. In other words, excess Ag can act as an electron donor while superfluous Te can play a role as an electron acceptor.45
Finefrock et al.46 synthesized Ag2Te nanomaterials coated on a nylon fiber. After annealing the flexible film, the Seebeck coefficient changed from negative to positive, which indicated that Ag2Te converted the conduction type from n-type to p-type. This is mainly due to the large changes in the stoichiometric ratio of Ag and Te before and after annealing. Compared with n-type Ag2Te, the TE performance of the p-type one is much lower.14,16 Therefore, mainstream research on the Ag2Te TE material is n-type.
As for wearable devices application, the Ag2Te-based films need to show competitive TE properties as well as good flexibility, i.e., excellent durability. By introducing a novel glass fiber-auxiliary cold pressing method, Gao et al.47 fabricated a Ag2Te nanowire film supported on a copy-paper substrate that has a maximum PF of 192 μW m−1 K−2 at 468 K. The film showed good flexibility with a PF value decreasing by 20% after 500 bending cycles. However, its PF at RT is mediocre (88 μW m−1 K−2) due to poor connection between Ag2Te nanowires. To fix this problem, Zeng et al.48 welded the Ag2Te nanowires by vacuum filtration and drop-coating methods. The PF of the film is 152 μW m−1 K−2 at RT, which is almost twice as large as the film reported by Gao. The welding methods maintain the morphology of Ag2Te nanowires, which preserves low thermal conductivity. However, the flexibility is poor with electrical resistance increasing 30% after 1000 bending cycles.
Besides single component thermoelectric films, multiple component films have also shown their potential. Usually, Bi2Te3/Sb2Te3 thin films are not perfect candidates for flexible TE materials due to their deficient nanostructure, which can be—but are not limited to—poor interconnections and too many nanopores. Poor interconnection can result in the deduction of electrical conductivity and strain force concentration which makes thin films brittle.
To improve the interfacial bonding, Feng et al.29 introduced Sb2Te3 as a nano-solder into Bi2Te3 films via screen-printing. The sample had excellent endurance and anti-oxidation (electric resistivity only changed by 3.6% after 6 months in the air). The porous structure of the film can withstand moderate deformation, which results in good flexibility and can effectively scatter the phonons. This special porous structure can reduce the thermal conductivity as well as the electrical conductivity.49 Thus, efforts need to be made to find a moderate number of nanopores that can keep the balance of these parameters to optimize the flexible TE performance of materials. Jin et al.50 used unbalanced magnetron sputtering technology to prepare Bi2Te3/cellulose fibers as flexible composites. Cellulose fibers have a porous structure, and the composite film can withstand deformation leading to high flexibility. The porous structure can also enhance phonon scattering, thus decreasing thermal conductivity. The PF values of the composite films are about 250–375 μW m−1 K−2 from RT to 473 K. The sample has a ZT value of 0.38 at 473 K.
The (Bi, Sb)2(Te, Se)3 TE bulk material has a high ZT value near RT, which is also a good candidate for flexible films. Researchers previously attempted to fabricate TE thin films by depositing (Bi, Sb)2(Te, Se)3 on different kinds of substrates (such as silica27,51 and alumina52,53) using magnetron sputtering and screen printing processes.54,55 However, the mechanical properties still cannot meet the requirements of flexible devices. Here, the flexibility of the TE thin film can be drastically improved by using organic substrates like polyimide (PI),56–58 polyethylene terephthalate (PET),59,60 and polycarbonate.61 Nevertheless, the ZT value of the films is much lower than that of the bulks, because the poor crystallinity and texture on the substrate impede the enhancement of the ZT value. Furthermore, due to the thermolability of organic substrate, traditional annealing and sintering processes (>500 °C) cannot be employed on most organic substrates to improve the crystallinity and texture.62,63 Thus, Danaei64et al. and Saeidi-Javash et al.65 introduced a photonic sintering method into n-type Bi2Te2.7Se0.3 to enhance its TE performance while maintaining flexibility. Although the PF of the n-type film that Saeidi-Javash et al. synthesized is not the highest Bi2Te2.7Se0.3 film, the photonic sintering method can also dramatically reduce the synthesis time, which is beneficial to industrial mass production.65
