Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Methylene- and thioether-linked cyanobiphenyl-based liquid crystal dimers CBnSCB exhibiting room temperature twist-bend nematic phases and glasses†
Yuki
Arakawa
 *,
Kenta
Komatsu
,
Takuma
Shiba
and
Hideto
Tsuji
*,
Kenta
Komatsu
,
Takuma
Shiba
and
Hideto
Tsuji

Department of Applied Chemistry and Life Science, Graduate School of Engineering, Toyohashi University of Technology, 1-1 Hibarigaoka, Tempaku-cho, Toyohashi, Aichi 441-8580, Japan. E-mail: arakawa@tut.jp
First published on 15th February 2021
Abstract
The twist-bend nematic (NTB) phase is a new heliconical liquid crystal (LC) phase that is associated with spontaneous symmetry breaking for achiral bent LC molecules. Herein, we demonstrate a homologous series of LC dimers exhibiting the stable NTB phases, which undergo vitrification to give NTB glass (NTBG) phases upon cooling below room temperature. Methylene- and thioether-linked cyanobiphenyl-based LC dimer homologs, i.e., CBnSCB (carbon number of the central oligomethylene spacer, n = 2, 4, 6, 8, and 10, for a bent molecular geometry) were developed for the first time. The phase-transition behavior and phase characteristics of CBnSCB were investigated by polarized light optical microscopy (POM), differential scanning calorimetry (DSC), and X-ray diffractometry (XRD). All CBnSCB homologs were found to be mesogenic, wherein CBnSCB (n = 4, 6, 8, and 10) exhibited NTB phases below the temperatures of conventional nematic (N) phases. Additionally, CB2SCB, which possesses the shortest spacer, showed a kinetically induced, monotropic mesophase that was formed directly from the isotropic (I) phase with a strong first order phase-transition property. Although POM observations of CB2SCB revealed the presence of optical textures similar to modulated N or pseudo-layered NTB phases, the XRD results indicated its apparently non-layered and liquid-like nature. In addition, the mesophase of CB2SCB was assumed to be miscible with the NTB phase of CB4SCB. It was therefore suggested that CB2SCB exhibited a NTB phase formed directly from the I phase. It is noteworthy that the NTB phases of CBnSCB (n = 2, 4, 6, and 8) underwent vitrification upon supercooling below room temperature, giving the NTBG phases. In particular, CBnSCB (n = 6 and 8) exhibited remarkably stable NTB and N phases, undergoing no crystallization upon re-heating from the NTBG phase up to the I phase. Furthermore, we investigated the phase-transition behaviors of previously reported analogs, i.e., bis(thioether)-linked CBSnSCB and ether- and thioether-linked CBOnSCB LC dimers (odd numbered n = 3, 5, 7, 9, and 11, for a bent geometry) using POM, and observed NTB phase formation from the small supercooled N domains of CBS11SCB, CBO3SCB, and CBO11SCB. It was therefore indicated that all reported bent CBSnSCB and CBOnSCB dimers (n = 3, 5, 7, 9, and 11) form the NTB phase. It was found that the CBnSCB homologs exhibited remarkably stable NTB phases and vitrifiable tendencies compared with the previously reported CBSnSCB and CBOnSCB analogs.
Introduction
Liquid crystals (LCs) are fascinating mesophases between solid crystals and isotropic liquids, which inherently possess both anisotropic and fluidic physical properties. Such unique features have contributed to the development of current spread LC display technologies. Usually, molecules that produce thermotropic LCs are composed of rigid aromatic and aliphatic rings and flexible alkyl chains, which promote the microphase separation of each part to form mesophases.In addition, the so-called LC dimers (or bimesogens), which are typically based on two rigid mesogenic structures linked by a flexible oligomethylene spacer, have also been actively researched. The molecular geometries of LC dimers are strongly influenced by the parity of the carbon or total atom numbers in the central spacers along the chains, giving a stretched Z-like (or an approximately linear) shape for even spacers, and a bent shape for odd spacers.1–5 This parity effect additionally bestows the LC dimers affluent mesomorphism6–9 and clear odd–even effects on physical properties.10,11
Furthermore, an emerging heliconical nematic (N) phase, the twist-bend nematic (NTB) phase, has offered a new insight into the diverse mesomorphism observed in LC dimers. Initially, the NTB phase was independently predicted by Meyer12 and Dozov,13 and was supported by a simulation by Memmer.14 Later, in-depth investigation of a few LC dimers including cyanobiphenyl (CB) dimers consisting of an odd carbon atom number on the oligomethylene spacer (n), i.e., CBnCB, which exhibits a unknown nematic (NX) phase below the temperature of the conventional N phase, was conducted,15–18 and the NTB phase was identified with respect to the NX phase.15 In addition, several bent molecules exhibiting such NX phases, which are also reminiscent of NTB phases,7,8,19–22 have been reported. Nevertheless, the detailed structures of the NTB phases are still under debate.23–26
The representative features of the NTB phase include the spontaneous formation of right- and left-handed helical nanostructured domains formed by achiral molecules, and much shorter helical pitches of ∼10 nm27–32 compared with those of helicoidal chiral N (N*) or cholesteric (Ch) phases, which usually have pitch ranges over hundreds of nanometers (see Fig. 1). In achiral molecular systems, both chiral domains are equivalently formed and are considered to be macroscopically achiral. In contrast, a macroscopic chirality was detected in the NTB phase of CB7CB by using circular dichroism spectroscopy.33 Scanning electron microscopy observations of the photo-crosslinked samples of the NTB phase revealed a helical organization of the nanostructures.34–36 In addition to the NTB phase, other structurally associated mesophases, such as heliconical smectic (Sm) phases based on bent-core molecules37,38 and bent LC dimers,39,40 the twist grain-boundary-twist-bend nematic phase,41 and the splay-bend N (NSB) phase electrically induced from the NTB phase42–44 have been experimentally verified. Furthermore, the splay N (NS) phase with the modulation rotated 90° with respect to the NSB phase was discovered in some rod-like mesogens.45,46
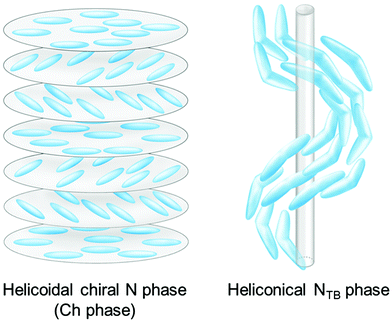 | ||
| Fig. 1 Schematic models of the helicoidal chiral nematic (N*) or cholesteric (Ch) phase and the heliconical twist-bend nematic (NTB) phases. Reproduced from ref. 67 and 72 with permission. | ||
The NTB phase is usually observed for bent LC dimers consisting of flexible spacers with an odd number of atoms. To date, a wide variety of bent LC dimers,47–72 oligomers,64,73–77 polymers,78 and bent-core molecules79,80 have been found to exhibit the NTB phase. In addition, NTB materials have been used for diverse applications such as photonic and optical devices,81–84 gels,85 elastic bodies,86 and photoalignment technologies.87
For characterization and application purposes, it is necessary to develop materials that exhibit the NTB phase over a wide temperature range, the room-temperature NTB phase, and its glass (G) phase or NTB glass (NTBG). Using mixtures with other mesogens is a useful method to lower the N–NTB phase-transition temperature,18 ultimately achieving a metastable room temperature NTB phase88 and its photocrosslinked state.35 However, the formation of room-temperature NTB materials from their single-component systems indeed remains a rarity.50,61,64,67,68,70,72 This has restricted deep structural analyses and examination of the physical properties of the NTB phases, in addition to the development of new applications. Therefore, novel LC dimers that can maintain NTB phases at room temperature and produce the corresponding glasses are in high demand.
On the other hand, in our previous work, we revealed that thioether-linked LC dimers have a beneficial effect in the realization of the NTB phase over a wide temperature range and the room-temperature NTB and NTBG phases.61,64,67,68,70 The asymmetrical methylene- and thioether-linked CB-based dimers bearing ethylene or hexamethylene spacers, namely CB2SCB and CB6SCB, respectively, was also reported previously.64 The latter exhibited an NTB phase, which gave the NTBG phase upon supercooling to room temperature. However, its homologous series with other oligomethylene spacers has yet to be developed. Such systems are expected to be candidates for LC materials that exhibit room temperature NTB and NTBG phases.
