 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Experimental validation of high electrical conductivity in Ni-rich LaNi1−xFexO3 solid solutions (x ≤ 0.4) in high-temperature oxidizing atmospheres†
Yoshinobu
Adachi‡
a,
Naoyuki
Hatada
 *a,
Masaki
Kato
b,
Ken
Hirota
b and
Tetsuya
Uda
*a
*a,
Masaki
Kato
b,
Ken
Hirota
b and
Tetsuya
Uda
*a
aDepartment of Materials Science and Engineering, Kyoto University, Yoshida-honmachi, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: hatada.naoyuki.8u@kyoto-u.jp; uda.tetsuya.5e@kyoto-u.ac.jp
bDepartment of Molecular Chemistry and Biochemistry, Faculty of Science and Engineering, Doshisha University, Kyo-Tanabe, Kyoto 610-0321, Japan
First published on 29th March 2021
Abstract
LaNi1−xFexO3 solid solutions are an interesting system exhibiting a composition-controlled metal–insulator transition and are also potential cathode materials for solid oxide fuel cells, but the composition dependence of their electrical conductivity is still an open question due to the difficulty in synthesis and sintering. Here, in contrast to previous studies, it is demonstrated that the electrical conductivity of LaNi1−xFexO3 monotonically increases with decreasing x (increasing Ni content), reaching as high as 1.0 × 104 S cm−1 at room temperature and 2.5 × 103 S cm−1 at 800 °C in 0.2 bar O2 when x = 0. The accurate electrical conductivity measurements of LaNi1−xFexO3 with high Ni contents (0 ≤ x ≤ 0.4) are realized using fully dense single-phase polycrystalline samples prepared by the post-sintering oxidation process. The results suggest that LaNi1−xFexO3 with a higher Ni content might be more suitable as the cathodes than the widely-studied composition LaNi0.6Fe0.4O3. Furthermore, LaNiO3 is now considered to have the highest electrical conductivity among precious-metal-free oxides in high-temperature oxidizing atmospheres and can find more applications.
LaNiO3, LaFeO3, and their solid solutions LaNi1−xFexO3 (LNF, 0 ≤ x ≤ 1) have been extensively studied in the field of solid state chemistry/physics for over 50 years owing to their interesting electrical properties.1–21 LaNiO3 is a metallic oxide with high electrical conductivity, which is rather unusual for undoped 3d-transition-metal-based perovskite oxides.6,10 LaNiO3 contains a low-spin configuration of Ni3+ (t2g6eg1).1,7,8,11 Due to the strong covalent bonding between Ni3+ and O2−, the eg orbitals are transformed into one-quarter filled itinerant σ* band states, and it results in the metallic behaviors. By contrast, LaFeO3 is a charge-transfer insulator which contains a high-spin, localized electron configuration of Fe3+ (t2g3eg2).1,11 Consequently the LaNi1−xFexO3 system exhibits a composition-controlled metal–insulator (m–i) transition at x = ∼0.3.2,3,5,12–14,22,23 Although no existing theory is sufficient to describe entirely the evolution of the electronic structure across the transition, a number of experimental and theoretical studies suggest that the metal–insulator transition is driven by increasing disorder effects arising from the random substitution for Ni with Fe in a metallic system with long–range Coulomb interactions.12,16
In recent decades, LaNi1−xFexO3 (in particular x = 0.4) has also been recognized as a promising cathode material for solid oxide fuel cells (SOFCs)22–42 because of its high electrical conductivity,22–26,28,32,34,37 good catalytic activity for oxygen reduction reaction,24,27,29,40 high durability against chromium poisoning,30 and thermal expansion coefficient (TEC) close to those of typical electrolyte materials.24–26,28,31–33,37
Here, the structural and physical properties of LaNi1−xFexO3 is briefly reviewed, which vary rather monotonically with iron content x. At room temperature, the solid solutions with 0 ≤ x < 0.5 are isostructural with LaNiO3 and have a rhombohedral perovskite structure, while those with composition 0.5 < x ≤ 1 are isostructural with LaFeO3 and have an orthorhombic perovskite structure.2,14,15,20,22,24,31,32,43,44 At 800 °C, the stable region of the rhombohedral phase extends up to x = ∼0.9.31 The molar volume of LaNi1−xFexO3 increases with x due to the larger ionic radius of Fe3+ compared with that of Ni3+.14,15,22,24,31,32,33,38,43 The thermal expansion coefficient is normally reported to be in the range of 8–14 × 10−6 K−1, close to those of typical electrolyte materials yttrium-stabilized zirconia (YSZ) and gadolinium-doped ceria (GDC), and it decreases with x.24–26,28,31–33,37 LaNiO3 decompose in air at around 1000 °C or higher to form phases with oxidation states of Ni below +3 according to the following equation:45