The TE performance of p-type Bi0.5Sb1.5Te3 bulk is higher than that of n-type Bi2Te2.7Se0.3, and thus there are more studies on p-type systems. Like the n-type film, most studies focused on adjusting the nanostructure of the film.66 If the length of the porous structure is longer than the mean free path of electrons but shorter than that of phonons, then the high electrical conductivity can be maintained while simultaneously decreasing the thermal conductivity and thus improving the TE performance.50,67 Kato et al.68,69 introduced a porous structure using a block copolymer to reduce the thermal conductivity and obtain bulk-like ZT (about 1.34) for Bi0.4Sb1.6Te3: this is an effective method to enhance the ZT value on a flexible thin film. However, the thermal conductivity is calculated by a model rather than an experiment; hence, the actual ZT value needs to be further confirmed. Besides, strengthening the connection of the interface can be another effective way to promote ZT value while also maintaining favorable flexibility.29 Varghese et al.70 added Te particles as nano-solders to successfully bridge the interface to Bi0.4Sb1.6Te3 TE films by a screen printing method. Besides the extraordinary flexibility and endurance (electrical resistance only increases 3% after 1000 bending cycles with 10 mm radius), the printed films also exhibit the highest TE performance at RT: The PF is 3000 μW m−1 K−2 and ZT value is about 1, which demonstrates a significant leap for wearable TE applications.70
For the practical flexible TE devices, people usually combine the excellent performance of n-type Bi2Te2.7Se0.3 and p-type Bi0.5Sb1.5Te3 into flexible TEGs.71–75 Meanwhile, due to the difficulty of measuring the thermal conductivity on thin films, the output power, power density, and other parameters of flexible TEGs become an important reference for the TE performance of materials.76–78 Zhao et al.74 introduced a honeycomb structure into the paper-based TEG fabricated by (Bi, Sb)2(Te, Se)3 and bacterial cellulose (Fig. 2b): this greatly improves the flexibility and water resistance and makes TEG applicable for a wide variety of surfaces. Feng et al.79 prepared a flexible TEG through the combination with n-Bi2Te2.7Se0.3 and p-Bi0.5Sb1.5Te3 on PET film. The samples had an excellent output voltage (0.8 V) versus other flexible TEGs based on the same materials. Both flexible TEGs need about 50 K of a temperature difference to achieve a high power density, which is too large for wearable energy harvesting applications; however, this is acceptable for wearable temperature sensing79 or wearable coolers.80
2.2 Organic-chalcogenide composites
Polymers possess an intrinsically low κ, which is independent of electrical optimization. Therefore, preparing organic/inorganic hybrids can be expected to combine the high PF of the inorganic filler and simultaneously low thermal conductivity of the polymer. Of the polymers, conducting polymers (CP) have an intrinsically high electrical conductivity. Suitable systems include poly(3,4-ethylenedioxythiophene) (PEDOT)81 and poly(styrenesulfonate)-doped PEDOT (PEDOT:PSS)46,82–85—these can dramatically increase the flexibility of the chalcogenide-based composites. More importantly, some results show that TE performance improves due to the energy filtering effect of the organic/inorganic heterointerface.For instance, Jao et al.85 prepared Ag2Te nanowires/PEDOT:PSS composites via physical mixing and developed a flexible TE nanogenerator. The nanogenerator showed a Seebeck coefficient of 100 μV K−1. However, because of the low efficiency, the nanogenerators were only aimed at temperature measurement in a multicomplex environment rather than wearable generators on the human body. Xu et al.86 demonstrated the effectiveness of a dimensionality-morphology strategy on the improvement of flexible thermoelectric performance. They optimized the power density of (PVDF)/Ta4SiTe4 organic–inorganic composites to 13 W m−2 with a temperature difference of 35.5 K (Fig. 3a).
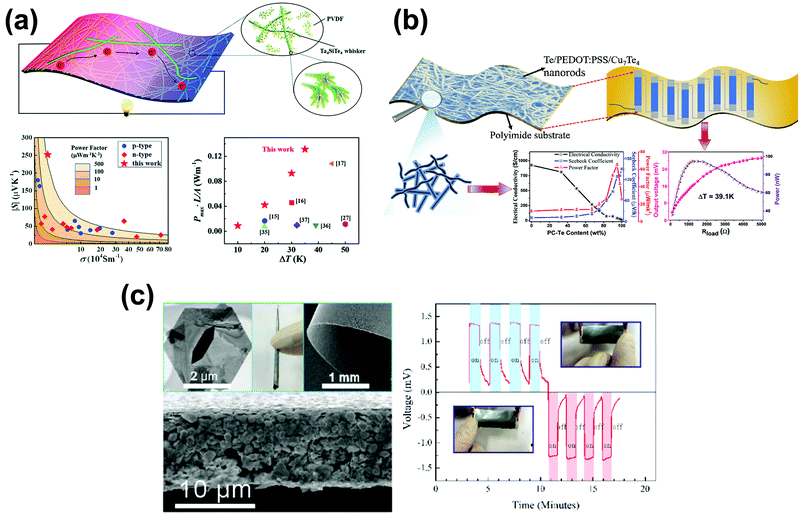 | ||
| Fig. 3 (a) Synthesis and TE performance of the flexible PVDF/Ta4SiTe4 composite film (reproduced with permission from ref. 86, copyright (2020) Royal Society of Chemistry). (b) Performance of the flexible PC-Cu7Te4/PC-Te nanocomposite film and TE device (reproduced with permission from ref. 89, copyright (2018) American Chemical Society). (c) Flexible and freestanding n-type Cu0.1Bi2Se3 nanoplatelet/PVDF TE composite film. Fingertip touch response of the Cu0.1Bi2Se3 nanoplate based flexible thermoelectric films, which shows the thermoelectric films could continuously convert the body heat into electricity (reproduced with permission from ref. 94, copyright (2015) Elsevier.) | ||
The organic solvent-assisted method can exfoliate inorganic compound nanosheets such as SnSe and Bi2Te3. Ju et al. combined tin selenide nanosheets with PEDOT:PSS.87 First, a small amount of Te was added during the sintering process of the SnSe ingot. Then, SnSe1−xTex powders were intercalated by Li-ions, and SnSe1−xTex nanosheets were exfoliated from the powder. Finally, the nanosheets were combined with PEDOT:PSS through physical mixing, and a composite film was obtained by filtration. The introduction of trace Te during the sintering process can prepare an optimized hole concentration of the sample. The SnSe0.97Te0.03 nanosheet/PEDOT:PSS composite showed a PF of 14.7 μW m−1 K−2 with excellent durability after 1000 bending cycles. Du et al.88 prepared Bi2Te3-based alloy nanosheets/PEDOT:PSS composites via exfoliation and drop-casting routes. The composite film containing 4.1 wt% Bi2Te3 showed higher electrical conductivity and a PF value of 32.3 μW m−1 K−2.