Herein we demonstrate LC dimer homologs that exhibit the room-temperature NTB and NTBG phases. A homologous series of asymmetrically methylene- and thioether-linked CB-based LC dimers with oligomethylene spacers containing different numbers of carbon atoms (n), i.e., CBnSCB, is established for the first time (Scheme 1). In this system, even numbers of carbon atoms were selected for the oligomethylene spacers, i.e., n = 2, 4, 6, 8, and 10, to give odd total numbers of atoms in the spacer n and the sulfur linkage (S) along the linear chain to ensure bent molecular geometries. In the present study, the previously reported CBnSCB homologs (n = 2 and 6) were also re-prepared and reinvestigated. The phase-transition behaviors, mesomorphism, and mesophase structures are evaluated using polarized light optical microscopy (POM), differential scanning calorimetry (DSC), and X-ray diffractometry (XRD). In addition, the phase-transition behaviors of the previously reported bent analogs, namely bis(thioether)-linked CBSnSCB and ether- and thioether-linked CBOnSCB dimers (n = 3, 5, 7, 9, and 11, see Scheme 1), were also re-evaluated using POM. Moreover, the phase transitions of CBnSCB, CBSnSCB, and CBOnSCB are comprehensively described.
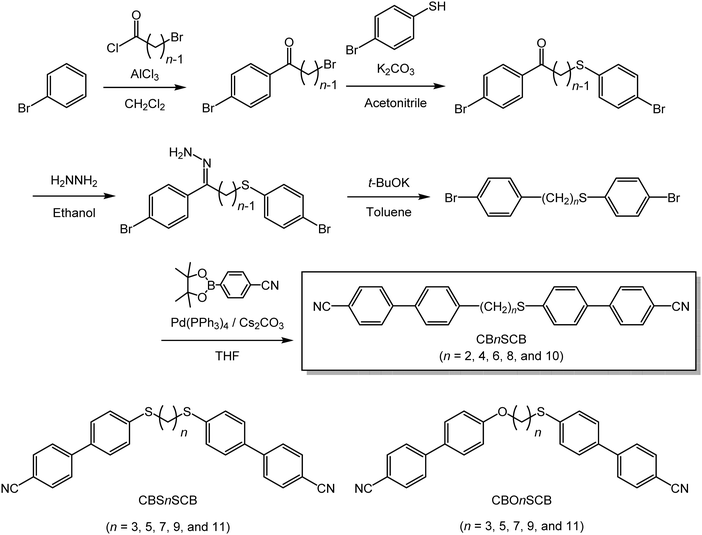 | ||
| Scheme 1 Synthetic pathway to CBnSCB (n = 2, 4, 6, 8, and 10) and molecular structures of the previously reported CBSnSCB and CBOnSCB analogs (n = 3, 5, 7, 9, and 11). | ||
Experimental
The CBnSCB series was synthesized according to the route outlined in Scheme 1. The corresponding procedure for CB4SCB is described in the ESI.† The other homologs were synthesized by similar procedures, and their characterization data are also shown in the ESI.† The molecular structures were confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy using JNM-ECS400 (400 MHz for 1H and 100 MHz for 13C) and JNM-ECX500 spectrometers (500 MHz for 1H and 126 MHz for 13C) (JEOL Ltd, Tokyo, Japan). Phase identification was carried out by POM observations using an Olympus polarized optical microscope (BX50, Tokyo, Japan) equipped with a Linkam temperature controller (LK-600PM, Surrey, UK). The phase-transition temperatures and the associated entropy changes (ΔS) were determined by DSC using a Shimadzu DSC 60 instrument at a heating and cooling rate of 3, 10, or 30 °C min−1 under a flow of nitrogen gas (rate = 50 mL min−1). The POM images and DSC curves of the dimers are shown either in the main text or in the ESI.† Powder XRD measurements for the mesophase and cold crystallization samples of CB2SCB were performed using a Rigaku Rint 2500 instrument with a Cu Kα X-ray source. The mesophase and cold crystallization samples were prepared by rapidly cooling to 25 °C from the I phase at 140 °C and by heating from the mesophase at 25 °C, respectively. The XRD measurements for both samples were conducted on a silicon substrate at 25 °C. Temperature-variable XRD measurements for the crystal and mesophases of CB4SCB were conducted using a Bruker D8 DISCOVER diffractometer equipped with a Vantec-500 detector. Cu Kα was used as the X-ray radiation source. CB4SCB powder was added and melted in a capillary glass tube of 1.5 mm diameter (WJM-Glass Müller GmbH). The capillary specimen was sandwiched between permanent magnets (B = 300 mT) in a folder. The capillary samples were set on a Bruker TCPU-P stage to control the sample temperature during X-ray irradiation. Following heating of the specimen to the isotropic (I) phase to remove its thermal history, the measurements were carried out at different mesophase temperatures. Binary mixtures of the CBnSCB homologs (n = 2 and 4) were prepared according to the literature.89 After dissolving the weighed dimers in dichloromethane to obtain a solution with a concentration of 10 mg mL−1, the solvent was slowly evaporated under reduced pressure. The mixed samples, which had been dried sufficiently under reduced pressure, were heated to 150 °C (to give the I phase), and then cooled to ambient temperature to produce homogeneous mixtures. Subsequently, the phase transitions of the mixtures upon cooling from the I phases were investigated by POM at a cooling rate of 3 °C min−1.Results and discussion
The thermal phase sequences and the phase-transition temperatures at the crystal (Cr) phase to the N or I phases (melting points, Tm) and the N to I phases upon first heating, and at the I to N phases (TIN), the N to NTB phases (TNNTB), the glass transition (Tg), and the crystallization (TCr) upon cooling of CBnSCB are tabulated in Table 1. In addition, the phase diagram of CBnSCB as a function of n is shown in Fig. 2. Initially, we discuss the results mainly for CBnSCB (n = 4, 6, 8, and 10), and then move on to discuss the phase characterization of the shortest CB2SCB.| Code | n | Phase | T (°C) | ΔS/R | Phase | T (°C) | ΔS/R | Phase | T (°C) | ΔS/R | Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Re-evaluated in the present study. b The ΔS/R value is taken at a rate of 30 °C min−1 to prevent crystallization. | |||||||||||
| CBnSCB | 2a | Cr | 131.0 | 7.2 | I | ||||||
| Cr | NTB | 42.5 | 0.55b | I | |||||||
| 4 | Cr | 126.2 | 7.9 | I | |||||||
| G | 13.1 | NTB | 70.3 | 0.23 | N | 86.8 | 0.14 | I | |||
| 6a | Cr | 99.0 | 10.3 | N | 113.2 | 0.34 | I | ||||
| G | 10.4 | NTB | 89.6 | 0.25 | N | 110.7 | 0.37 | I | |||
| 8 | Cr | 92.6 | 12.4 | N | 119.8 | 0.48 | I | ||||
| G | 10.9 | NTB | 93.9 | N | 117.8 | 0.49 | I | ||||
| 10 | Cr | 103.4 | 12.1 | N | 119.0 | 0.69 | I | ||||
| Cr | 86.5 | 11.4 | NTB | 95.5 | N | 117.8 | 0.69 | I | |||
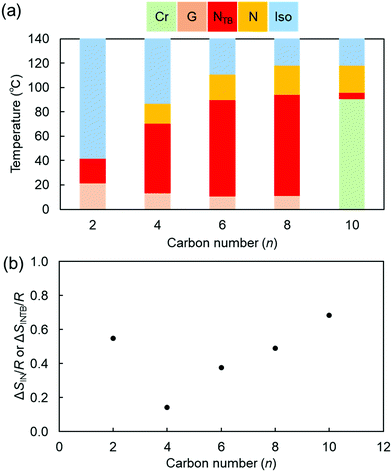 | ||
| Fig. 2 (a) Phase diagram and (b) ΔSIN/R (or ΔSINTB/R for CB2SCB) as a function of n upon the cooling of CBnSCB. | ||
Upon first heating, CBnSCB (n = 6, 8, and 10) exhibited an N phase, whereas CBnSCB (n = 2 and 4) showed only one Tm peak corresponding to the Cr to I phase transition (Fig. S1–S5 in the ESI†). It is clear that a relatively long spacer is a prerequisite to form the N phase. This trend could be associated with molecular geometrical biaxiality and anisotropy, which is enhanced for LC dimers with short and long spacers, respectively. In other words, in comparison with short CBnSCB (n = 2 and 4) exhibiting a more bent or biaxial geometry, intermediate and long CBnSCB dimers (n = 6, 8, and 10) with a more anisotropic geometry are advantageous in that they form LC phases upon heating.