| 4LaNiO3 (s) → La4Ni3O10 (s) + NiO (s) + 1/2O2 (g) | (1) |
This makes synthesis and sintering of LaNiO3 difficult. The decomposition temperature of LaNi1−xFexO3 becomes higher with x due to the higher stability of Fe3+ compared with Ni3+, and thus LaNi0.6Fe0.4O3 (x = 0.4) is stable up to around 1200 °C in air.15,22,38,43,44
Despite many works to characterize LaNi1−xFexO3, the composition dependence of the electrical conductivity of LaNi1−xFexO3 is still an open question. Some studies have shown a monotonic increase of the electrical conductivity with decreasing x (increasing Ni content),3,12,13,28 while other recent studies have indicated a conductivity maximum at x = 0.4.19,22,24,32 The overpotential of LaNi1−xFexO3 cathodes on scandia-stabilized zirconia electrolytes reached a minimum also at x = 0.4, which suggested a correlation between the electrical conductivity and the cathode performance.24 Based on this, most of the studies aiming at the development of LaNi1−xFexO3 cathodes for SOFCs have focused on LaNi1−xFexO3 with x = 0.4.26,27,29,33,34,36,37,40,41 However, it is worth mentioning that the decrease in the electrical conductivity while entering into the metallic region (x < ∼0.3) is rather unusual. In this context, the origin of the apparent conductivity maximum has been suggested to be lower sintering density22 or decomposition24,32 of LaNi1−xFexO3 samples with x < 0.4 due to their poor thermal stability. Therefore, it is quite important to re-evaluate accurately the electrical conductivity of LaNi1−xFexO3 with x < 0.4 to validate the picture of the electronic conduction in the LaNi1−xFexO3 system and to find the optimal composition as cathodes.
In this study, we have prepared dense polycrystalline samples of single-phase LaNi1−xFexO3 with high Ni contents (0 ≤ x ≤ 0.4) by virtue of the post-sintering oxidation process.46 In this process, pre-sintered dense tablets of mixed oxides were converted to single-phase LaNi1−xFexO3 under high oxygen partial pressures. Then their accurate electrical conductivity has been evaluated to reveal the composition dependence.
Dense samples of LaNi1−xFexO3 with x = 0.4 and 0.36 were readily prepared by conventional solid-state reaction of La2O3, NiO, and Fe2O3 and sintering under ordinary pressure, while that with x = 0.2 could not be obtained in the same way. Fig. 1(a) shows powder X-ray diffraction patterns and SEM images of LaNi1−xFexO3 tablets with compositions x = 0.4, 0.36, and 0.2 after sintered at 1300 °C in air, oxygen gas, and air, respectively. The sintered tablets with x = 0.4 and 0.36 were almost single-phase perovskite, while the tablet with x = 0.2 contained impurity phases La4Ni3O10 and NiO in addition to the perovskite phase.§ The upper temperature limit of the stability of the perovskite phase LaNi1−xFexO3 has been reported to become lower as x or oxygen partial pressure (pO2) decreases.38,44 The present results follow this trend and reconfirm the difficulty in sintering LaNi1−xFexO3 with high Ni contents (x ≤ 0.2) under ordinary pressure.
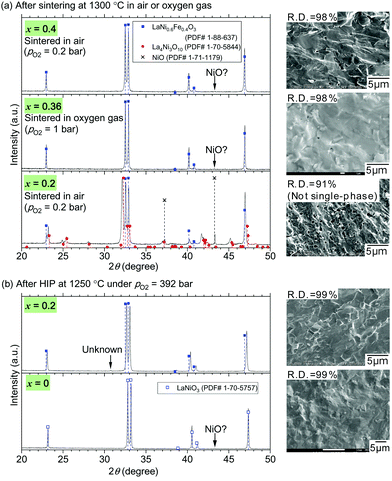 | ||
| Fig. 1 Powder X-ray diffraction patterns and SEM images of LaNi1−xFexO3 tablets (a) after sintered at 1300 °C in air or oxygen gas and (b) after HIP treatments at 1250 °C under pO2 = 392 bar. The SEM images are those of fracture surfaces of the tablets. Their relative densities measured by the Archimedes method are indicated on the SEM images. The data of LaNi1−xFexO3 with x = 0 are from our previous work.46 Part of the figure was presented at conferences.47,48 | ||
The post-sintering oxidation realized dense samples of LaNi1−xFexO3 with x = 0.2 and 0. The above mixed-phase tablet with x = 0.2 was subsequently oxidized at 1250 °C under pO2 = 392 bar using hot isostatic pressing (HIP). As evident from its powder X-ray diffraction pattern in Fig. 1(b), the sample with x = 0.2 was converted to almost single-phase perovskite owing to this high-pressure oxidation treatment. A dense sample of single-phase LaNiO3 (x = 0) was also obtained similarly by oxidizing a pre-sintered tablet composed of fine La2NiO4 and NiO grains as reported in our previous paper.46 Its XRD pattern in Fig. 1(b) confirms the almost single-phase nature. SEM images of the fracture surfaces of the samples with x = 0, 0.2, 0.36, and 0.4, and their relative densities measured by the Archimedes method are also shown in Fig. 1(a) and (b). There are few pores in their fracture surfaces after the final heat treatments, and their relative densities finally exceeded 98%.