In situ synthesis is an effective method to adequately disperse inorganic nanoparticles in a CP matrix, thus enhancing wettability and making the two phases robustly tethered. In 2016, Zaia et al. prepared controlled growth of PEDOT:PSS-coated Te–Cu1.75Te heterostructures using a facile solution-based synthesis method. They showed a creative synthetic method to grow nanoscale islands of Cu1.75Te alloy within hybrid PEDOT:PSS-Te nanowires. The PEDOT:PSS/Te/Cu1.75Te heterostructure nanocomposite was drop-cast on a glass substrate; this exhibited a 22% PF (84 μW m−1 K−2) enhancement compared with PEDOT:PSS/Te. However, the film is not flexible. Lu et al.89 prepared PEDOT:PSS-coated Te (PC-Te) nanorods in situ; PEDOT:PSS-coated polycrystal Cu7Te4 (PC-Cu7Te4) nanorods were then prepared using the PC-Te nanorods as templates. Finally, PC-Cu7Te4/PC-Te composite films were fabricated through physical mixing and drop-casting. The flexible ternary composite film showed an enhanced PF of 65.3 μW m−1 K−2 at 300 K, which is about five-fold as large as that of the PC-Cu7Te4 nanorod film and about three times as large as that of the PC-Te nanorod film.
The enhancement of the TE properties is ascribed to the synergetic effect of the two kinds of nanorods and the carrier filtering effect at the two heterointerfaces of Te/PEDOT:PSS and Cu7Te4/PEDOT:PSS. An eight single-leg flexible TEG consisting of the optimized composite film was fabricated: It produced a voltage of 31.2 mV and a maximum output power density of 0.395 W m−2 at a temperature gradient of 39 K (Fig. 3b). Inspired by the synthesis method of the PC-Cu7Te4 nanorod, they90 synthesized PEDOT:PSS-coated Se (PC-Se) nanowires for the first time via an in situ chemical synthesis method. PEDOT:PSS-coated CuxSey (PC-CuxSey) nanowires were prepared by a template-directed synthesis method. Finally, PEDOT:PSS/CuxSey nanocomposite films on flexible nylon membranes were fabricated by vacuum-assisted filtration and cold-pressing. An optimized PF ∼270.3 μW m−1 K−2 was obtained at 300 K.
The film exhibited superior flexibility with 83% of the original PF retention after bending for 1000 cycles around a rod with a diameter of 5 mm. Finally, a TE prototype composed of nine legs of the optimized hybrid film generated a voltage and a maximum power density of 15 mV and 0.91 W m−2, respectively, at a temperature gradient of 30 K. To further improve the TE performance of the composite material, Cu2+ and Ag+ were added to PC-Se nanowires in solution to perform a complete reaction. Then, an n-type PEDOT:PSS/Ag2Se/CuAgSe TE composite film supported by porous nylon membrane was fabricated through vacuum-assisted filtration followed by hot-pressing. With PEDOT added, the RT thermal conductivity of the composite film remained low (0.458–0.800 W m−1 K−1). As a result, the corresponding ZT value is 0.6 < ZT < 1.1. After bending for 1000 cycles around a 4-mm radius rod, the PF decreases by only 8%. It showed excellent flexibility, which is attributed to the synergetic effect of the flexible nylon membrane and the porous PEDOT:PSS/Ag2Se/CuAgSe composite film. Moreover, a flexible TE prototype composed of 11 single legs generated a voltage and a maximum power of 45.8 mV and 3.2 μW with a power density of 8.4 W m−2 at a temperature difference of 36 K.91
Most CP-based materials are p-type semiconductors and only generate positive TE power. To develop flexible n-type TE materials with negative TE power, researchers often use insulating polymers such as polyvinylidene fluoride (PVDF) and (polyvinylpyrrolidone) PVP to fabricate TE composites. Although the insulating polymer matrix can decrease the electrical conductivity of the composites, they also have some advantages over CPs, including lower cost, superior flexibility, and ultralow thermal conductivity.
Meng et al.92 prepared an n-type PVP/Ag/Ag2Te flexible composite film supported by a nylon filter membrane through vacuum-assisted filtration and heat treatment, where the PF is 217 μW m−1 K−2 and ZT is about 0.15–0.3 at 300 K. These show the highest TE performance among Ag2Te-based composites. After 1000 rounds of bending tests, the electrical conductivity only decreased by 9.4%, which illustrates the excellent flexibility. A flexible TE device was assembled on a polyimide substrate using five n-type legs. The device has a maximum output power of 469 nW at a temperature difference of 39.6 K and a corresponding power density of 3.41 W m−2.