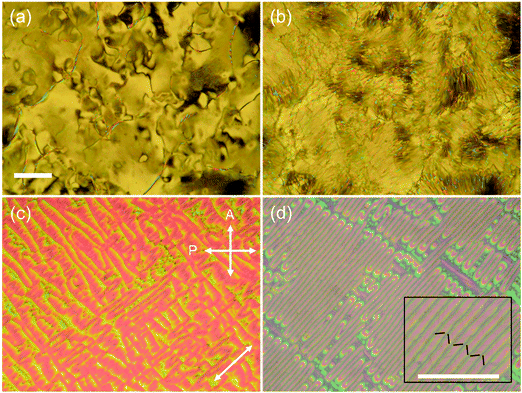
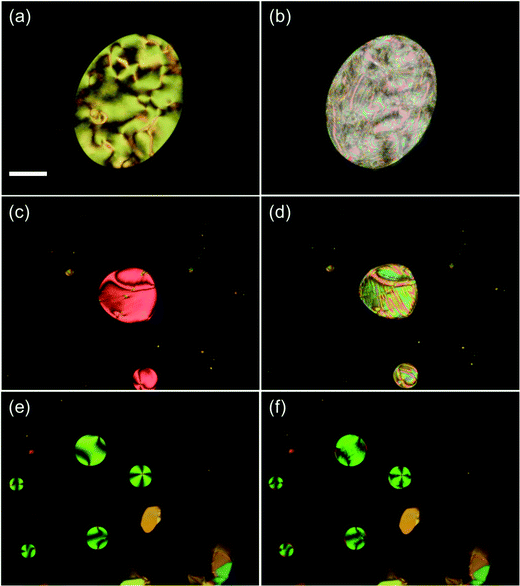
Upon cooling, CBnSCB (n = 4, 6, 8, and 10) clearly exhibited the NTB phases below the N phases, which follows the usual thermal phase sequence of NTB–N–I; this is not the case for CB2SCB, as discussed later. Phase identification was achieved by POM observations and XRD measurements. In the non-treated glass cells, the marble and schlieren N textures of CBnSCB (n = 4, 6, 8, and 10) transformed into blocky, focal conic, polygonal, and striped textures below the N phases [Fig. 3 and Fig. S6, S7, ESI†]. Such optical textures are typical of the pseudo-layered NTB phase. In addition, the POM images of CB4SCB and CB8SCB obtained using a uniaxially rubbed polyimide-surface cell for planar alignment displayed a striped texture below the N phase [Fig. 3(c) and (d), respectively], which was similar to that previously reported for CB6SCB,64 further supporting evidence of the NTB phase. This striped texture is assumed to arise from the undulation of the pseudo layers based on the Helfrich-Hurault buckling instability.28,36 The helical axes and pseudo layers orient parallel and perpendicular to the rubbing (or stripe) direction, respectively. As shown in the magnified image of CB8SCB in the inset of Fig. 3(d), the diagonal line textures represented by zig-zag black solid lines in each stripe are oriented in the same direction in alternate stripes.
Temperature-variable XRD measurements for the N and NTB phases of CB4SCB were also conducted, for which the obtained one-dimensional (1D) XRD profiles are shown in Fig. S8 (ESI†). It was found that the XRD profiles in the NTB phase of CB4SCB at 30 °C did not show any peaks in the small-angle region, and were similar to those of the N phase at 85 °C. The observed broad wide-angle diffraction (2θ = 20.8°, which corresponds to d-spacing = 4.27 Å) in the NTB phase at 30 °C suggests their fluid liquid-like correlation to the molecular transverse direction. The XRD results therefore support the NTB phase, and indicate that the Sm-like optical textures based on POM could be attributable to the presence of pseudo-layered structures rather than unambiguous layered Sm phases. In addition to the observed POM textures, the fact that the 1D-XRD profile of the NTB phase at 30 °C does not contain any discernible crystallization peaks confirms that the NTB phase stably cooled to room temperature without crystallization, to provide the NTBG phase. When the sample was allowed to stand for 1 week, the XRD pattern displayed several crystalline peaks. The d-spacing of a main small-angle peak at 2θ = 7.32° corresponded to 12.1 Å, which is approximately half of the molecular length, indicating the so-called intercalated structures of the dimers. Furthermore, a very minor small-angle peak was observed at 2θ = 3.69°, and its d-spacing was 23.9 Å, which is close to the full molecular length.
The DSC curves of second heating and first cooling of CBnSCB (n = 2, 4, 6, and 8) are shown in Fig. 4. Interestingly, the NTB phases of CBnSCB (n = 4, 6, and 8) were supercooled to room temperature without any discernible crystallization peaks, and these samples vitrified to yield the NTBG phase upon cooling [Fig. 4(b)–(d)]. The mesophase of CB2SB was also vitrified upon cooling to room temperature [Fig. 4(a)]. In the present study, DSC measurements were re-performed for CBnSCB (n = 2 and 6), and compared with those reported in our previous report.64 The present batch of CB6SCB did not show any crystallization upon cooling to 0 °C. This is different from the results for the previous batch, which exhibited crystallization upon cooling and subsequent heating. The NTB phase of CB10SCB underwent crystallization upon cooling [Fig. S5, ESI†]. The mesophases of CB4SCB were crystallized under similar second heating courses, as shown in Fig. 4(b). However, it is noteworthy that upon second heating from the NTBG phase at 0 °C after cooling from the I phase, the NTB and N phases of CBnSCB (n = 6 and 8) did not undergo cold crystallization, retaining the mesophases up to the I phase transition [Fig. 4(c) and (d)]. Such a prominent NTB and N phase stability observed for CBnSCB (n = 6 and 8) is similar to that of thioether-linked naphthalene-based LC dimers, which were reported in our previous work.67 In addition, to verify the stability of the NTB and NTBG phases of CBnSCB (n = 4, 6, and 8), their DSC samples were rapidly cooled from their I phases to their corresponding NTBG phases, and allowed to stand at room temperature for 3 d. Subsequently, they were re-evaluated upon heating from 0 °C to the temperatures of their I phases. As can be seen in Fig. S2–S4 (ESI†), the obtained DSC curves of CBnSCB (n = 4 and 6) revealed that glass transition and cold crystallization took place from the LC phases (NTB or N) to the Cr phases, indicating that the NTB or NTBG phases could be maintained for 3 d. To our surprise, CB8SCB exhibited no discernible cold crystallization peak, but did undergo an NTB–N phase transition at 95.8 °C and an N–I phase transition at 119.3 °C with a small enthalpy change of 1.69 kJ mol−1, highlighting the prominent stability of the NTB and/or NTBG phases of CB8SCB.
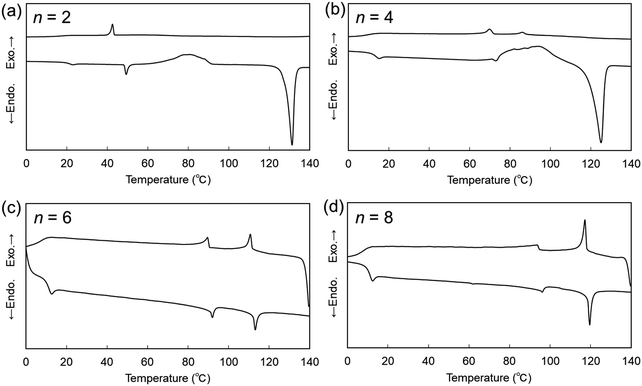 | ||
| Fig. 4 DSC curves of (a) CB2SCB (b) CB4SCB, (c) CB6SCB, and (d) CB8SCB upon second heating and first cooling at a rate of 10 °C min−1. | ||
It is known that the N–NTB phase transition depends on the DSC scanning rate, and the phase-transition behavior gradually changes from first-order-like to second-order-like upon increasing the rate. For example, at a relatively high rate, such as the 10 °C min−1 used in the present study, second-order-like transitions were observed in the LC dimers.61,67,68,70,71,92 In addition, in a few cases of the LC dimers homologs, it was reported that the DSC peak on the N–NTB phase transition alters from second-order-like to first-order-like with decreasing the spacer chain length,57,61,70,71 which could be associated with a larger structural difference between the N and NTB phases for shorter spacer homologs than for the longer counterparts, implying the molecular rigidity and packing manner in the NTB phases for shorter spacer LC dimers.70 In the present study, the N–NTB transitions of CBnSCB gradually transformed from second-order-like to first-order-like peak shapes with decreasing values of n (from 10 to 4), as shown in (Fig. 4 and Fig. S5, ESI†). It is noteworthy that CBnSCB (n = 4 and 6) exhibited first-order-like peaks with ΔSNNTB/R values of 0.23 and 0.25, respectively, even though the DSC scanning rate was 10 °C min−1. In particular, the ΔSNNTB/R of 0.23 for CB4SCB is larger than the corresponding ΔSIN/R, i.e., 0.14. Such phase transitions also indicate the largely different structures between the N and NTB phases (especially in the presence of unique characteristics in the NTB phase).70 These results may imply that this trend could eventually lead to a strong first order property with a ΔS/R of 0.55 on the phase-transition peak from the I phase to the mesophase of CB2SCB, as discussed later. As shown in Fig. 2(b), the ΔSIN/R value decreases with decreasing n (from 10 to 4). This behavior is associated with the enhanced bent or biaxial molecular geometries resulting from reduced spacer lengths.