Electrical conductivity measurements using the dense LaNiO3 sample revealed that the electrical conductivity of LaNiO3 has been much underestimated in the literature due to insufficient sintering. The electrical conductivity measurements of LaNi1−xFexO3 were carried out by the four-probe technique using bar-like tablets with silver electrodes (illustrated in Fig. 2) in air or a mixture of O2 and Ar gases. Fig. 3 shows the electrical conductivity values of LaNiO3 either obtained in this study using the dense sample or reported in the literature.5,12,14,18,28,49,50,51,52,53,54 While the temperature dependences (or the slopes) of the present and the reported data are consistent, significant discrepancies are seen in the electrical conductivity values. The present data and those obtained from a single-crystal LaNiO353 are almost one order of magnitude higher than the other literature values. In general, the apparent electrical conductivity of a pressed/sintered material is heavily dependent on its relative density:32,55 The apparent electrical conductivity varies from the inherent value of the material to almost an order of magnitude lower values as its relative density changes from 100% to 50%. The LaNiO3 samples used in the cited studies in Fig. 3 except the single crystal were only pressed or sintered at around 1000 °C or lower5,12,14,18,28,49,50,51,52,54 due to the poor thermal stability of LaNiO3 under an ordinary partial pressure of oxygen (1 bar O2 or less). Based on previous studies12,49,51,56 and our recent study on the La–Ni–O system,46 it is thought to be difficult to obtain fully dense samples (relative density over 95%) under such conditions. Therefore, the literature values of the electrical conductivity of LaNiO3 are likely to be much underestimated due to the lower relative densities. It is worth mentioning that this large discrepancy cannot be attributed to the difference in the atmosphere where the electrical conductivity measurements were carried out, as the variation of the electrical conductivity of LaNiO3 is less than 12% in the pO2 range of 10−3–1 bar at 800 °C (see Fig. S1, ESI†).
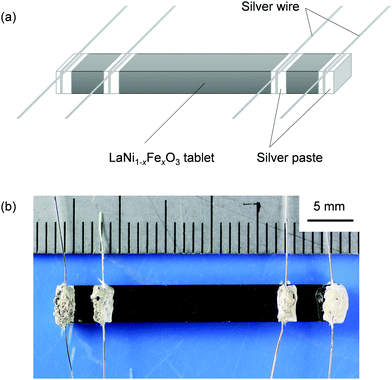 | ||
| Fig. 2 (a) Schematic of LaNi1−xFexO3 samples used for electrical conductivity measurements. (b) Photograph of a LaNi0.6Fe0.4O3 sample used for electrical conductivity measurements. | ||
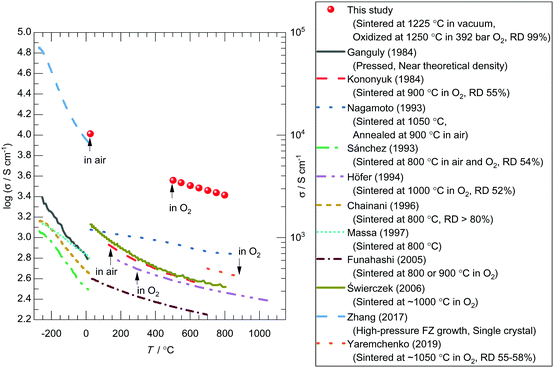 | ||
| Fig. 3 Temperature dependence of the electrical conductivity (σ) of LaNiO3 measured using a dense sample in this study or published in the literature.5,12,14,18,28,49–54 Atmospheres where measurements were carried out are indicated in the figure. Preparation conditions and relative densities of the specimen are summarized in the legend. The sintering condition of Świerczek et al.28 was given in their paper as “1000–1200 °C range, depending on the sample chemical composition”. As LaNiO3 is considered to be thermally most unstable composition in their paper, we guess the sintering temperature of LaNiO3 was approximately 1000 °C. | ||
The composition dependence of the electrical conductivity of LaNi1−xFexO3 is proved to be monotonic. Fig. 4(a) and (b) shows the iron content dependence of the electrical conductivity of LaNi1−xFexO3 at room temperature and 800 °C in 0.2 bar O2. The electrical conductivity values at x = 0.4 obtained in this study agree reasonably with literature values. At x < 0.4, the electrical conductivity increases with decreasing x (increasing Ni content), reaching 1.0 × 104 S cm−1 and 2.5 × 103 S cm−1 at x = 0 at room temperature and 800 °C, respectively, while the literature data do not show a clear trend.¶,|| This discrepancy can be due to the underestimation of the conductivity of Ni-rich samples in the past studies due to the lower relative densities as discussed in the previous paragraph or the existence of secondary phases.24,32 The increasing trend of the electrical conductivity with Ni content could be attributed to the previously proposed picture that the metal–insulator transition is driven by disorder effects arising from the substitution for Ni in metallic LaNiO3 with Fe. However, further experimental and theoretical studies using appropriately synthesized samples are desirable to elucidate the nature of M–I transition in this system. Referring also to literature data on Ni-poor compositions, we can see that the electrical conductivity of LaNi1−xFexO3 increases monotonically with decreasing x in the whole composition range.