PVDF can guarantee the robustness and flexibility of the composite film. In 2015, Dun et al.93 reported a free-standing Bi2Se3 nanoplate/PVDF composite film through a drop-casting method. The PF is 30 μW m−1 K−2 at RT and dropped by 15% after 1500 rounds of bending. They also prepared a freestanding n-type Cu0.1Bi2Se3 nanoplatelet/PVDF TE composite film (Fig. 3c). The optimized PF and ZT of the composites are 103 μW m−1 K−2 and 0.1 at RT, respectively. They showed that PVDF can not only guarantee the flexibility but also introduce the energy barrier at the Cu0.1Bi2Se3/PVDF heterointerface. Thus, high TE properties are maintained.94
2.3 Free-standing chalcogenide-based films
Organic substrates/components have poor stability at temperatures higher than 473 K, while free-standing inorganic flexible TE films can be applied at high temperatures. Liang et al.95 reported high organic material-like flexibility and state-of-the-art ZT (up to 0.44 at 300 K and 0.63 at 450 K) in Ag2S-based inorganic materials. Besides, a full-inorganic flexible TEG based on Ag2(S, Se) and Ag2(S, Te) was fabricated (Fig. 4a). The flexible full-inorganic device generated a high normalized maximum power density of 5.56 W m−2 under a temperature difference of 20 K. The discovery of intrinsic plastic deformability in bulk Ag2S enabled fabrication of a fully inorganic Ag2S-based flexible TEG.96 More recently, Wei et al.97 discovered exceptional deformability and plasticity in bulk single-crystalline InSe, which can be used in flexible TEG, sensors, photodetectors, etc. (Fig. 4b). Such extraordinary mechanical properties stem from both the interlayer pliability and the interlayer long-range In–Se coulombic interaction across the van der Waals gap.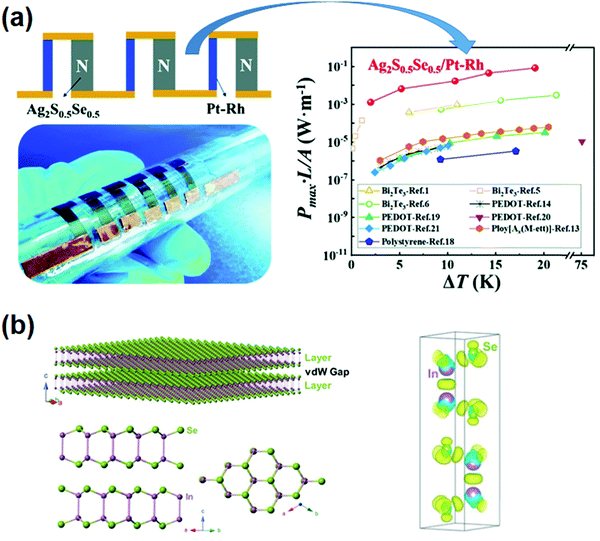 | ||
| Fig. 4 (a) Six-couple flexible Ag2S0.5Se0.5/Pt-Rh TE device and maximum power density (reproduced and adapted with permission from ref. 95, copyright (2019) Royal Society of Chemistry). (b) The InSe crystal structure and differential charge density (reproduced and adapted with permission from ref. 97, copyright (2020) American Association for the Advancement of Science). | ||
Besides flexible materials, changing the process conditions to alter the form of the product is also a feasible solution. Jin et al.98 designed a creative new approach to produce high-performance TE materials using the SWCNT network as a scaffold to guide the deposition and growth of layer-structured Bi2Te3 nanocrystals to form a freestanding composite film. The ordered Bi2Te3/SWCNT interfaces can suppress the scattering of carriers during interfacial transport while dampening thermal conductivity due to porosity. The flexible films not only show excellent flexibility (bending radius <5 mm) but also exhibit high TE performance (bulk-like ZT value of 0.9 at RT).
3. Conclusion and outlook
In this review, we summarized recent progress on chalcogenide-based flexible TE films including organic/inorganic composites, inorganic films supported on flexible substrates, and full-inorganic films. Most bulk inorganic semiconductors are intrinsically brittle at ambient conditions. The high flexibility of the traditional inorganic materials can be achieved by depositing nanostructures on the flexible substrates or combining them with flexible materials, e.g. PEDOT:PSS. For preparing inorganic films supported by flexible substrates, the power factor seems relatively high, and the flexibility is sometimes worse. For preparing organic/inorganic nanocomposite films, the TE property of inorganic material is higher than organic material, and it is important that the amount of the inorganic component is as high as possible, and because the two components have significantly different chemical properties, it is a great challenge to form a composite with perfect interfaces between the two phases. The discovery of intrinsic plastic deformability in bulk Ag2S and InSe greatly promoted the development and application of all-inorganic flexible TE materials, which may be the focus of future research directions. Significant progress has been made toward developing these candidate materials with high TE performance and high flexibility.Despite the promising performance given in the literature, the overall performance of flexible TE materials is still far from commercial application. Here, we give our outlook on future research:
1. To date, flexible TE films are mainly supported by flexible substrates. Since the desired device should be as small or as thin as possible, the substrate (which is often thicker than the film) should not use much space in the device. Therefore, a true free-standing flexible device should be the ultimate solution.