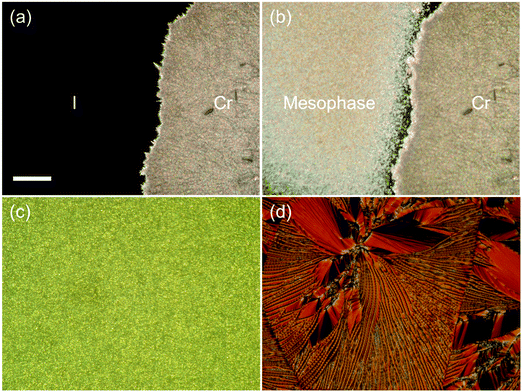
We then moved on to examine the phase behavior and characterization of the shortest CB2SCB homolog. CB2SCB shows unique phase-transition behavior depending on the thermal treatment conditions outlined in our previous study.64 The DSC curves of CB2SCB upon first heating and cooling are shown in Fig. S1 (ESI†) and Fig. 5, respectively. It can be seen that CB2SCB exhibited one Tm at ∼131 °C, which corresponded to the Cr–I phase transition upon the first heating of the pristine solution-crystallized sample (Fig. S1, ESI†).64 Upon cooling at a high rate, i.e., 10 or 30 °C min−1, the exothermic peak at ∼43 °C with a first order transition property was observed by DSC [Fig. 4(a) and 5]. On the other hand, at a low cooling rate (i.e., 3 °C min−1), a broadened crystallization peak was also observed between ∼85 and 50 °C (Fig. 5). In this study, we further reinvestigated the phase-transition behavior of CB2SCB and found that the former peak at ∼43 °C corresponds to a transition from the I phase to a kind of mesophase that was not the Cr phase; thus, CB2SCB is mesogenic. This phase, which was observed as a turbid liquid, was a highly viscous, which was confirmed by pressing and sliding.
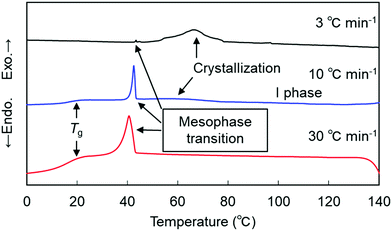 | ||
| Fig. 5 DSC curves of CB2SCB upon cooling at different rates, which were re-evaluated in the present study. | ||
POM observations of CB2SCB revealed birefringent domains gradually growing in a dark texture of the I phase [Fig. 6(a)] upon cooling at a relatively low rate of 3 °C min−1 from the high temperature I phase over 100 °C, which were attributed to partial crystallization and corresponded to the above-mentioned broadening peak at ∼85–50 °C in the DSC curve (Fig. 5). During the slow growth of these partial Cr domains, the remaining I phase domain (or dark region) transitioned to the mesophase at ∼43 °C [Fig. 6(b)]. Fig. 6(c) shows the birefringent texture that emerged at ∼43 °C during cooling from the I phase at a rate of 10 °C min−1. However, the individual domain observed in the optical texture by POM is particularly small due to its highly viscoelastic nature, which does not allow us to identify the type of mesophase. However, it is interesting to note that during holding at temperature in the vicinity of the I-to-mesophase phase transition after cooling to 50 °C at a high cooling rate over 30 °C min−1 to kinetically prevent the gradual crystallization at ∼85–50 °C in the I phase, discernible rope-like or striped, fan-shaped-like, and polygonal-like textures were gradually formed from circular domains [Fig. 6(d)]. This result is reminiscent of the presence of a modulated N phase and similar to the pseudo layered NTB phase. Although we attempted to observe the mesophase of CB2SCB via POM using a uniaxially rubbed polyimide-surface glass cell for planar alignment, only the small-domain optical texture similar to that seen in Fig. 6(c) was observed, and the alignment could not be achieved.
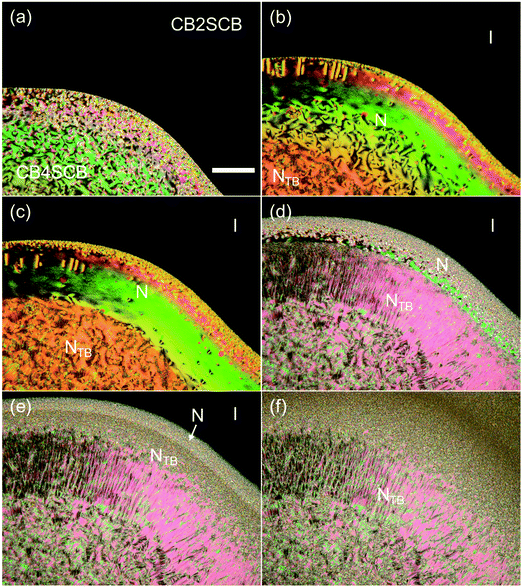
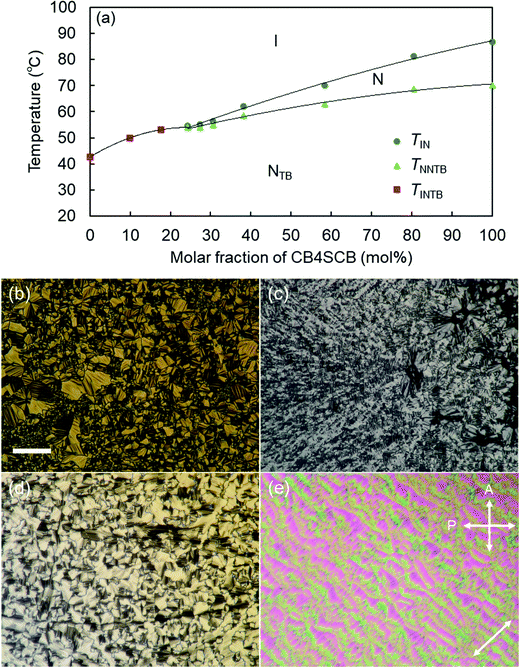
Subsequently, we employed POM to examine the miscibility of CB2SCB blended with CB4SCB as a reference sample for their contact preparation and binary mixtures. The CB4SCB analog forms the NTB phase and is structurally similar to CB2SCB; therefore, it is assumed that the possibility of their phase separation being attributed to a difference in molecular structures is prevented. The POM images of their contact preparation are shown in Fig. 7. As shown in Fig. 7(a), the N phase texture (bottom left) and the I phase region (top right) correspond to the CB4SCB-rich region and the CB2SCB-rich region, respectively. The N phase texture in the CB4SCB-rich region transformed to the blocky texture of the NTB phase [Fig. 7(b)]. In the intermediate regions between the CB4SCB-rich and CB2SCB-rich regions in Fig. 7(b)–(e), the N phase texture gradually formed from the CB4SCB-rich to the CB2SCB-rich regions, and subsequently, the N phase gradationally transitioned to the NTB phase, ultimately resulting in the appearance of an ambiguous texture in the CB2SCB-rich region, similar to the mesophase of CB2SCB, as shown in Fig. 7(e) and (f). These gradual changes in the POM textures could be ascribed to the concentration gradient between CB4SCB and CB2SCB. It is interesting to note that the unclear optical texture similar to that of the mesophase of CB2SCB continuously emerged from the N or NTB borders in the intermediate regions and at higher temperatures than ∼43 °C of the I-to-mesophase phase-transition temperature of CB2SCB. Subsequently, the binary mixtures of CB2SCB blended with CB4SCB were investigated. The phase diagram of the binary mixture upon cooling as a function of the molar fraction of CB4SCB was determined by POM observations and is illustrated in Fig. 8(a) along with the representative optical textures [Fig. 8(b)–(e)]. Both molecules were miscible in one another without phase separation. Surprisingly, the binary mixtures containing a low mol% of CB4SCB (or a high mol% of CB2SCB) exhibited direct NTB phase formation from the I phase at temperatures higher than that of the I-to-mesophase phase transition of CBS2CB (∼43 °C). Fig. 8(b) and (c) show the blocky texture typical of the NTB phase observed for the binary mixtures of 9.9 and 17.7 mol% CB4SCB; these emerged upon holding at a temperature in the vicinity of the I–NTB phase transitions of the binary mixtures. The optical textures of the NTB phases of the binary mixtures became discernible upon increasing the mol% of CB4SCB (or decreasing the mol% of CB2SCB) [see Fig. 8(b)–(d)]. As shown in Fig. 8(a), the TIN, TNNTB, and N phase temperature ranges of the binary mixtures gradually decreased with a decrease in the mol% of CB4SCB (or an increase in the mol% of CB2SCB). Eventually, the N phase of the binary mixture disappeared at low CB4SCB contents of ∼20 mol%. The I–NTB phase-transition temperature for each binary mixture is abbreviated to TINTB, as is the I-to-mesophase phase-transition temperature of CB2SCB [Fig. 8(a)]. Interestingly, the TNNTB and TINTB values of the binary mixtures were continuously connected from the TNNTB of CB4SCB alone (∼70 °C) to the I-to-mesophase phase-transition temperature (treated as the TINTB) of CB2SCB (∼43 °C). As shown in Fig. 8(e), in addition, a striped texture was also observed for the binary mixture (∼30 mol% CB4SCB) showing a narrow temperature range for the N phase above that of the NTB phase when POM was carried out using a uniaxially rubbed surface cell, although this texture could not be observed for the mesophase of CB2SCB alone. This indicates that the presence of the upper N phase could have a beneficial effect in helping the NTB phase align in a uniaxially rubbed cell. Through such POM observations, it was confirmed that the optical textures of the mesophases observed for all prepared binary mixtures were maintained (or supercooled) below room temperature without crystallization.