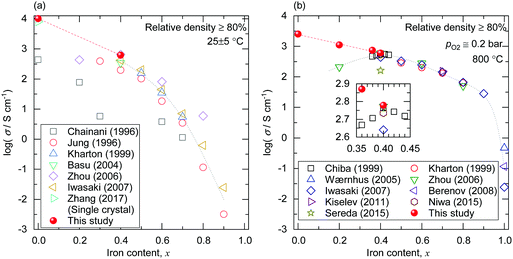 | ||
| Fig. 4 (a) Iron content dependence of the electrical conductivity of LaNi1−xFexO3 at 25 ± 5 °C. The literature values by Chainani et al.,12 Jung and Iguchi,13 Kharton et al.,25 Basu et al.,26 Zhou et al.,19 Iwasaki et al.,20 and Zhang et al.53 are also shown. The figure shows only the electrical conductivities of the samples whose relative densities were over 80% or that of a single crystal.53 (b) Iron content dependence of the electrical conductivity of LaNi1−xFexO3 at 800 °C. The literature values by Chiba et al.,32 Kharton et al.,25 Wærnhus et al.,57 Zhou et al.,19 Iwasaki et al.,20 Berenov et al.,58 Kiselev and Cherepanov,59 Niwa et al.,23 and Sereda et al.37 are also shown. The figure shows only the electrical conductivities of the samples whose relative densities were over 80%. The inset shows a zoom around x = 0.4. Part of the figure was presented at a conference.47 | ||
In Fig. 5(a), the electrical conductivity of LaNi1−xFexO3 is compared with those of simple perovskite or perovskite-related (layered-perovskite) oxides based on 3d transition metals Cr, Mn, Fe, Co, Ni, and/or Cu. These oxides are stable in air and at high temperatures (∼500–800 °C), and have potential applications as the cathodes and interconnects of SOFCs. The electrical conductivity of LaNiO3 (LaNi1−xFexO3 with x = 0) is found to be the highest among the above oxides including popular cathode materials such as La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF), Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF), La0.5Sr0.5MnO3−δ (LSM), and La0.6Sr0.4CoO3−δ (LSC). More broadly, Fig. 5(b) compares the electrical conductivities of LaNi1−xFexO3 and other representative electrically conducting oxides out of the above category, based on 3d, 4d, or 5d transition metals. ReO3 exhibits 1–2 orders of magnitude higher conductivity than that of LaNiO3. Also, RuO2 with rutile structure, TiOx (x = ∼1) with NaCl structure, Ti2O3 with corundum structure, and NaxWO3, La1−xSrxVO3, SrMoO3, and SrRuO3 with perovskite structure exhibit equal or higher conductivity than LaNiO3. However, many of them (ReO3,60 NaCl-type TiOx,61 Ti2O3,61 NaxWO3,62,63 La1−xSrxVO3,64,65 and SrMoO366) are unstable in high-temperature air and the rest of them (SrRuO3 and RuO2) are based on precious metal, both can be drawbacks for practical use. Therefore LaNiO3 is now considered as the precious-metal-free oxide with the highest electrical conductivity in high-temperature air.