2. Since we have established an understanding of many other traditional bulk materials, endeavors should be made to apply these materials to flexible devices. Various approaches to boosting the TE performance of bulk thermoelectric materials have been proposed and can guide further enhancement of flexible TE materials.
3. Most papers mainly focus on increasing TE performance. However, the mechanical performance, i.e., the origin of flexibility, has not yet been fully discussed or systematically explored. While many articles claimed that they have good flexibility, the damping of performance is still far from daily application. Thus, rediscovering the flexibility might be a new way to enhance the overall performance of flexible TE materials. Also, involving new geometrical architectures can bring several advantages.
4. The papers reviewed here rarely provided thermal properties; others provided the thermal conductivity of both the material and the film. These facts indicate a limitation of measuring methods. To fully understand the thermal properties, a new method for direct thermal conductivity measurement is needed.
With satisfying performances of wearable TEGs, it is predictable that many personal medical or sports devices will no longer need to charge a battery. Such new personal devices can remove worries about the power source and will increase convenience and quality of life.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 11934007, 11874194 and 51632005), the leading talents of Guangdong Province Program (Grant No. 00201517), the Science and Technology Innovation Committee Foundation of Shenzhen (Grant No. KQTD2016022619565991, JCYJ20200109141205978, and RCBS20200714114959040), the fellowship of China National Postdoctoral Program for Innovative Talents (BX20200160), the fellowship of China Postdoctoral Science Foundation (2020M682758), high level of special funds (G02206302), the Guangdong Provincial Key Laboratory of Energy Materials for Electric Power (No. 2018B030322001), and the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds) (Grant No. pdjh2021c0086).References
- T. M. Tritt and M. A. Subramanian, MRS Bull., 2006, 31, 188–198 CrossRef.
- E. D. M. Rowe, D. Ph, D. Sc and F. Group, Thermoelectrics Handbook: Macro to Nano (Google eBook), 2010 Search PubMed.
- K. K. Jung, Y. Jung, C. J. Choi, J. M. Lee and J. S. Ko, Curr. Appl. Phys., 2016, 16, 1442–1448 CrossRef.
- H. An, D. Karas, B. W. Kim, S. Trabia and J. Moon, Nanotechnology, 2018, 29, 275403 CrossRef.
- K. Ahmad and C. Wan, Nanotechnology, 2017, 28, 415402 CrossRef PubMed.
- S. B. Riffat and X. Ma, Appl. Therm. Eng., 2003, 23, 913–935 CrossRef.
- B. Yang, H. Ahuja and T. N. Tran, HVACR Res., 2008, 14, 635–653 CrossRef.
- L. Fu, M. Yin, D. Wu, W. Li, D. Feng, L. Huang and J. He, Energy Environ. Sci., 2017, 10, 2030–2040 RSC.
- L. Fu, J. Cui, Y. Yu, Y. Huang, Y. Wang, Y. Chen and J. He, J. Mater. Chem. A, 2019, 7, 6304–6311 RSC.
- J. Zhang, D. Wu, D. He, D. Feng, M. Yin, X. Qin and J. He, Adv. Mater., 2017, 29, 1703148 CrossRef PubMed.
- Y. Pei, X. Shi, A. Lalonde, H. Wang, L. Chen and G. J. Snyder, Nature, 2011, 473, 66–69 CrossRef CAS PubMed.
- Y. Xiao, H. Wu, W. Li, M. Yin, Y. Pei, Y. Zhang, L. Fu, Y. Chen, S. J. Pennycook, L. Huang, J. He and L. D. Zhao, J. Am. Chem. Soc., 2017, 139, 18732–18738 CrossRef CAS.
- T. Starner, IBM Syst. J., 1996, 35, 618–629 Search PubMed.
- S. Yang, K. Cho, J. Yun, J. Choi and S. Kim, Thin Solid Films, 2017, 641, 65–68 CrossRef CAS.
- C. Zhou, C. Dun, B. Ge, K. Wang, Z. Shi, G. Liu, D. L. Carroll and G. Qiao, Nanoscale, 2018, 10, 14830–14834 RSC.
- W. Wang, J. Liu, X. Li, Q. Jiang, J. Xu, C. Luo, P. Liu, R. Tan, Y. Du and F. Jiang, J. Nanopart. Res., 2019, 21, 131 CrossRef.
- S. Liu, N. Peng, Y. Bai, D. Ma, F. Ma and K. Xu, RSC Adv., 2016, 6, 31668–31674 RSC.
- T. Inamoto and M. Takashiri, J. Appl. Phys., 2016, 120, 1–8 CrossRef.
- P. Nuthongkum, A. Sakulkalavek and R. Sakdanuphab, J. Electron. Mater., 2017, 46, 2900–2907 CrossRef CAS.
- P. Nuthongkum, R. Sakdanuphab, M. Horprathum and A. Sakulkalavek, J. Electron. Mater., 2017, 46, 6444–6450 CrossRef CAS.
- S. Kianwimol, P. Wanarattikan, R. Sakdanuphab, P. Pluengphon, T. Bovornratanaraks and A. Sakulkalavek, J. Electron. Mater., 2019, 48, 3490–3496 CrossRef CAS.