To further characterize the mesophase of CB2SCB, we conducted XRD measurements using mesophase and cold crystallization samples of CB2SCB. The mesophase and cold crystallization samples were prepared by rapidly cooling to 25 °C from the I phase at 140 °C, and by heating from the mesophase at 25 °C, respectively. The XRD measurements for both samples were conducted at 25 °C. The obtained 1D XRD profiles of the mesophase and cold crystallization samples of CB2SCB at 25 °C are shown in Fig. 9. As can be seen in the figure, the diffraction peaks of the two samples are clearly different to one another. The mesophase sample formed by rapid cooling from the I phase exhibited only a broadened peak in the wide-angle region. The broadened peak centered at 2θ = 20.8° corresponds to approximately 4.27 Å, which is comparable to a lateral molecular distance observed in usual N phases. Additionally, no other peaks were observed in the small-angle region. These observations indicate that this state is likely a type of nonlayered mesophase with a liquid-like correlation or an N-type nature. If this mesophase of CB2SCB has a periodic electron density modulation such as in the case of Sm phases, Bragg scattering peaks should be observed in the small-angle region. On the other hand, the cold crystallization sample was found to exhibit many sharp diffraction peaks due to the presence of a crystal structure.
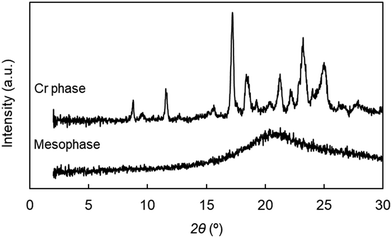 | ||
| Fig. 9 1D-XRD profiles of CB2SCB for the mesophase sample at 25 °C (bottom) and the cold crystallization sample at 25 °C (top). | ||
With respect to the mesophase of CB2SCB, POM observations revealed modulated N-like or pseudo-layered NTB-like textures, and the XRD results indicated its non-layered and fluidic nature. In addition, DSC measurements indicated its first order phase-transition property. Furthermore, POM observations of the contact preparation and binary mixtures revealed that the mesophase of CB2SCB was miscible with the NTB phase of CB4SCB, and their blends with a high mol% of CB2SCB showed optical textures typical of the NTB phase, which were formed directly from each I phase. It was therefore suggested that the mesophase of CB2SCB may also be the NTB phase. In other words, the NTB phase of CB2SCB directly formed from the I phase without any intermediate N phase, which is particularly rare.54,55,90 There have been only a few examples of single component54,55 and mixed90 LC dimer systems that exhibited this direct NTB formation from the I phase. For example, Dawood et al. reported an imine-linked LC dimer that showed direct formation of the NTB phase from the I phase, which was observed for a dimer homolog with a short propylene (C3H6) spacer, and this species was expected to possess a larger biaxiality than its longer spacer homologs.54,55 The observed I–NTB phase transition indicated a strong first order property, as was proposed theoretically within a restricted region of the molecular bend angles.91 As shown in Fig. 4(a) and 5, the present DSC results for the I-to-mesophase phase transition of CB2SCB also revealed a first order property. The ΔS/R at the I–NTB phase transition (abbreviated to ΔSINTB/R) value of 0.55, which was recorded at a rate of 30 °C min−1, is clearly larger than those of other CBnSCB homologs. Such behavior is in agreement with the literature.54,55,90,91 The previously reported ΔS/R value of 1.78 for CB2SCB was smaller than the present value because it was evaluated at a rate of 10 °C min−1, and so resulted in prior crystallization. In addition, as mentioned above, alignment of the mesophase of CB2SCB was not achieved in a uniaxially rubbed cell. This may also imply a macroscopic feature of the mesophase formed directly from the I phase, which is more similar to the Sm phase than the N phase. Furthermore, as shown in Fig. 8(e), the observed stripes are thin and small compared with those of typical stripes, which are similar to those observed for the ester-linked analogs exhibiting very short helical pitches.70 Further characterization of this mesophase of CB2SCB will be required together with that of the related materials.