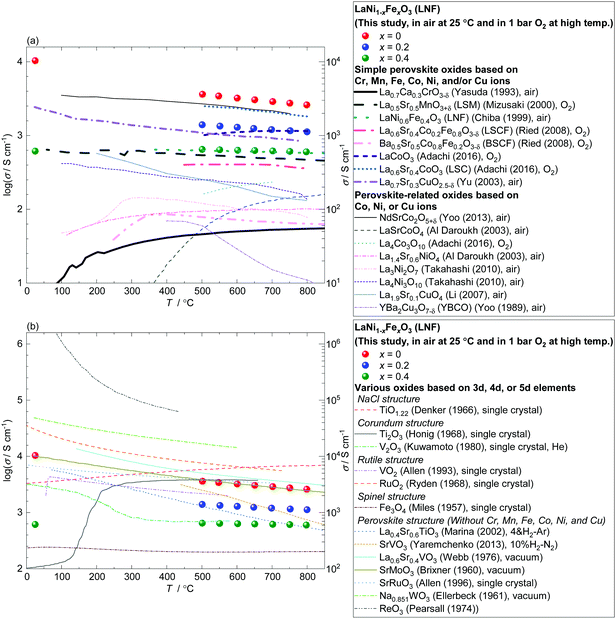 | ||
| Fig. 5 Comparison of the electrical conductivities of LaNi1−xFexO3 (x = 0, 0.2, 0.4) with those of representative electrically conducting oxides at temperatures between room temperature and 800 °C. Measurement conditions are summarized in the legend. (a) Comparison with simple perovskite or perovskite-related oxides based on 3d transition metals Cr, Mn, Fe, Co, Ni, and/or Cu: La0.7Ca0.3CrO3−δ,67 La0.5Sr0.5MnO3+δ,68 LaNi0.6Fe0.4O3,32 La0.6Sr0.4Co0.2Fe0.8O3−δ,69 Ba0.5Sr0.5Co0.8Fe0.2O3−δ,69 LaCoO3,70 La0.6Sr0.4CoO3−δ,70 La0.7Sr0.3CuO2.5−δ,71 NdSrCo2O5+δ,72 LaSrCoO4,73 La4Co3O10,70 La1.4Sr0.6NiO4,73 La3Ni2O7,74 La4Ni3O10,74 La1.9Sr0.1CuO4,75 and YBa2Cu3O7−δ.76 (b) Comparison with other representative electrically conducting oxides out of the above category, based on 3d, 4d, or 5d transition metals: TiO1.22,77 Ti2O3,78 V2O3,79 VO2,80 RuO2,81 Fe3O4,82 La0.4Sr0.6TiO3,83 SrVO3,84 La0.6Sr0.4VO3,85 SrMoO3,86 SrRuO3,87 Na0.851WO3,88 and ReO3.89 Part of the figure was presented at a conference.48 | ||
Conclusions
In conclusion, the electrical conductivity of Ni-rich LaNi1−xFexO3 (0 ≤ x ≤ 0.4) is found to monotonically increase with decreasing x (increasing Ni content) at both room temperature and 800 °C by use of the fully dense single-phase polycrystalline samples prepared by the post-sintering oxidation process. The confirmed trend suggests that LaNi1−xFexO3 with smaller x (higher Ni content) might be more suitable for the cathodes of SOFCs than the widely-studied composition LaNi0.6Fe0.4O3 (x = 0.4). It should be noted, however, that a higher conductivity does not necessarily lead to a better electrode performance. Therefore, it is desirable to investigate the electrochemical properties of Ni-rich LaNi1−xFexO3 (0 ≤ x ≤ 0.4). One concern with Ni-rich LaNi1−xFexO3 is the thermal instability during operation at high temperatures,35 but it may be less problematic for the applications in low-temperature SOFCs, which are under active development. In addition, Ni-rich LaNi1−xFexO3 can be more readily applicable as oxygen electrodes in solid oxide electrolyzer cells (SOECs) because the electrodes are operated in highly oxidative conditions, where LaNi1−xFexO3 is more stable.38,46 (see Fig. S2, ESI†) Furthermore, in a broader context, LaNiO3 is now considered as the precious-metal-free oxide with the highest electrical conductivity in high-temperature air (2.5 × 103 S cm−1 at 800 °C in 0.2 bar O2) and can find other applications as an oxidation-resistant, high-temperature electrical conductor.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by Grant-in-Aid for JSPS Research Fellow Grant Number 16J09427. We are grateful to Shin-Etsu Chemical Co., Ltd for providing lanthanum oxide.Notes and references
- J. B. Goodenough, Czech. J. Phys., 1967, 17, 304–336 CrossRef CAS.
- C. N. R. Rao, O. Parkash and P. Ganguly, J. Solid State Chem., 1975, 15, 186–192 CrossRef CAS.
- O. Parkash, Proc. Indian Natl. Sci. Acad., Part A, 1978, 87, 331–335 Search PubMed.
- K. Asai and H. Sekizawa, J. Phys. Soc. Jpn., 1980, 49, 90–98 CrossRef CAS.
- P. Ganguly, N. Y. Vasanthacharya and C. N. R. Rao, J. Solid State Chem., 1984, 54, 400–406 CrossRef CAS.
- J. B. Torrance, P. Lacorre, A. I. Nazzal, E. J. Ansaldo and C. Niedermayer, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 8209–8212 CrossRef CAS PubMed.
- J. L. García-Muñoz, J. Rodríguez-Carvajal, P. Lacorre and J. B. Torrance, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 4414–4425 CrossRef PubMed.
- K. Sreedhar, J. M. Honig, M. Darwin, M. McElfresh, P. M. Shand and J. Xu, et al. , Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6382–6386 CrossRef CAS PubMed.
- D. D. Sarma, O. Rader, T. Kachel, A. Chainani, M. Mathew and K. Holldack, et al. , Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14238–14243 CrossRef CAS PubMed.
- T. Arima and Y. Tokura, J. Phys. Soc. Jpn., 1995, 64, 2488–2501 CrossRef CAS.