- K. Kusagaya, H. Hagino, S. Tanaka, K. Miyazaki and M. Takashiri, J. Electron. Mater., 2015, 44, 1632–1636 CrossRef CAS.
- K. Kusagaya and M. Takashiri, J. Alloys Compd., 2015, 653, 480–485 CrossRef CAS.
- E. Schwyter, W. Glatz, L. Durrer and C. Hierold, in 2008 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS, IEEE, 2008, pp. 46–48.
- E. Koukharenko, X. Li, I. Nandhakumar, N. Frety, S. P. Beeby, D. Cox, M. J. Tudor, B. Schiedt, C. Trautmann, A. Bertsch and N. M. White, J. Micromech. Microeng., 2008, 18, 1–9 CrossRef.
- W. Glatz, E. Schwyter, L. Durrer and C. Hierold, J. Microelectromech. Syst., 2009, 18, 763–772 CAS.
- E. Symeou, C. Nicolaou, T. Kyratsi and J. Giapintzakis, J. Appl. Phys., 2019, 125, 0–9 CrossRef CAS.
- E. M. F. Vieira, J. Figueira, A. L. Pires, J. Grilo, M. F. Silva, A. M. Pereira and L. M. Goncalves, J. Alloys Compd., 2019, 774, 1102–1116 CrossRef CAS.
- J. Feng, W. Zhu, Y. Deng, Q. Song and Q. Zhang, ACS Appl. Energy Mater., 2019, 2, 2828–2836 CrossRef CAS.
- S. J. Kim, J. H. We and B. J. Cho, Energy Environ. Sci., 2014, 7, 1959–1965 RSC.
- C. Dun, Y. Liu, A. Al-Qawasmeh, C. A. Hewitt, Y. Guo, J. Xu, Q. Jiang, J. Wang, G. Marcus, D. Cadavid, D. Montgomery, H. Wang, K. Kovnir, A. Cabot and D. L. Carroll, 2D Mater., 2018, 5, 045008 CrossRef CAS.
- J. A. Perez-Taborda, L. Vera, O. Caballero-Calero, E. O. Lopez, J. J. Romero, D. G. Stroppa, F. Briones and M. Martin-Gonzalez, Adv. Mater. Technol., 2017, 2, 1700012 CrossRef.
- J. A. Perez-Taborda, O. Caballero-Calero, L. Vera-Londono, F. Briones and M. Martin-Gonzalez, Adv. Energy Mater., 2018, 8, 1702024 CrossRef.
- Y. Ding, Y. Qiu, K. Cai, Q. Yao, S. Chen, L. Chen and J. He, Nat. Commun., 2019, 10, 841 CrossRef PubMed.
- Y. Lu, Y. Qiu, K. Cai, Y. Ding, M. Wang, C. Jiang, Q. Yao, C. Huang, L. Chen and J. He, Energy Environ. Sci., 2020, 13, 1240–1249 RSC.
- W. Yang, S. Mao, J. Yang, T. Shang, H. Song, J. Mabon, W. Swiech, J. R. Vance, Z. Yue, S. J. Dillon, H. Xu and B. Xu, Sci. Rep., 2016, 6, 1–9 CrossRef PubMed.
- J. A. Perez-Taborda, O. Caballero-Calero, L. Vera-Londono, F. Briones and M. Martin-Gonzalez, Adv. Energy Mater., 2018, 8, 1702024 CrossRef.
- C. Dun, C. A. Hewitt, Q. Li, J. Xu, D. C. Schall, H. Lee, Q. Jiang and D. L. Carroll, Adv. Mater., 2017, 29, 1700070 CrossRef PubMed.
- Y. Zhou, H. Wu, D. Wang, L. Fu, Y. Zhang, J. He, S. J. Pennycook and L. D. Zhao, Mater. Today Phys., 2018, 7, 77–88 CrossRef.
- C. Jiang, Y. Ding, K. Cai, L. Tong, Y. Lu, W. Zhao and P. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 9646–9655 CrossRef CAS PubMed.
- J. Gao, L. Miao, H. Lai, S. Zhu, Y. Peng, X. Wang, K. Koumoto and H. Cai, iScience, 2020, 23, 100753 CrossRef CAS PubMed.
- Z. Lin, C. Hollar, J. S. Kang, A. Yin, Y. Wang, H. Y. Shiu, Y. Huang, Y. Hu, Y. Zhang and X. Duan, Adv. Mater., 2017, 29, 1606662 CrossRef PubMed.
- J. Yun, K. Cho, Y. Park, S. Yang, J. Choi and S. Kim, Nano Res., 2017, 10, 683–689 CrossRef CAS.
- N. A. Rongione, M. Li, H. Wu, H. D. Nguyen, J. S. Kang, B. Ouyang, H. Xia and Y. Hu, Adv. Electron. Mater., 2019, 5, 1800774 CrossRef.
- P. F. Taylor and C. Wood, J. Appl. Phys., 1961, 32, 1–3 CrossRef CAS.
- S. W. Finefrock, X. Zhu, Y. Sun and Y. Wu, Nanoscale, 2015, 7, 5598–5602 RSC.