Finally, the TIN, TNNTB, and ΔSIN/R values of all CBnSCB homologs were compared with those of the previously reported bis(thioether)-linked CBSnSCB analogs and the ether- and thioether-linked CBOnSCB analogs (Scheme 1) with respect to individual homologs containing the same total chain length of the oligomethylene spacer and linkage atoms along the linear chain, meaning that the total chain length refers to n + 1 for CBnSCB and n + 2 for CBSnSCB and CBOnSCB (Fig. 10). The specific values are listed in Table 2, as obtained from the literature.61 In the present study, we also reinvestigated the phase transitions of CBSnSCB and CBOnSCB using POM, and observed that CBS11SCB and CBOnSCB (n = 3 and 11) also exhibited NTB formation in the small supercooled N domains (Fig. 11), giving TNNTB values of 91 °C for CBS11SCB, 47 °C for CBO3SCB, and 92 °C for CBO11SCB. It was therefore apparent that all the reported bent CBSnSCB and CBOnSCB analogs (odd n = 3, 5, 7, 9, and 11) can form the NTB phase. It is interesting to note that the TNNTB value of 47 °C observed for CBO3SCB is significantly low compared with that of usual LC dimers (∼80–110 °C). Such low TNNTB tendency is similar to that of another analog with a short chain CBS3SCB (see Table 2)61 and a selenium-linked CBSe3SeCB analog,62 which is ascribed to the more bent geometry for shorter spacer dimers, as described later. As can be seen in Fig. 10(a), in addition to the fact that the TIN of CBOnSCB was significantly higher than the corresponding values for CBnSCB and CBSnSCB, the values of CBnSCB and CBSnSCB were similar to one another. Furthermore, the TNNTB and ΔSIN/R values of CBSnSCB are lower than those of CBnSCB and CBOnSCB in the small and middle values of n, as shown in Fig. 10. These trends are in good agreement with those observed for other rod-like mesogens with alkyl, alkoxy, and alkylthio groups.93–96 These could be ascribed to the steric hindrance associated with the bond angles and rotation barriers of the methylene C–CH2–C, the ether C–O–C, and the thioether C–S–C linkage bonds. The bond angles of the thioether C–S–C, methylene C–C–C, and ether C–O–C bonds are ∼100, 110, and 118°, respectively; the dimers should therefore be bent in the order of CBSnSCB (107°) > CBnSCB (111°) > CBOnSCB (126°).61,64 In addition, C–O–C has a higher rotation barrier compared with that of C–S–C.97 Hence, the less bent (or more straightened) CBOnSCB, which is assumed to have a lower steric hindrance or molecular biaxiality, exhibits higher TIN and ΔSIN/R values compared with CBnSCB and CBSnSCB. Meanwhile, the more bent CBSnSCB, which exhibits a higher steric hindrance or molecular biaxiality, displays lower TIN, TNNTB, and ΔSIN/R values compared with CBnSCB and CBOnSCB. Nevertheless, the comparably similar TIN values of CBnSCB and CBSnSCB could be ascribed to their molecular symmetries. The asymmetric and symmetric linkage bonds of CBnSCB and CBSnSCB contribute to the relative decrease and increase in the phase-transition temperature, respectively, which result in comparably similar TIN values. It should be noted that compared to CBSnSCB and CBOnSCB, the methylene-linked CBnSCB homologs exhibit a distinctly prominent stability in the NTB phase, and the ability to vitrify as NTBG. For example, CBnSCB (n = 4, 6, and 8) exhibited a perfect NTBG phase without partial crystallization. This behavior of CBnSCB could be attributed to the following multiple effects. Firstly, the lower molecular polarizability of CBnSCB compared to those of the thioether (CBSnSCB) and ether (CBOnSCB) analogs should reduce the crystallization ability of the molecules. In addition, asymmetric structures can effectively contribute to prevent crystallization, resulting in formation of the NTBG phase.67,68 The intermediate molecular bend (111°) of CBnSCB may also contribute to the stability of the NTB phase, since bent molecules fill the space, forming Sm layers.98,99
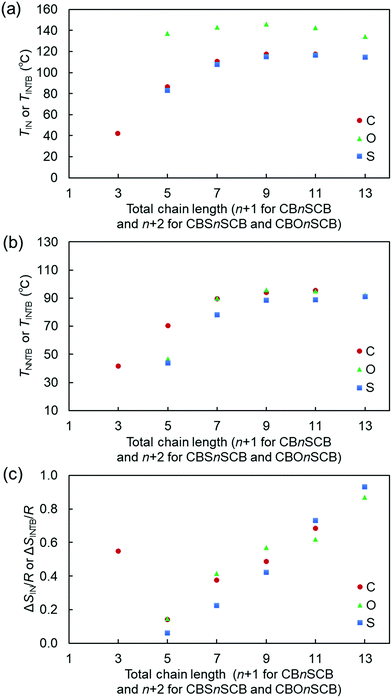 | ||
| Fig. 10 (a) TIN (or TINTB for CB2SCB), (b) TNNTB (or TINTB for CB2SCB), and (c) ΔSIN/R (or ΔSINTB/R for CB2SCB) values of CBnSCB, CBSnSCB, and CBOnSCB upon cooling as a function of the linkage bonds present in the structures. Here, C (i.e., CH2), O, and S represent the linkage bonds of CBnSCB, CBSnSCB, and CBOnSCB, respectively. The horizontal axes (total chain length) in panels (a)–(c) denote the total atom numbers of the linkage and the spacer along the linear chains, i.e., n + 1 for CBnSCB and n + 2 for CBSnSCB and CBOnSCB. The phase-transition data recorded at a rate of 3 °C min−1 for CBSnSCB and CBOnSCB were obtained from the literature.61 In addition, the present study observed NTB phase formation for CBS11SCB, CBO3SCB, and CBO11SCB in the small supercooled N domains using POM, giving TNNTB values of 91 °C for CBS11SCB, 47 °C for CBO3SCB, and 92 °C for CBO11SCB. | ||
| Code | n | Phase | T (°C) | Phase | T (°C) | Phase | T (°C) | ΔS/R | Phase |
|---|---|---|---|---|---|---|---|---|---|
| a Determined using POM. | |||||||||
| CBSnSCB | 3 | Cr | 70.1 | NTB | 44.0a | N | 83.2 | 0.06 | I |
| 5 | Cr | 68.9 | NTB | 78.0 | N | 107.8 | 0.23 | I | |
| 7 | G | 15.9 | NTB | 88.3 | N | 115.2 | 0.42 | I | |
| 9 | Cr | 100.8 | NTB | 89a | N | 116.7 | 0.73 | I | |
| 11 | Cr | 101.4 | NTB | 91a | N | 114.4 | 0.93 | I | |
| CBOnSCB | 3 | Cr | 101.1 | NTB | 47a | N | 137.5 | 0.15 | I |
| 5 | Cr | 59.5 | NTB | 90.1 | N | 143.8 | 0.42 | I | |
| 7 | G | 16.0 | NTB | 95.9 | N | 146.7 | 0.57 | I | |
| 9 | Cr | 98.0 | NTB | 95a | N | 143.0 | 0.62 | I | |
| 11 | Cr | 102.5 | NTB | 92a | N | 134.1 | 0.87 | I | |
Conclusions
A homologous series of cyanobiphenyl-based LC dimers linked with methylene and thioether linkages, i.e., CBnSCB, where n = 2, 4, 6, 8, and 10, were developed for the first time. CBnSCB homologs (n = 4, 6, 8, and 10) formed the NTB phase below the N phase upon cooling. Interestingly, the results obtained for CB2SCB implied a direct formation of the NTB phase from the I phase, which is in marked contrast to the common NTB–N–I phase sequence. It is noteworthy that the CBnSCB homologs (n = 2, 4, 6, and 8) exhibited NTB phases which underwent vitrification to form the NTBG phase upon cooling below room temperature. In particular, CBnSCB (n = 6 and 8) exhibited prominent stability of the NTB and N phases, and did not undergo cold crystallization upon heating from the NTBG phase up to the I phase. In addition, the present study reinvestigated the phase-transition behaviors of the CBSnSCB and CBOnSCB analogs (n = 3, 5, 7, 9, and 11) using POM, and NTB phase formation was observed from the small supercooled N domains of CBS11SCB, CBO3SCB, and CBO11SCB, indicating that all reported bent CBSnSCB and CBOnSCB dimers (n = 3, 5, 7, 9, and 11) potentially exhibit the NTB phase. Overall, the CBnSCB homologs presented remarkably stable NTB phases and vitrifiable tendencies compared with the previously reported CBSnSCB and CBOnSCB analogs. This study demonstrates a new LC homologous series exhibiting a stable NTB phase over a wide range, which can be supercooled to room temperature. Such characteristic dimers will lead to more detailed evaluations of the NTB phases, and will likely lead to the discovery of interesting structural and physical properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 17K14493 and 20K15351), the Naito Research Grant, and research grants from the Toukai Foundation for Technology and Toyohashi University of Technology. We would like to thank Prof. Masatoshi Tokita for permitting the temperature-variable XRD measurements at Tokyo Institute of Technology, and thank the Cooperative Research Facility Center at the Toyohashi University of Technology for assisting with the 1D-XRD measurements. We would also like to thank Ms Tsugumi Shiokawa and Dr Hiroko Tada in the Division of Instrumental Analysis (Okayama University) for carrying out the mass measurements.References
- J. W. Emsley, G. R. Luckhurst, G. N. Shilstone and I. Sage, Mol. Cryst. Liq. Cryst., 1984, 102, 223 CrossRef CAS.
- P. J. Barnes, A. G. Douglass, S. K. Heeks and G. R. Luckhurst, Liq. Cryst., 1993, 13, 603 CrossRef CAS.
- G. R. Luckhurst, Macromol. Symp., 1995, 96, 1 CrossRef CAS.
- A. Ferrarini, G. R. Luckhurst and P. L. Nordio, Mol. Phys., 1995, 85, 131 CrossRef CAS.
- C. T. Imrie and P. A. Henderson, Chem. Soc. Rev., 2007, 36, 2096 RSC.
- J. Watanabe, H. Komura and T. Niori, Liq. Cryst., 1993, 13, 455 CrossRef CAS.
- M. Šepelj, A. Lesac, U. Baumeister, S. Diele, D. W. Bruce and Z. Hameršak, Chem. Mater., 2006, 18, 2050 CrossRef.
- M. Šepelj, A. Lesac, U. Baumeister, S. Diele, H. L. Nguyen and D. W. Bruce, J. Mater. Chem., 2007, 17, 1154 RSC.
- E. Białecka-Florjańczyk, I. Śledzińska, E. Górecka and J. Przedmojski, Liq. Cryst., 2008, 35, 401–406 CrossRef.