- G. Pari, S. Mathi Jaya, G. Subramoniam and R. Asokamani, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 16575–16581 CrossRef CAS PubMed.
- A. Chainani, D. D. Sarma, I. Das and E. V. Sampathkumaran, J. Phys.: Condens. Matter, 1996, 8, L631–L636 CrossRef.
- W. H. Jung and E. Iguchi, Philos. Mag. B, 1996, 73, 873–891 CrossRef CAS.
- N. E. Massa, H. Falcón, H. Salva and R. E. Carbonio, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 10178–10191 CrossRef CAS.
- H. Falcón, A. E. Goeta, G. Punte and R. E. Carbonio, J. Solid State Chem., 1997, 133, 379–385 CrossRef.
- D. D. Sarma, A. Chainani, S. R. Krishnakumar, E. Vescovo, C. Carbone and W. Eberhardt, et al. , Phys. Rev. Lett., 1998, 80, 4004–4007 CrossRef CAS.
- R. Kumar, R. J. Choudhary, M. Wasi Khan, J. P. Srivastava, C. W. Bao and H. M. Tsai, et al. , J. Appl. Phys., 2005, 97, 093526 CrossRef.
- R. Funahashi, M. Mikami, S. Urata, M. Kitawaki, T. Kouuchi and K. Mizuno, Meas. Sci. Technol., 2005, 16, 70–80 CrossRef CAS.
- X.-D. Zhou, J. B. Yang, E.-C. Thomsen, Q. Cai, B. J. Scarfino and Z. Nie, et al. , J. Electrochem. Soc., 2006, 153, J133–J138 CrossRef CAS.
- K. Iwasaki, T. Ito, M. Yoshino, T. Matsui, T. Nagasaki and Y. Arita, J. Alloys Compd., 2007, 430, 297–301 CrossRef CAS.
- M. Wasi Khan, S. Husain, M. A. Majeed Khan, M. Gupta, R. Kumar and J. P. Srivastava, Philos. Mag., 2010, 90, 3069–3079 CrossRef.
- E. Niwa, C. Uematsu, E. Miyashita, T. Ohzeki and T. Hashimoto, Solid State Ionics, 2011, 201, 87–93 CrossRef CAS.
- E. Niwa, H. Maeda, C. Uematsu and T. Hashimoto, Mater. Res. Bull., 2015, 70, 241–247 CrossRef CAS.
- R. Chiba, F. Yoshimura and Y. Sakurai, Proc. - Electrochem. Soc., 1999, 99-19, 453–462 CrossRef CAS.
- V. V. Kharton, A. P. Viskup, E. N. Naumovich and V. N. Tikhonovich, Mater. Res. Bull., 1999, 34, 1311–1317 CrossRef CAS.
- R. N. Basu, F. Tietz, E. Wessel, H. P. Buchkremer and D. Stöver, Mater. Res. Bull., 2004, 39, 1335–1345 CrossRef CAS.
- H. Orui, K. Watanabe, R. Chiba and M. Arakawa, J. Electrochem. Soc., 2004, 151, A1412–A1417 CrossRef CAS.
- K. Świerczek, J. Marzec, D. Pałubiak, W. Zając and J. Molenda, Solid State Ionics, 2006, 177, 1811–1817 CrossRef.
- M. Bevilacqua, T. Montini, C. Tavagnacco, E. Fonda, P. Fornasiero and M. Graziani, Chem. Mater., 2007, 19, 5926–5936 CrossRef CAS.
- T. Komatsu, R. Chiba, H. Arai and K. Sato, J. Power Sources, 2008, 176, 132–137 CrossRef CAS.
- T. Ohzeki, T. Hashimoto, K. Shozugawa and M. Matsuo, Solid State Ionics, 2010, 181, 1771–1782 CrossRef CAS.
- R. Chiba, F. Yoshimura and Y. Sakurai, Solid State Ionics, 1999, 124, 281–288 CrossRef CAS.
- N. Sukpirom, S. Iamsaard, S. Charojrochkul and J. Yeyongchaiwat, J. Mater. Sci., 2011, 46, 6500–6507 CrossRef CAS.
- J. Y. Chen, J. Rebello, V. Vashook, D. M. Trots, S. R. Wang and T. L. Wen, et al. , Solid State Ionics, 2011, 192, 424–430 CrossRef CAS.
- R. A. Budiman, S. Hashimoto, T. Nakamura, K. Yashiro, K. Amezawa and T. Kawada, ECS Trans., 2015, 66, 177–183 CrossRef CAS.
- R. A. Budiman, T. Miyazaki, S. Hashimoto, T. Nakamura, K. Yashiro and K. Amezawa, et al. , J. Electrochem. Soc., 2015, 162, F1445–F1450 CrossRef CAS.
- V. V. Sereda, D. S. Tsvetkov, I. L. Ivanov and A. Y. Zuev, J. Mater. Chem. A, 2015, 3, 6028–6037 RSC.