- J. Gao, L. Miao, C. Liu, X. Wang, Y. Peng, X. Wei, J. Zhou, Y. Chen, R. Hashimoto, T. Asaka and K. Koumoto, J. Mater. Chem. A, 2017, 5, 24740–24748 RSC.
- X. Zeng, L. Ren, J. Xie, D. Mao, M. Wang, X. Zeng, G. Du, R. Sun, J. B. Xu and C. P. Wong, ACS Appl. Mater. Interfaces, 2019, 11, 37892–37900 CrossRef CAS PubMed.
- Z. Wu, E. Mu, Z. Che, Y. Liu, F. Sun, X. Wang and Z. Hu, J. Alloys Compd., 2020, 828, 154239 CrossRef CAS.
- Q. Jin, W. Shi, Y. Zhao, J. Qiao, J. Qiu, C. Sun, H. Lei, K. Tai and X. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 1743–1751 CrossRef CAS PubMed.
- B. G. Kim, C. H. Lim, S. M. Choi, W. S. Seo, H. L. Lee, S. H. Hyun and S. M. Jeong, CrystEngComm, 2015, 17, 7522–7527 RSC.
- M. Kashiwagi, S. Hirata, K. Harada, Y. Zheng, K. Miyazaki, M. Yahiro and C. Adachi, Appl. Phys. Lett., 2011, 98, 023114 CrossRef.
- S. J. Kim, H. Choi, Y. Kim, J. H. We, J. S. Shin, H. E. Lee, M. W. Oh, K. J. Lee and B. J. Cho, Nano Energy, 2017, 31, 258–263 CrossRef CAS.
- J. Lee, H. Kim, L. Chen, S. H. Choi and V. K. Varadan, Nanosyst. Eng. Med., 2012, 8548, 85481C Search PubMed.
- J. Lee, H. J. Kim, L. Chen, S. H. Choi, G. N. Mathur and V. K. Varadan, Nanosensors, Biosensors, Info-Tech Sensors Syst., 2013, vol. 8691, p. 86910R.
- L. X. Liang, Y. Deng, Y. Wang, H. L. Gao and J. Cui, J. Nanopart. Res., 2014, 16, 1–7 CAS.
- T. T. Shen and C. N. Liao, Mater. Lett., 2017, 186, 314–317 CrossRef CAS.
- E. Koukharenko, S. A. Boden, N. P. Sessions, N. Frety, I. Nandhakumar and N. M. White, J. Mater. Sci.: Mater. Electron., 2018, 29, 3423–3436 CrossRef CAS.
- S. H. Kim, T. Min, J. W. Choi, S. H. Baek, J. P. Choi and C. Aranas, Energy, 2018, 144, 607–618 CrossRef CAS.
- R. Feng, F. Tang, N. Zhang and X. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 38616–38624 CrossRef CAS PubMed.
- A. Datta, A. Sangle, N. Hardingham, C. Cooper, M. Kraan, D. Ritchie, V. Narayan and S. Kar-Narayan, Materials, 2017, 10, 553 CrossRef PubMed.
- E. B. Secor, T. Z. Gao, M. H. Dos Santos, S. G. Wallace, K. W. Putz and M. C. Hersam, ACS Appl. Mater. Interfaces, 2017, 9, 29418–29423 CrossRef CAS PubMed.
- E. B. Secor, B. Y. Ahn, T. Z. Gao, J. A. Lewis and M. C. Hersam, Adv. Mater., 2015, 27, 6683–6688 CrossRef CAS PubMed.
- R. Danaei, T. Varghese, M. Ahmadzadeh, J. McCloy, C. Hollar, M. Sadeq Saleh, J. Park, Y. Zhang and R. Panat, Adv. Eng. Mater., 2018, 21, 1800800 CrossRef.
- M. Saeidi-Javash, W. Kuang, C. Dun and Y. Zhang, Adv. Funct. Mater., 2019, 29, 1901930 CrossRef.
- P. Gao, L. Chen, B. Wu, Q. Zhang, C. Xue, C. Hou and Q. Sun, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2019, 34, 781–786 CrossRef CAS.
- B. J. Last and D. J. Thouless, Phys. Rev. Lett., 1971, 27, 1719–1721 CrossRef CAS.
- K. Kato, Y. Hatasako, M. Kashiwagi, H. Hagino, C. Adachi and K. Miyazaki, J. Electron. Mater., 2014, 43, 1733–1739 CrossRef CAS.
- K. Kato, Y. Hatasako, M. Uchino, Y. Nakata, Y. Suzuki, T. Hayakawa, C. Adachi and K. Miyazaki, Adv. Mater. Interfaces, 2014, 1, 1300015 CrossRef.
- T. Varghese, C. Dun, N. Kempf, M. Saeidi-Javash, C. Karthik, J. Richardson, C. Hollar, D. Estrada and Y. Zhang, Adv. Funct. Mater., 2020, 30, 1905796 CrossRef CAS.
- G. Delaizir, J. Monnier, M. Soulier, R. Grodzki, B. Villeroy, J. Testard, J. Simon, C. Navone and C. Godart, Sens. Actuators, A, 2012, 174, 115–122 CrossRef CAS.