- T. Yoshida, A. Sugimoto, A. Ikoma, T. Matsuoka, S. Kang, K. Sakajiri, J. Watanabe and M. Tokita, Liq. Cryst., 2015, 42, 463 CrossRef CAS.
- Y. Arakawa, S. Sasaki, K. Igawa, M. Tokita, G. Konishi and H. Tsuji, New J. Chem., 2020, 44, 17531 RSC.
- R. B. Meyer, in Structural Problems in Liquid Crystal Physics, Molecular Fluids: Summer School in Theoretical Physics, les Houches lectures 1973, ed. R. Balian and G. Weil, Gordon and Breach, New York, 1976, pp. 271–343 Search PubMed.
- I. Dozov, Europhys. Lett., 2001, 56, 247 CrossRef CAS.
- R. Memmer, Liq. Cryst., 2002, 29, 483 CrossRef CAS.
- M. Cestari, S. Diez-Berart, D. A. Dunmur, A. Ferrarini, M. R. de La Fuente, D. J. B. Jackson, D. O. Lopez, G. R. Luckhurst, M. A. Perez-Jubindo, R. M. Richardson, J. Salud, B. A. Timimi and H. Zimmermann, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 031704 CrossRef CAS.
- V. P. Panov, M. Nagaraj, J. K. Vij, Y. P. Panarin, A. Kohlmeier, M. G. Tamba, R. A. Lewis and G. H. Mehl, Phys. Rev. Lett., 2010, 105, 167801 CrossRef CAS.
- V. P. Panov, R. Balachandran, M. Nagaraj, J. K. Vij, M. G. Tamba, A. Kohlmeier and G. H. Mehl, Appl. Phys. Lett., 2011, 99, 261903 CrossRef.
- C. S. P. Tripathi, P. Losada-Pérez, C. Glorieux, A. Kohlmeier, M. G. Tamba, G. H. Mehl and J. Leys, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 041707 CrossRef.
- G. Ungar, J. L. Feijoo, A. Keller, R. Yourd and V. Percec, Macromolecules, 1990, 23, 3411 CrossRef CAS.
- G. Ungar, V. Percec and M. Zuber, Macromolecules, 1992, 25, 75 CrossRef CAS.
- M. W. Schröder, S. Diele, G. Pelzl, U. Dunemann, H. Kresse and W. Weissflog, J. Mater. Chem., 2003, 13, 1877 RSC.
- C. V. Yelamaggad, I. S. Shashikala and Q. Li, Chem. Mater., 2007, 19, 6561 CrossRef CAS.
- A. G. Vanakaras and D. J. Photinos, Soft Matter, 2016, 12, 2208 RSC.
- L. M. Heist, E. T. Samulski, C. Welch, Z. Ahmed, G. H. Mehl, A. G. Vanakaras and D. J. Photinos, Liq. Cryst., 2020, 47, 2058 CrossRef CAS.
- E. T. Samulski, A. G. Vanakaras and D. J. Photinos, Liq. Cryst., 2020, 47, 2092 CrossRef CAS.
- I. Dozov and G. Luckhurst, Liq. Cryst., 2020, 47, 2098 CrossRef CAS.
- D. Chen, J. H. Porada, J. B. Hooper, A. Klittnick, Y. Shen, M. R. Tuchband, E. Korblova, D. Bedrov, D. M. Walba, M. A. Glaser, J. E. Maclennan and N. A. Clark, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15931 CrossRef CAS.
- V. Borshch, Y.-K. Kim, J. Xiang, M. Gao, A. Jákli, V. P. Panov, J. K. Vij, C. T. Imrie, M. G. Tamba, G. H. Mehl and O. D. Lavrentovich, Nat. Commun., 2013, 4, 2635 CrossRef CAS.
- C. Zhu, M. R. Tuchband, A. Young, M. Shuai, A. Scarbrough, D. M. Walba, J. E. Maclennan, C. Wang, A. Hexemer and N. A. Clark, Phys. Rev. Lett., 2016, 116, 147803 CrossRef.
- W. D. Stevenson, Z. Ahmed, X. B. Zeng, C. Welch, G. Ungar and G. H. Mehl, Phys. Chem. Chem. Phys., 2017, 19, 13449 RSC.
- Y. Cao, J. Feng, A. Nallapaneni, Y. Arakawa, K. Zhao, G. H. Mehl, F. Liu and C. Zhu, arXiv:1907.11330.
- Y. Cao, C. Feng, A. Jakli, C. Zhu and F. Liu, Giant, 2020, 2, 100018 CrossRef.
- W. D. Stevenson, X. Zeng, C. Welch, A. K. Thakur, G. Ungar and G. H. Mehl, J. Mater. Chem. C, 2020, 8, 1041 RSC.
- V. P. Panov, S. P. Sreenilayam, Y. P. Panarin, J. K. Vij, C. J. Welch and G. H. Mehl, Nano Lett., 2017, 17, 7515 CrossRef CAS.
- N. Trbojevic, D. J. Read and M. Nagaraj, Phys. Rev. E, 2019, 99, 062704 CrossRef CAS.
- J. Zhou, W. Tang, Y. Arakawa, H. Tsuji and S. Aya, Phys. Chem. Chem. Phys., 2020, 22, 9593 RSC.
- S. P. Sreenilayam, Y. P. Panarin, J. K. Vij, V. P. Panov, A. Lehmann, M. Poppe, M. Prehm and C. Tschierske, Nat. Commun., 2016, 7, 11369 CrossRef CAS.
- S. P. Sreenilayam, Y. P. Panarin, J. K. Vij, A. Lehmann, M. Poppe and C. Tschierske, Phys. Rev. Mater., 2017, 1, 035604 CrossRef.
- M. G. Tamba, S. M. Salili, C. Zhang, A. Jákli, G. H. Mehl, R. Stannarius and A. Eremin, RSC Adv., 2015, 5, 11207 RSC.
- J. P. Abberley, R. Killah, R. Walker, J. M. D. Storey, C. T. Imrie, M. Salamończyk, C. Zhu, E. Gorecka and D. Pociecha, Nat. Commun., 2018, 9, 228 CrossRef.
- M. T. Murachver, A. Nemati, M. Salamończyk, C. Bullock, Z. Sabata, H. Rahmani, T. Vorobiova, A. Izadnegahdar, S. M. Salili, V. Norman, C. Zhu, T. Hegmann, S. N. Sprunt, J. T. Gleeson and A. Jákli, Soft Matter, 2019, 15, 3283 RSC.
- K. Merkel, A. Kocot, J. K. Vij and G. Shanker, Phys. Rev. E, 2018, 98, 022704 CrossRef CAS.
- K. Merkel, A. Kocot, G. H. Mehl and C. Welch, Phys. Chem. Chem. Phys., 2019, 21, 22839 RSC.
- C. Meyer, C. Blanc, G. R. Luckhurst, P. Davidson and I. Dozov, Sci. Adv., 2020, 6, eabb8212 CrossRef CAS.
- A. Mertelj, L. Cmok, N. Sebastián, R. J. Mandle, R. R. Parker, A. C. Whitwood, J. W. Goodby and M. Čopič, Phys. Rev. X, 2018, 8, 041025 CAS.
- N. Chaturvedi and R. D. Kamien, Phys. Rev. E, 2019, 100, 022704 CrossRef CAS.
- P. A. Henderson and C. T. Imrie, Liq. Cryst., 2011, 38, 1407 CrossRef CAS.
- R. J. Mandle, E. J. Davis, S. A. Lobato, C.-C. A. Vol, S. J. Cowling and J. W. Goodby, Phys. Chem. Chem. Phys., 2014, 16, 6907 RSC.
- R. J. Mandle, E. J. Davis, C. C. A. Voll, C. T. Archbold, J. W. Goodby and S. J. Cowling, Liq. Cryst., 2015, 42, 688 CAS.
- N. Sebastián, D. O. López, B. Robles-Hernández, M. R. de la Fuente, J. Salud, M. A. Pérez-Jubindo, D. A. Dunmur, G. R. Luckhurst and D. J. B. Jackson, Phys. Chem. Chem. Phys., 2014, 16, 21391 RSC.
- Z. Ahmed, C. Welch and G. H. Mehl, RSC Adv., 2015, 5, 93513 RSC.
- R. J. Mandle, C. C. A. Voll, D. J. Lewis and J. W. Goodby, Liq. Cryst., 2016, 43, 13 CrossRef CAS.
- R. J. Mandle, C. T. Archbold, J. P. Sarju, J. L. Andrews and J. W. Goodby, Sci. Rep., 2016, 6, 36682 CrossRef.
- A. A. Dawood, M. C. Grossel, G. R. Luckhurst, R. M. Richardson, B. A. Timimi, N. J. Wells and Y. Z. Yousif, Liq. Cryst., 2016, 43, 2 CrossRef CAS.
- A. A. Dawood, M. C. Grossel, G. R. Luckhurst, R. M. Richardson, B. A. Timimi, N. J. Wells and Y. Z. Yousif, Liq. Cryst., 2017, 44, 106 CAS.
- T. Ivšić, U. Baumeister, I. Dokli, A. Mikleušević and A. Lesac, Liq. Cryst., 2017, 44, 93 Search PubMed.
- D. A. Paterson, J. P. Abberley, W. T. A. Harrison, J. M. D. Storey and C. T. Imrie, Liq. Cryst., 2017, 44, 127 CAS.
- A. Lesac, U. Baumeister, I. Dokli, Z. Hameršak, T. Ivšić, D. Kontrec, M. Viskić, A. Knežević and R. J. Mandle, Liq. Cryst., 2018, 45, 1101 CrossRef CAS.
- A. Knežević, M. Sapunar, A. Buljan, I. Dokli, Z. Hameršak, D. Kontrec and A. Lesac, Soft Matter, 2018, 14, 8466 RSC.
- K. Watanabe, T. Tamura, S. Kang and M. Tokita, Liq. Cryst., 2018, 45, 924 CrossRef CAS.
- Y. Arakawa, K. Komatsu and H. Tsuji, New J. Chem., 2019, 43, 6786 RSC.
- Y. Arakawa and H. Tsuji, J. Mol. Liq., 2019, 289, 111097 CrossRef.
- E. Cruickshank, M. Salamończyk, D. Pociecha, G. J. Strachan, J. M. D. Storey, C. Wang, J. Feng, C. Zhu, E. Gorecka and C. T. Imrie, Liq. Cryst., 2019, 46, 1595 CrossRef CAS.
- Y. Arakawa, K. Komatsu, S. Inui and H. Tsuji, J. Mol. Struct., 2020, 1199, 126913 CrossRef CAS.
- R. J. Mandle and J. W. Goodby, Chem. – Eur. J., 2019, 25, 14454 CrossRef CAS.
- A. Zep, K. Pruszkowska, Ł. Dobrzycki, K. Sektas, P. Szałański, P. H. Marek, M. K. Cyrański and R. R. Sicinski, CrystEngComm, 2019, 21, 2779 RSC.
- Y. Arakawa, Y. Ishida and H. Tsuji, Chem. – Eur. J., 2020, 26, 3767 CrossRef CAS.
- Y. Arakawa, K. Komatsu, Y. Ishida and H. Tsuji, Liq. Cryst., 2020 DOI:10.1080/02678292.2020.1800848.
- A. Knežević, I. Dokli, J. Novak, D. Kontrec and A. Lesac, Liq. Cryst., 2020 DOI:10.1080/02678292.2020.1817585.
- Y. Arakawa, K. Komatsu, J. Feng, C. Zhu and H. Tsuji, Mater. Adv., 2021, 2, 261 RSC.
- Y. Arakawa, K. Komatsu, Y. Ishida, K. Igawa and H. Tsuji, Tetrahedron, 2021, 81, 131870 CrossRef CAS.
- R. Walker, M. Majewska, D. Pociecha, A. Makal, J. M. D. Storey, E. Gorecka and C. T. Imrie, ChemPhysChem, 2021 DOI:10.1002/cphc.202000993.
- Y. Wang, G. Singh, D. M. Agra-Kooijman, M. Gao, H. K. Bisoyi, C. Xue, M. R. Fisch, S. Kumar and Q. Li, CrystEngComm, 2015, 17, 2778 RSC.
- S. M. Jansze, A. Martínez-Felipe, J. M. D. Storey, A. T. M. Marcelis and C. T. Imrie, Angew. Chem., Int. Ed., 2015, 54, 643 CAS.
- R. J. Mandle and J. W. Goodby, ChemPhysChem, 2016, 17, 967 CrossRef CAS.
- A. Al-Janabi, R. J. Mandle and J. Goodby, RSC Adv., 2017, 7, 47235 RSC.
- R. J. Mandle and J. W. Goodby, Angew. Chem., Int. Ed., 2018, 57, 7096 CrossRef CAS.
- W. D. Stevenson, J. An, X. B. Zeng, M. Xue, H. X. Zou, Y. S. Liu and G. Ungar, Soft Matter, 2018, 14, 3003 RSC.
- D. Chen, M. Nakata, R. Shao, M. R. Tuchband, M. Shuai, U. Baumeister, W. Weissflog, D. M. Walba, M. A. Glaser, J. E. Maclennan and N. A. Clark, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 89, 022506 CrossRef.
- P. Sreenilayam, V. P. Panov, J. K. Vij and G. Shanker, Liq. Cryst., 2017, 44, 244 Search PubMed.
- J. Xiang, A. Varanytsia, F. Minkowski, D. A. Paterson, J. M. D. Storey, C. T. Imrie, O. D. Lavrentovich and P. Palffy-Muhoray, Electrically tunable laser based on oblique heliconical cholesteric liquid crystal, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 12925 CrossRef CAS.
- Y. Wang, Z. Zheng, H. K. Bisoyi, K. G. Gutierrez-Cuevas, L. Wang, R. S. Zola and Q. Li, Mater. Horiz., 2016, 3, 442 RSC.
- M. Mrukiewicz, O. S. Iadlovska, G. Babakhanova, S. Siemianowski, S. V. Shiyanovskii and O. D. Lavrentovich, Liq. Cryst., 2019, 46, 1544 CrossRef CAS.
- S. Krishna Prasad, P. Lakshmi Madhuri, P. Satapathy and C. V. Yelamaggad, Appl. Phys. Lett., 2018, 112, 253701 CrossRef.
- V. Sridurai, M. B. Kanakala, C. V. Yelamaggad and G. G. Nair, Soft Matter, 2019, 15, 9982 RSC.
- S. Aya, P. Salamon, D. A. Paterson, J. M. D. Storey, C. T. Imrie, F. Araoka, A. Jákli and Á. Buka, Adv. Mater. Interfaces, 2019, 6, 1802032 CrossRef.
- C. Feng, J. Feng, R. Saha, Y. Arakawa, J. Gleeson, S. Sprunt, C. Zhu and A. Jákli, Phys. Rev. Res., 2020, 2, 032004 CrossRef CAS.
- A. Aouini, M. Nobili, E. Chauveau, P. Dieudonné-George, G. Damême, D. Stoenescu, I. Dozov and C. Blanc, Crystals, 2020, 10, 1110 CrossRef CAS.
- Y. Arakawa, Y. Sasaki and H. Tsuji, J. Mol. Liq., 2019, 280, 153 CrossRef CAS.
- C. T. Archbold, E. J. Davis, R. J. Mandle, S. J. Cowling and J. W. Goodby, Soft Matter, 2015, 11, 7547 RSC.
- C. Greco, G. R. Luckhurst and A. Ferrarini, Soft Matter, 2014, 10, 9318 RSC.
- R. J. Mandle, E. J. Davis, C. T. Archbold, S. J. Cowling and J. W. Goodby, J. Mater. Chem. C, 2014, 2, 556 RSC.
- Y. Arakawa, S. Kang, H. Tsuji, J. Watanabe and G. Konishi, RSC Adv., 2016, 6, 16568 RSC.
- Y. Arakawa and H. Tsuji, Mol. Cryst. Liq. Cryst., 2017, 647, 422 CrossRef CAS.
- D. Węgłowska, P. Kula and J. Herman, RSC Adv., 2016, 6, 403 RSC.
- Y. Arakawa, S. Inui and H. Tsuji, Liq. Cryst., 2018, 45, 811 CrossRef CAS.
- X. D. Li, S. K. Lee, S. Kang, M. Tokita, S. Kawauchi and J. Watanabe, Chem. Lett., 2009, 38, 424 CrossRef CAS.
- T. Akutagawa, Y. Matsunaga and K. Yashuhara, Liq. Cryst., 1994, 17, 659 CrossRef CAS.
- T. Niori, T. Sekine, J. Watanabe, T. Furukawa and H. Takezoe, J. Mater. Chem., 1996, 1231 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00990c |
| This journal is © The Royal Society of Chemistry 2021 |