- Y. Morishima, E. Niwa and T. Hashimoto, J. Therm. Anal. Calorim., 2016, 123, 1769–1775 CrossRef CAS.
- R. A. Budiman, H. J. Hong, S. Hashimoto, T. Nakamura, K. Yamaji and K. Yashiro, et al. , Solid State Ionics, 2017, 310, 148–153 CrossRef CAS.
- K. Chen, H. Dai, S. He and L. Bi, Fuel Cells, 2018, 18, 561–565 CrossRef CAS.
- N. Soltani, R. Martínez-Bautista, A. Bahrami, L. H. Arcos, M. Cassir and J. C. Carvayar, Chem. Phys. Lett., 2018, 700, 138–144 CrossRef CAS.
- Y. Chen, W. Zhou, D. Ding, M. Liu, F. Ciucci and M. Tade, et al. , Adv. Energy Mater., 2015, 5, 1500537 CrossRef.
- E. A. Kiselev, N. V. Proskurnina, V. I. Voronin and V. A. Cherepanov, Inorg. Mater., 2007, 43, 167–175 CrossRef CAS.
- E. A. Kiselev and V. A. Cherepanov, J. Solid State Chem., 2010, 183, 1992–1997 CrossRef CAS.
- D. O. Bannikov and V. A. Cherepanov, J. Solid State Chem., 2006, 179, 2721–2727 CrossRef CAS.
- Y. Adachi, N. Hatada, K. Hirota, M. Kato and T. Uda, J. Am. Ceram. Soc., 2019, 102, 7077–7088 CrossRef CAS.
- Y. Adachi, N. Hatada, K. Hirota, M. Kato and T. Uda, Abstracts of the 83rd Spring Meeting of the Electrochemical Society of Japan, 2016, 1N20 Search PubMed.
- N. Hatada and T. Uda, The 45th Symposium on Solid State Ionics of Japan Extended Abstracts, 2019, 256–257 Search PubMed.
- I. F. Kononyuk, N. G. Surmach, S. P. Tolochko and T. V. Kugan, Inorg. Mater., 1984, 20, 1036–1039 Search PubMed.
- H. Nagamoto, I. Mochida, K. Kagotani, H. Inoue and A. Negishi, J. Mater. Res., 1993, 8, 3158–3162 CrossRef CAS.
- H. E. Höfer and R. Schmidberger, J. Electrochem. Soc., 1994, 141, 782–786 CrossRef.
- R. D. Sánchez, M. T. Causa, J. Sereni, M. Vallet-Regí, M. J. Sayagués and J. M. González-Calbet, J. Alloys Compd., 1993, 191, 287–289 CrossRef.
- J. Zhang, H. Zheng, Y. Ren and J. F. Mitchell, Cryst. Growth Des., 2017, 17, 2730–2735 CrossRef CAS.
- A. A. Yaremchenko, B. I. Arias-Serrano, K. Zakharchuk and J. R. Frade, ECS Trans., 2019, 91, 2399–2408 CrossRef CAS.
- J. Mizusaki, S. Tsuchiya, K. Waragai, H. Tagawa, Y. Arai and Y. Kuwayama, J. Am. Ceram. Soc., 1996, 79, 109–113 CrossRef CAS.
- H. Obayashi and T. Kudo, Jpn. J. Appl. Phys., 1975, 14, 330–335 CrossRef CAS.
- I. Wærnhus, T. Grande and K. Wiik, Solid State Ionics, 2005, 176, 2609–2616 CrossRef.
- A. Berenov, E. Angeles, J. Rossiny, E. Raj, J. Kilner and A. Atkinson, Solid State Ionics, 2008, 179, 1090–1093 CrossRef CAS.
- E. A. Kiselev and V. A. Cherepanov, Solid State Ionics, 2011, 191, 32–39 CrossRef CAS.
- H. Oppermann, Z. Anorg. Allg. Chem., 1985, 523, 135–144 CrossRef CAS.
- M. Cancarevic, M. Zinkevich and F. Aldinger, CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2007, 31, 330–342 CrossRef CAS.
- M. E. Straumanis, J. Am. Chem. Soc., 1949, 71, 679–683 CrossRef CAS.
- F. H. Potter and R. G. Egdell, J. Mater. Chem., 1994, 4, 1647–1651 RSC.
- T. Nakamura, G. Petzow and L. J. Gauckler, Mater. Res. Bull., 1979, 143, 649–659 CrossRef.
- R. Pankajavalli and O. M. Sreedharan, Mater. Lett., 1995, 24, 247–251 CrossRef CAS.
- T. Maekawa, K. Kurosaki, H. Muta, M. Uno and S. Yamanaka, J. Alloys Compd., 2005, 390, 314–317 CrossRef CAS.
- I. Yasuda and T. Hikita, J. Electrochem. Soc., 1993, 140, 1699–1704 CrossRef CAS.
- J. Mizusaki, Y. Yonemura, H. Kamata, K. Ohyama, N. Mori and H. Takai, et al. , Solid State Ionics, 2000, 132, 167–180 CrossRef CAS.
- P. Ried, P. Holtappels, A. Wichser, A. Ulrich and T. Graule, J. Electrochem. Soc., 2008, 155, B1029–B1035 CrossRef CAS.
- Y. Adachi, N. Hatada and T. Uda, J. Electrochem. Soc., 2016, 163, F1084–F1090 CrossRef CAS.
- H.-C. Yu and K.-Z. Fung, Mater. Res. Bull., 2003, 38, 231–239 CrossRef CAS.
- S. Yoo, S. Choi, J. Kim, J. Shin and G. Kim, Electrochim. Acta, 2013, 100, 44–50 CrossRef CAS.
- M. Al Daroukh, V. V. Vashook, H. Ullmann, F. Tietz and I. Arual Raj, Solid State Ionics, 2003, 158, 141–150 CrossRef CAS.
- S. Takahashi, S. Nishimoto, M. Matsuda and M. Miyake, J. Am. Ceram. Soc., 2010, 93, 2329–2333 CrossRef CAS.
- Q. Li, H. Zhao, L. Huo, L. Sun, X. Cheng and J.-C. Grenier, Electrochem. Commun., 2007, 9, 1508–1512 CrossRef CAS.
- H.-I. Yoo, J. Mater. Res., 1989, 4, 23–27 CrossRef CAS.
- S. P. Denker, J. Appl. Phys., 1966, 37, 142–149 CrossRef CAS.
- J. M. Honig and T. B. Reed, Phys. Rev., 1968, 174, 1020–1026 CrossRef CAS.
- H. Kuwamoto, J. M. Honig and J. Appel, Phys. Rev. B: Condens. Matter Mater. Phys., 1980, 22, 2626–2636 CrossRef CAS.
- P. B. Allen, R. M. Wentzcovitch, W. W. Schulz and P. C. Canfield, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 4359–4363 CrossRef CAS.
- W. D. Ryden, A. W. Lawson and C. C. Sartain, Phys. Lett. A, 1968, 26, 209–210 CrossRef CAS.
- P. A. Miles, W. B. Westphal and A. von Hippel, Rev. Mod. Phys., 1957, 29, 279–307 CrossRef CAS.
- O. A. Marina, N. L. Canfield and J. W. Stevenson, Solid State Ionics, 2002, 149, 21–28 CrossRef CAS.
- A. A. Yaremchenko, B. Brinkmann, R. Janssen and J. R. Frade, Solid State Ionics, 2013, 247–248, 86–93 CrossRef CAS.
- J. B. Webb and M. Sayer, J. Phys. C: Solid State Phys., 1976, 9, 4151–4164 CrossRef CAS.
- L. H. Brixner, J. Inorg. Nucl. Chem., 1960, 14, 225–230 CrossRef CAS.
- P. B. Allen, H. Berger, O. Chauvet, L. Forro, T. Jarlborg and A. Junod, et al. , Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 4393–4398 CrossRef CAS.
- L. D. Ellerbeck, H. R. Shanks, P. H. Sidles and G. C. Danielson, J. Chem. Phys., 1961, 35, 298–302 CrossRef CAS.
- T. P. Pearsall and C. A. Lee, Phys. Rev. B: Condens. Matter Mater. Phys., 1974, 10, 2190–2194 CrossRef CAS.
- V. V. Kharton, F. M. B. Marques, J. A. Kilner and A. Atkinson, in Solid State Electrochemistry I, ed. V. V. Kharton, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2009, ch. 9, pp. 301–334 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, oxygen partial pressure dependence of the electrical conductivity of LaNi1−xFexO3, thermodynamic stability of lanthanum nickelates. See DOI: 10.1039/d0ma00983k |
| ‡ Deceased. |
| § Fe may partially occupy the Ni-sites in La4Ni3O10 and NiO.44 |
| ¶ Based on XRD patterns (Fig. 1), the amount of secondary phases (NiO and an unknown phase) is estimated to be ∼1 wt% or less. Therefore the secondary phases would not constitute a long-range conduction network and the high electrical conductivity observed in this study can be attributed to the main phase, LaNi1−xFexO3. Also, the fact that the conductivity value of the LaNiO3 polycrystal obtained in this study are almost identical to that of a single crystal at room temperature (Fig. 3(a)) suggests that the influence of secondary phases is not significant. |
| || In LaNi1−xFexO3 (0 ≤ x ≤ 0.4) solid solutions, electronic conduction should dominate over ionic conduction. Although they contain oxygen vacancies9,35,54 and can conduct oxide ions, the oxide ion conductivity at 800 °C is assumed to be less than 1 S cm−1 as in the case of other best oxide-ion-conducting perovskite oxides (LSC, LSGM, etc.).90. |
| This journal is © The Royal Society of Chemistry 2021 |