- Z. Lu, M. Layani, X. Zhao, L. P. Tan, T. Sun, S. Fan, Q. Yan, S. Magdassi and H. H. Hng, Small, 2014, 10, 3551–3554 CrossRef CAS PubMed.
- W. Zhu, Y. Deng, M. Gao and Y. Wang, Energy Convers. Manage., 2015, 106, 1192–1200 CrossRef CAS.
- X. Zhao, W. Han, Y. Jiang, C. Zhao, X. Ji, F. Kong, W. Xu and X. Zhang, Nanoscale, 2019, 11, 17725–17735 RSC.
- J. Luo, Z. Cao, M. Yuan and X. Chou, Results Phys., 2019, 12, 1304–1310 CrossRef.
- B. Chen, M. Kruse, B. Xu, R. Tutika, W. Zheng, M. D. Bartlett, Y. Wu and J. C. Claussen, Nanoscale, 2019, 11, 5222–5230 RSC.
- J. Li, X. Tang, Y. Liu, Z. Yuan, Z. Xu and K. Liu, Energy Technol., 2019, 7, 1800707 CrossRef.
- S. Kumar, H. H. Singh and N. Khare, Energy Convers. Manage., 2019, 198, 111783 CrossRef CAS.
- R. Feng, F. Tang, N. Zhang and X. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 38616–38624 CrossRef CAS PubMed.
- T. Zhang, K. Li, J. Zhang, M. Chen, Z. Wang, S. Ma, N. Zhang and L. Wei, Nano Energy, 2017, 41, 35–42 CrossRef CAS.
- J. Na, Y. Kim, T. Park, C. Park and E. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 32392–32400 CrossRef CAS PubMed.
- E. J. Bae, Y. H. Kang, K. S. Jang, C. Lee and S. Y. Cho, Nanoscale, 2016, 8, 10885–10890 RSC.
- Y. Du, X. Liu, J. Xu and S. Z. Shen, Mater. Chem. Front., 2019, 3, 1328–1334 RSC.
- Y.-C. Li and Z.-H. Lin, ECS Trans., 2016, 72, 67–71 CrossRef CAS.
- Y.-T. Jao, Y.-C. Li, Y. Xie and Z.-H. Lin, ECS J. Solid State Sci. Technol., 2017, 6, N3055–N3057 CrossRef CAS.
- Q. Xu, S. Qu, C. Ming, P. Qiu, Q. Yao, C. Zhu, T.-R. Wei, J. He, X. Shi and L. Chen, Energy Environ. Sci., 2020, 13, 511–518 RSC.
- H. Ju, D. Park, K. Kim and J. Kim, J. Alloys Compd., 2019, 792, 638–643 CrossRef CAS.
- Y. Du, K. F. Cai, S. Chen, P. Cizek and T. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 5735–5743 CrossRef CAS.
- Y. Lu, Y. Qiu, Q. Jiang, K. Cai, Y. Du, H. Song, M. Gao, C. Huang, J. He and D. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 42310–42319 CrossRef CAS.
- Y. Lu, Y. Ding, Y. Qiu, K. Cai, Q. Yao, H. Song, L. Tong, J. He and L. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 12819–12829 CrossRef CAS PubMed.
- Y. Lu, Y. Qiu, K. Cai, X. Li, M. Gao, C. Jiang and J. He, Mater. Today Phys., 2020, 14, 100223 CrossRef.
- Q. Meng, Y. Qiu, K. Cai, Y. Ding, M. Wang, H. Pu, Q. Yao, L. Chen and J. He, ACS Appl. Mater. Interfaces, 2019, 11, 33254–33262 CrossRef CAS PubMed.
- C. Dun, C. A. Hewitt, H. Huang, J. Xu, D. S. Montgomery, W. Nie, Q. Jiang and D. L. Carroll, ACS Appl. Mater. Interfaces, 2015, 7, 7054–7059 CrossRef CAS PubMed.
- C. Dun, C. A. Hewitt, H. Huang, J. Xu, C. Zhou, W. Huang, Y. Cui, W. Zhou, Q. Jiang and D. L. Carroll, Nano Energy, 2015, 18, 306–314 CrossRef CAS.
- J. Liang, T. Wang, P. Qiu, S. Yang, C. Ming, H. Chen, Q. Song, K. Zhao, T. R. Wei, D. Ren, Y. Y. Sun, X. Shi, J. He and L. Chen, Energy Environ. Sci., 2019, 12, 2983–2990 RSC.
- X. Shi, H. Chen, F. Hao, R. Liu, T. Wang, P. Qiu, U. Burkhardt, Y. Grin and L. Chen, Nat. Mater., 2018, 17, 421–426 CrossRef CAS.
- T. R. Wei, M. Jin, Y. Wang, H. Chen, Z. Gao, K. Zhao, P. Qiu, Z. Shan, J. Jiang, R. Li, L. Chen, J. He and X. Shi, Science, 2020, 369, 542–545 CrossRef CAS PubMed.
- Q. Jin, S. Jiang, Y. Zhao, D. Wang, J. Qiu, D. M. Tang, J. Tan, D. M. Sun, P. X. Hou, X. Q. Chen, K. Tai, N. Gao, C. Liu, H. M. Cheng and X. Jiang, Nat. Mater., 2019, 18, 62–68 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |